Tactile Location Perception Encoded by Gamma-Band Power
Abstract
:1. Introduction
2. Methods
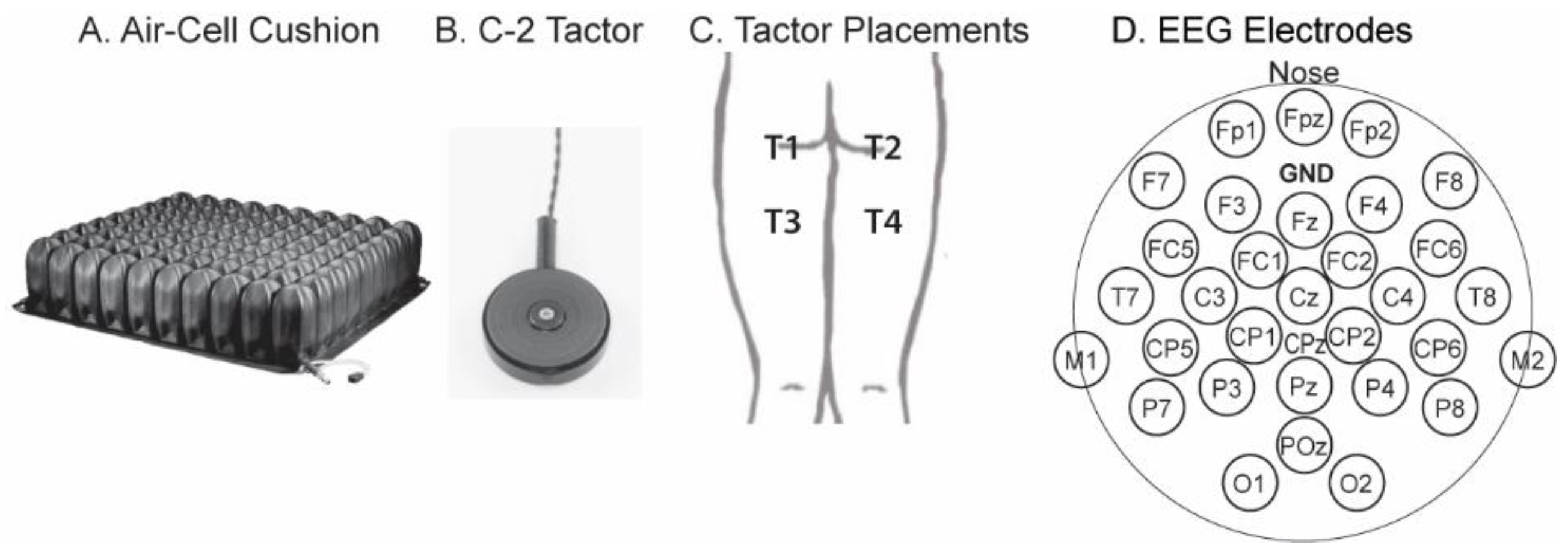
2.1. Experimental Setup
2.2. Signal Processing and Classification
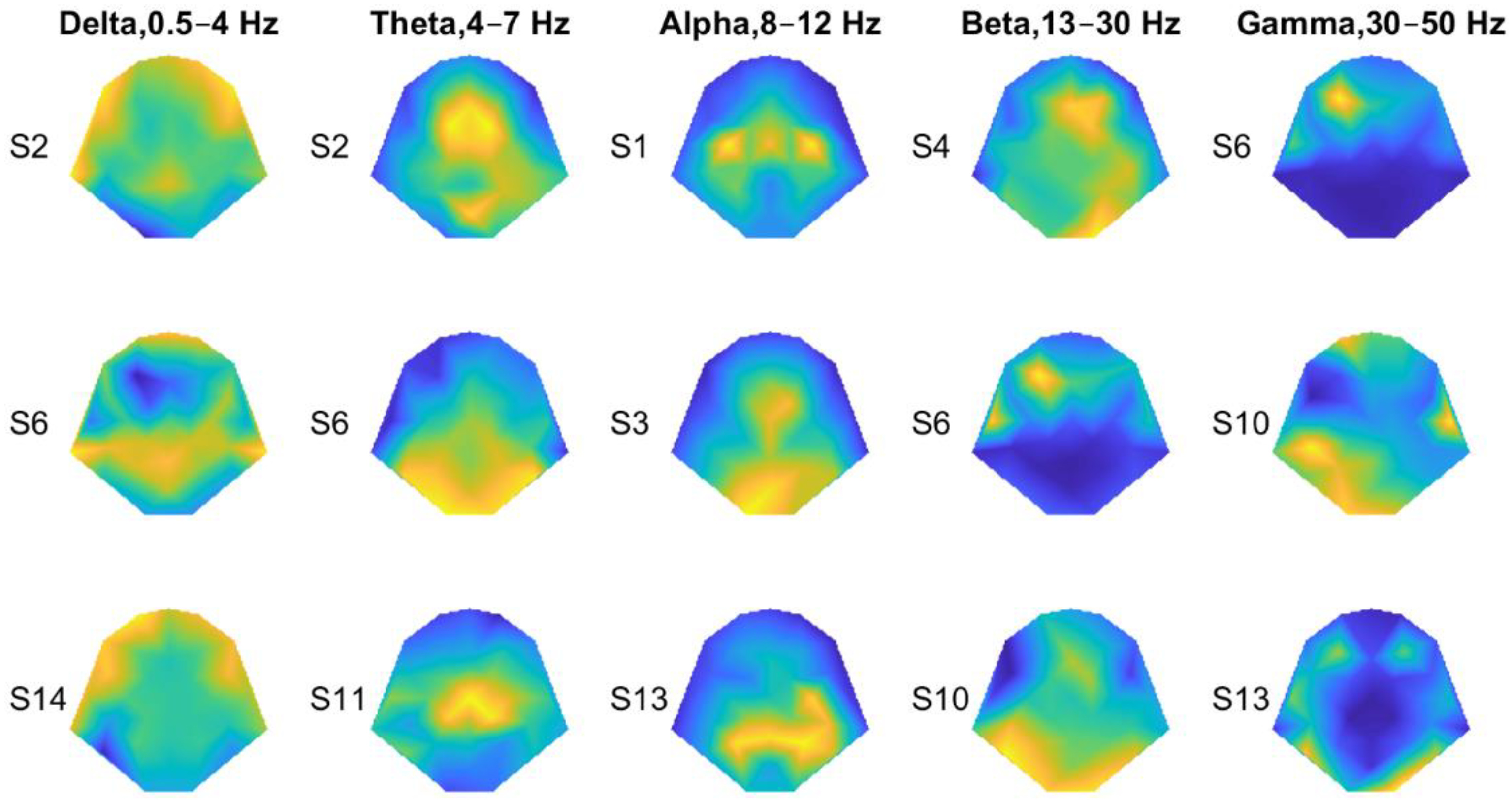
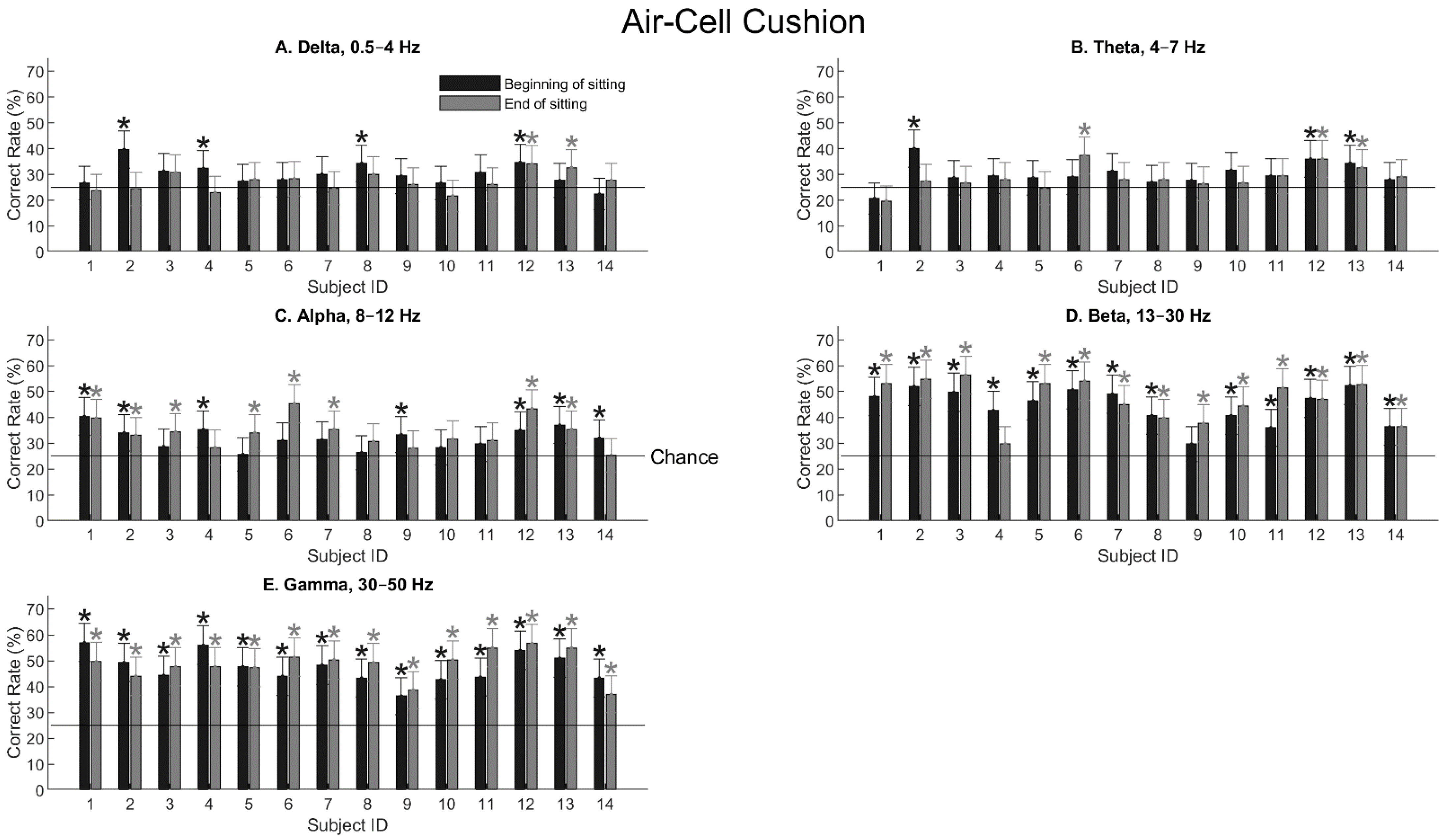
3. Results
3.1. Somatosensory-Evoked Potentials
3.2. Decoding Tactile Locations
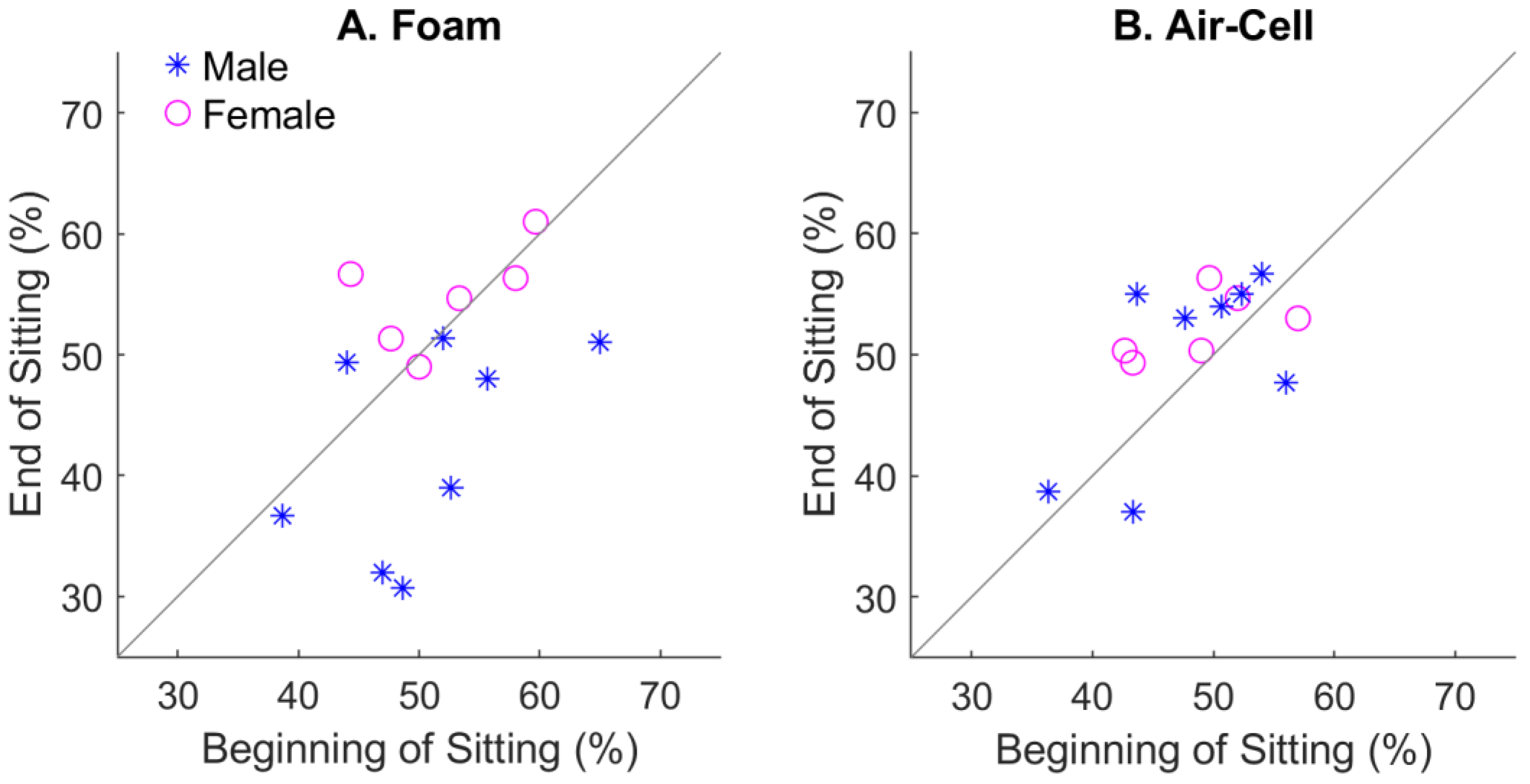
4. Discussion
4.1. Decoding Spatial Locations for Tactile Stimulation
4.2. Somatosensory Responses during Sitting
4.3. Gender Effect
4.4. Applications
4.5. Conclusions and Future Plans
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norman, J.F.; Norman, H.F.; Clayton, A.M.; Lianekhammy, J.; Zielke, G. The visual and haptic perception of natural object shape. Percept. Psychophys 2004, 66, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Helbig, H.B.; Ernst, M.O. Optimal integration of shape information from vision and touch. Exp. Brain Res. 2007, 179, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Nagano, H.; Yamada, Y. Psychophysical dimensions of tactile perception of textures. IEEE Trans. Haptics 2013, 6, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Green, B.G. Temperature perception on the hand during static versus dynamic contact with a surface. Atten. Percept. Psychophys. 2009, 71, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Harrar, V.; Harris, L.R. Eye position affects the perceived location of touch. Exp. Brain Res. 2009, 198, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Spence, C. Head orientation biases tactile localization. Brain Res. 2007, 1144, 136–141. [Google Scholar] [CrossRef]
- Pritchett, L.M.; Harris, L.R. Perceived touch location is coded using a gaze signal. Exp. Brain Res. 2011, 213, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Green, B.G. The perception of distance and location for dual tactile pressures. Percept. Psychophys 1982, 31, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfar, A.A.; Stambaugh, C.R.; Nicolelis, M.A. Encoding of tactile stimulus location by somatosensory thalamocortical ensembles. J. Neurosci. 2000, 20, 3761–3775. [Google Scholar] [CrossRef]
- Enander, J.M.D.; Jorntell, H. Somatosensory Cortical Neurons Decode Tactile Input Patterns and Location from Both Dominant and Non-dominant Digits. Cell Rep. 2019, 26, 3551–3560 e3554. [Google Scholar] [CrossRef]
- Lande, A.S.; Garvert, A.C.; Ebbesen, N.C.; Jordbraek, S.V.; Vervaeke, K. Representations of tactile object location in the retrosplenial cortex. Curr. Biol. 2023, 33, 4599–4610.e7. [Google Scholar] [CrossRef] [PubMed]
- Spitoni, G.F.; Galati, G.; Antonucci, G.; Haggard, P.; Pizzamiglio, L. Two forms of touch perception in the human brain. Exp. Brain Res. 2010, 207, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Hu, D.; Blohm, G. EEG-Based Perceived Tactile Location Prediction. IEEE Trans. Auton. Ment. Dev. 2015, 7, 342–348. [Google Scholar] [CrossRef]
- Gefen, A. The biomechanics of sitting-acquired pressure ulcers in patients with spinal cord injury or lesions. Int. Wound J. 2007, 4, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Pinto Pereira, S.M.; Ki, M.; Power, C. Sedentary behaviour and biomarkers for cardiovascular disease and diabetes in mid-life: The role of television-viewing and sitting at work. PLoS ONE 2012, 7, e31132. [Google Scholar] [CrossRef] [PubMed]
- Thosar, S.S.; Bielko, S.L.; Mather, K.J.; Johnston, J.D.; Wallace, J.P. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med. Sci. Sports Exerc. 2015, 47, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Ormeci, B.; Avci, E.; Kaspar, C.; Terim, O.E.; Erdogru, T.; Oge, A.E. A Novel Electrophysiological Method in the Diagnosis of Pudendal Neuropathy: Position-related Changes in Pudendal Sensory Evoked Potentials. Urology 2017, 99, 288.e1–288.e7. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.A.; Hajdu, M.; Gonzalez, E.G.; Michelsen, C.; Semedei, R. Cortical somatosensory evoked potentials: Effects of positional changes. Arch. Phys. Med. Rehabil. 1989, 70, 194–198. Available online: https://www.ncbi.nlm.nih.gov/pubmed/2923540 (accessed on 1 January 2023). [CrossRef]
- Gavrilenko, T.; Gatev, P.; Popivanov, D.; Gantchev, G.N. Effect of body and foot positions on the somatosensory evoked potentials. Act. Nerv. Super. 1990, 32, 81–86. Available online: https://www.ncbi.nlm.nih.gov/pubmed/2399802 (accessed on 1 January 2023).
- Fardo, F.; Spironelli, C.; Angrilli, A. Horizontal body position reduces cortical pain-related processing: Evidence from late ERPs. PLoS ONE 2013, 8, e81964. [Google Scholar] [CrossRef]
- Gefen, A. Tissue changes in patients following spinal cord injury and implications for wheelchair cushions and tissue loading: A literature review. Ostomy Wound Manag. 2014, 60, 34–45. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24515983 (accessed on 1 January 2023).
- Brienza, D.; Kelsey, S.; Karg, P.; Allegretti, A.; Olson, M.; Schmeler, M.; Zanca, J.; Geyer, M.J.; Kusturiss, M.; Holm, M. A randomized clinical trial on preventing pressure ulcers with wheelchair seat cushions. J. Am. Geriatr Soc. 2010, 58, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Ryun, S.; Kim, J.S.; Lee, H.; Chung, C.K. Tactile Frequency-Specific High-Gamma Activities in Human Primary and Secondary Somatosensory Cortices. Sci. Rep. 2017, 7, 15442. [Google Scholar] [CrossRef] [PubMed]
- Hansford, K.J.; Baker, D.H.; McKenzie, K.J.; Preston, C.E.J. Distinct neural signatures of multimodal resizing illusions. Neuropsychologia 2023, 187, 108622. [Google Scholar] [CrossRef] [PubMed]
- Valentini, E.; Shindy, A.; Witkovsky, V.; Stankewitz, A.; Schulz, E. Interindividual variability and individual stability of pain- and touch-related neuronal gamma oscillations. J. Neurophysiol. 2023, 129, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Trevarrow, M.; Gehringer, J.; Bergwell, H.; Arpin, D.; Heinrichs-Graham, E.; Wilson, T.W.; Kurz, M.J. Gamma somatosensory cortical oscillations are attenuated during the stance phase of human walking. Neurosci. Lett. 2020, 732, 135090. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Liu, C.Y.; Hsu, S.C.; Tseng, Y.J. Reduced coupling of somatosensory gating and gamma oscillation in panic disorder. Psychiatry Res. Neuroimaging 2021, 307, 111227. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.N.; Acharya, V.J. Overview of EEG Montages and Principles of Localization. J. Clin. Neurophysiol. 2019, 36, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gotman, J. Automatic removal of eye movement artifacts from the EEG using ICA and the dipole model. Prog. Nat. Sci. 2009, 19, 1165–1170. [Google Scholar] [CrossRef]
- Baura, G.D. Chapter 13—Electroencephalographs. In Medical Device Technologies; Baura, G.D., Ed.; Academic Press: Oxford, UK, 2012; pp. 275–295. [Google Scholar]
- Guliyev, N.J.; Ismailov, V.E. A Single Hidden Layer Feedforward Network with Only One Neuron in the Hidden Layer Can Approximate Any Univariate Function. Neural. Comput. 2016, 28, 1289–1304. [Google Scholar] [CrossRef]
- Lock, R.H.; Lock, P.F.; Morgan, K.L.; Lock, E.F.; Lock, D.F. Statistics: Unlocking the Power of Data, 2nd ed.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Dorfman, L.J. Sensory evoked potentials: Clinical applications in medicine. Annu. Rev. Med. 1983, 34, 473–489. [Google Scholar] [CrossRef]
- Smith, D.J.; Varghese, L.A.; Stepp, C.E.; Guenther, F.H. Comparison of steady-state visual and somatosensory evoked potentials for brain-computer interface control. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2014, 2014, 1234–1237. [Google Scholar] [CrossRef]
- Grundy, B.L. Monitoring of sensory evoked potentials during neurosurgical operations: Methods and applications. Neurosurgery 1982, 11, 556–575. Available online: https://www.ncbi.nlm.nih.gov/pubmed/6755296 (accessed on 1 January 2023). [CrossRef] [PubMed]
- Nuwer, M.R. Spinal cord monitoring. Muscle Nerve 1999, 22, 1620–1630. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Li, X.; Cui, H.; Xie, X.; Luk, K.D.; Hu, Y. Usefulness of Time-Frequency Patterns of Somatosensory Evoked Potentials in Identification of the Location of Spinal Cord Injury. J. Clin. Neurophysiol. 2015, 32, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Aminoff, M.J. Use of somatosensory evoked potentials to evaluate the peripheral nervous system. J. Clin. Neurophysiol. 1987, 4, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Macerollo, A.; Brown, M.J.N.; Kilner, J.M.; Chen, R. Neurophysiological Changes Measured Using Somatosensory Evoked Potentials. Trends Neurosci. 2018, 41, 294–310. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Staines, W.R. Modulatory effects of movement sequence preparation and covert spatial attention on early somatosensory input to non-primary motor areas. Exp. Brain Res. 2015, 233, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Sonoo, M.; Kobayashi, M.; Genba-Shimizu, K.; Mannen, T.; Shimizu, T. Detailed analysis of the latencies of median nerve somatosensory evoked potential components, 1: Selection of the best standard parameters and the establishment of normal values. Electroencephalogr Clin. Neurophysiol. 1996, 100, 319–331. [Google Scholar] [CrossRef]
- Webster, J.G. Medical Instrumentation: Application and Design, 4th ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Purves, D.; Augustine, G.; Fitzpatrick, D.; Katz, L.; LaMantia, A.; McNamara, J.; Williams, S. Neuroscience, 2nd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Kajimoto, H.; Kawakami, N.; Tachi, S. Psychophysical evaluation of receptor selective stimulation in electro-tactile display. SICE Div. Conf. Program Abstr. 2002, si2002, 418. [Google Scholar] [CrossRef]
- Custead, R.; Oh, H.; Rosner, A.O.; Barlow, S. Adaptation of the cortical somatosensory evoked potential following pulsed pneumatic stimulation of the lower face in adults. Brain Res. 2015, 1622, 81–90. [Google Scholar] [CrossRef]
- Abdouni, A.; Moreau, G.; Vargiolu, R.; Zahouani, H. Static and active tactile perception and touch anisotropy: Aging and gender effect. Sci. Rep. 2018, 8, 14240. [Google Scholar] [CrossRef] [PubMed]
- Sadato, N.; Ibanez, V.; Deiber, M.P.; Hallett, M. Gender difference in premotor activity during active tactile discrimination. Neuroimage 2000, 11, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gerling, G.J. An individual’s skin stiffness predicts their tactile acuity. J. Physiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Leban, B.; Fadda, P.; Fancello, G.; Nussbaum, M.A. Effect of prolonged sitting on body-seat contact pressures among quay crane operators: A pilot study. Work 2016, 55, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Kopplin, K.; Gefen, A. An air-cell-based cushion for pressure ulcer protection remarkably reduces tissue stresses in the seated buttocks with respect to foams: Finite element studies. J. Tissue Viability 2014, 23, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, Z.; Jiang, J. A 36-Class Bimodal ERP Brain-Computer Interface Using Location-Congruent Auditory-Tactile Stimuli. Brain Sci. 2020, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Savic, A.M.; Novicic, M.; Ethordevic, O.; Konstantinovic, L.; Miler-Jerkovic, V. Novel electrotactile brain-computer interface with somatosensory event-related potential based control. Front. Hum. Neurosci. 2023, 17, 1096814. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, L.; Syrov, N.; Miroshnikov, A.; Lebedev, M.; Kaplan, A. Event-Related Desynchronization Induced by Tactile Imagery: An EEG Study. eNeuro 2023, 10, ENEURO.0455-22.2023. [Google Scholar] [CrossRef]
- Chen, M.L.; Fu, D.; Boger, J.; Jiang, N. Age-Related Changes in Vibro-Tactile EEG Response and Its Implications in BCI Applications: A Comparison Between Older and Younger Populations. IEEE Trans. Neural. Syst. Rehabil. Eng. 2019, 27, 603–610. [Google Scholar] [CrossRef]


| Source | Sum Sq. | df | Mean Sq. | F | Prob > F |
|---|---|---|---|---|---|
| Sitting | 269.1 | 1 | 269.07 | 7.94 | 0.0056 |
| Frequency | 10,496.3 | 4 | 2624.08 | 77.42 | 0 |
| Gender | 496.9 | 1 | 496.9 | 14.66 | 0.0002 |
| Sitting:Frequency | 78.6 | 4 | 19.64 | 0.58 | 0.6781 |
| Sitting:Gender | 18 | 1 | 17.95 | 0.53 | 0.4681 |
| Frequency:Gender | 316.6 | 4 | 79.15 | 2.34 | 0.0592 |
| Error | 4202.8 | 124 | 33.89 | ||
| Total | 15,650.8 | 139 |
| Source | Sum Sq. | df | Mean Sq. | F | Prob > F |
|---|---|---|---|---|---|
| Sitting | 0.5 | 1 | 0.55 | 0.02 | 0.8937 |
| Frequency | 9462.5 | 4 | 2365.63 | 77.47 | 0 |
| Gender | 2.6 | 1 | 2.62 | 0.09 | 0.7701 |
| Sitting:Frequency | 152.5 | 4 | 38.12 | 1.25 | 0.294 |
| Sitting:Gender | 24.7 | 1 | 24.69 | 0.81 | 0.3703 |
| Frequency:Gender | 137 | 4 | 34.24 | 1.12 | 0.3496 |
| Error | 3786.2 | 124 | 30.53 | ||
| Total | 13,542.9 | 139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Dong, Y.; Gai, Y. Tactile Location Perception Encoded by Gamma-Band Power. Bioengineering 2024, 11, 377. https://doi.org/10.3390/bioengineering11040377
Chen Q, Dong Y, Gai Y. Tactile Location Perception Encoded by Gamma-Band Power. Bioengineering. 2024; 11(4):377. https://doi.org/10.3390/bioengineering11040377
Chicago/Turabian StyleChen, Qi, Yue Dong, and Yan Gai. 2024. "Tactile Location Perception Encoded by Gamma-Band Power" Bioengineering 11, no. 4: 377. https://doi.org/10.3390/bioengineering11040377






