Micro-Computed Tomography Analysis of Peri-Implant Bone Defects Exposed to a Peri-Implantitis Microcosm, with and without Bone Substitute, in a Rabbit Model: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
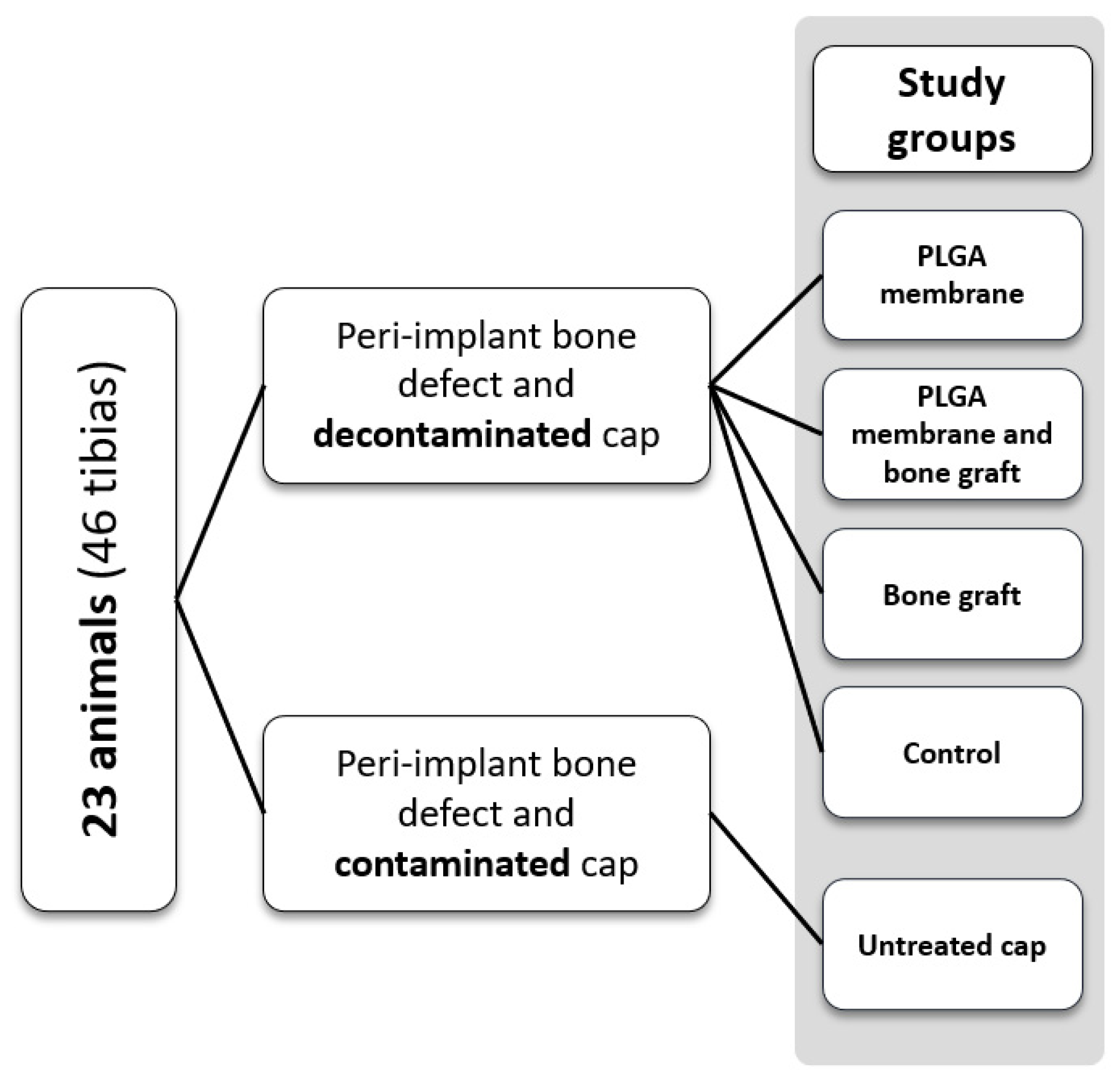
2.1. Study Design
2.2. Contamination of Titanium Caps
2.3. Surgical Procedures
2.4. Micro-CT Imaging Study
2.5. Statistical Analysis
3. Results
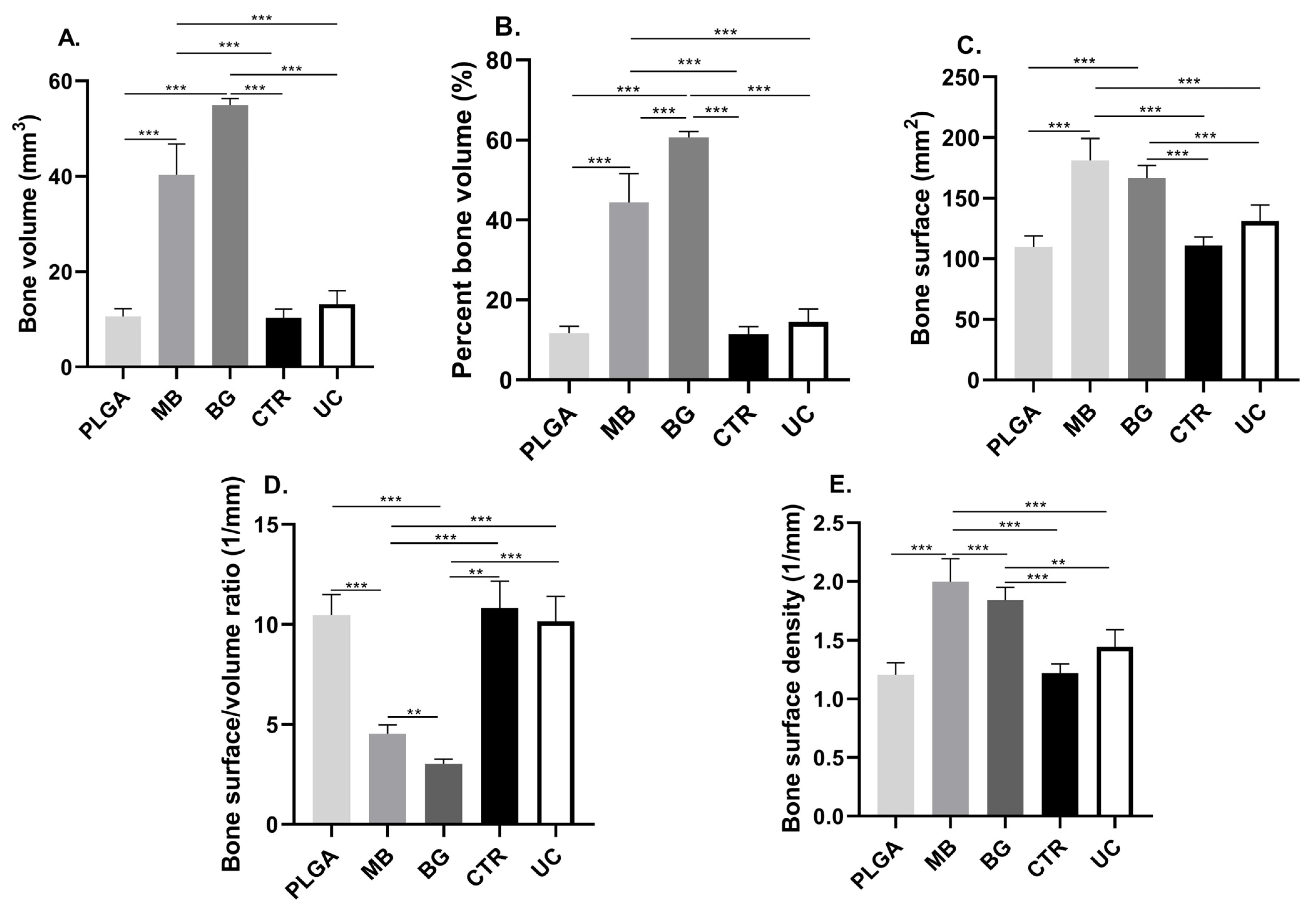
3.1. Bone Volume (BV) and Percent Bone Volume (BV/TV)
3.2. Bone Surface (BS)
3.3. Bone Surface Density (BS/TV)
| PLGA | MB | BG | CRT | UC | |
|---|---|---|---|---|---|
| BV (mm3) | 10.61 ± 1.60 | 40.32 ± 6.49 | 55.00 ± 1.32 | 10.40 ± 1.73 | 13.18 ± 2.86 |
| BV/TV (%) | 11.69 ± 1.77 | 44.44 ± 7.16 | 60.62 ± 1.46 | 11.46 ± 1.91 | 14.53 ± 3.15 |
| BS (mm2) | 109.80 ± 9.17 | 181.10 ± 18.26 | 166.40 ± 10.52 | 110.70 ± 7.17 | 131.20 ± 13.30 |
| BS/BV (1/mm) | 10.47 ± 1.01 | 4.53 ± 0.46 | 3.03 ± 0.23 | 10.82 ± 1.33 | 10.16 ± 1.23 |
| BS/TV (1/mm) | 1.20 ± 0.10 | 1.99 ± 0.19 | 1.83 ± 0.11 | 1.22 ± 0.07 | 1.44 ± 0.14 |
| PLGA | MB | BG | CRT | UC | |
|---|---|---|---|---|---|
| BV (mm3) | 11.01 ± 2.39 | 48.38 ± 4.83 | 49.29 ± 6.23 | 13.83 ± 6.00 | 9.66 ± 2.39 |
| BV/TV (%) | 12.14 ± 2.63 | 53.33 ± 5.32 | 54.33 ± 6.86 | 15.25 ± 6.61 | 10.65 ± 2.64 |
| BS (mm2) | 119.6 ± 20.86 | 149.80 ± 10.02 | 167.8 ± 12.95 | 124.90 ± 25.74 | 102.60 ± 19.49 |
| BS/BV (1/mm) | 10.98 ± 0.80 | 3.12 ± 0.39 | 3.46 ± 0.61 | 9.83 ± 2.49 | 10.75 ± 0.97 |
| BS/TV (1/mm) | 1.31 ± 0.23 | 1.65 ± 0.10 | 1.85 ± 0.14 | 1.37 ± 0.28 | 1.13 ± 0.21 |

| 15 Days | 30 Days | |
|---|---|---|
| PLGA membrane | ||
| BV (mm3) | 10.61 ± 1.60t | 11.01 ± 2.39 |
| BV/TV (%) | 11.69 ± 1.77t | 12.14 ± 2.63 |
| BS (mm2) | 109.80 ± 9.17t | 119.6 ± 20.86 |
| BS/BV (1/mm) | 10.47 ± 1.01mw | 10.98 ± 0.80 |
| BS/TV (1/mm) | 1.20 ± 0.10t | 1.31 ± 0.23 |
| Membrane and bone graft | ||
| BV (mm3) | 40.32 ± 6.49t | 48.38 ± 4.83 |
| BV/TV (%) | 44.44 ± 7.16t | 53.33 ± 5.32 |
| BS (mm2) | 181.10 ± 18.26t | 149.80 ± 10.02 |
| BS/BV (1/mm) | 4.53 ± 0.46mw | 3.12 ± 0.39 |
| BS/TV (1/mm) | 1.99 ± 0.19t | 1.65 ± 0.10 |
| Bone graft | ||
| BV (mm3) | 55.00 ± 1.32t | 49.29 ± 6.23 |
| BV/TV (%) | 60.62 ± 1.46t | 54.33 ± 6.86 |
| BS (mm2) | 166.40 ± 10.52t | 167.8 ± 12.95 |
| BS/BV (1/mm) | 3.03 ± 0.23mw | 3.46 ± 0.61 |
| BS/TV (1/mm) | 1.83 ± 0.11t | 1.85 ± 0.14 |
| Control group | ||
| BV (mm3) | 10.40 ± 1.73t | 13.83 ± 6.00 |
| BV/TV (%) | 11.46 ± 1.91t | 15.25 ± 6.61 |
| BS (mm2) | 110.70 ± 7.17t | 124.90 ± 25.74 |
| BS/BV (1/mm) | 10.82 ± 1.33mw | 9.83 ± 2.49 |
| BS/TV (1/mm) | 1.22 ± 0.07t | 1.37 ± 0.28 |
| Untreated cap | ||
| BV (mm3) | 13.18 ± 2.86t | 9.66 ± 2.39 |
| BV/TV (%) | 14.53 ± 3.15t | 10.65 ± 2.64 |
| BS (mm2) | 131.20 ± 13.30t | 102.60 ± 19.49 |
| BS/BV (1/mm) | 10.16 ± 1.23mw | 10.75 ± 0.97 |
| BS/TV (1/mm) | 1.44 ± 0.14t | 1.13 ± 0.21 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef]
- Wada, M.; Mameno, T.; Otsuki, M.; Kani, M.; Tsujioka, Y.; Ikebe, K. Prevalence and risk indicators for peri-implant diseases: A literature review. Jpn. Dent. Sci. Rev. 2021, 57, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Fragkioudakis, I.; Tseleki, G.; Doufexi, A.-E.; Sakellari, D. Current Concepts on the Pathogenesis of Peri-implantitis: A Narrative Review. Eur. J. Dent. 2021, 15, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Elefteriou, F.; Busse, B.; Fiedler, I.A.; Kwon, R.Y.; Farell, E.; Ahmad, M.; Ignatius, A.; Grover, L.M.; Geris, L.; et al. Why animal experiments are still indispensable in bone research: A statement by the European Calcified Tissue Society. J. Bone Miner. Res. 2023, 38, 1045–1061. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Sylvestre, N.; Bouchard, P.; Chaussain, C.; Bardet, C. Pre-Clinical Models in Implant Dentistry: Past, Present, Future. Biomedicines 2021, 9, 1538. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sculean, A.; Engebretson, S.P.; Becker, J.; Sager, M. Animal models for peri-implant mucositis and peri-implantitis. Periodontology 2015, 68, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Musskopf, M.L.; Stadler, A.F.; Wikesjö, U.M.; Susin, C. The Minipig Intraoral Dental Implant Model: A Systematic Review and Meta-analysis. PLoS ONE 2022, 17, e0264475. [Google Scholar] [CrossRef] [PubMed]
- Matos, F.G.; Stremel, A.C.A.; Lipinski, L.C.; Cirelli, J.A.; Santos, F.A.D. Dental implants in large animal models with experimental systemic diseases: A Systematic review. Lab. Anim. 2023, 57, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Chew, R.J.J.; Lu, J.X.; Sim, Y.F.; Yeo, A. Rodent Peri-implantitis models: A Systematic review and Meta-analysis of morphological changes. J. Periodontal Implant. Sci. 2022, 52, 479. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Rodriguez-Fontan, F.; Eckstein, K.; Muralidharan, A.; Uzcategui, A.C.; Fuchs, J.R.; Weatherford, S.; Erickson, C.B.; Bryant, S.J.; Ferguson, V.L. Rabbit Model of Physeal Injury for the Evaluation of Regenerative Medicine Approaches. Tissue Eng. Part C Methods 2019, 25, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.D.; Hiebert, B.D.; Panahifar, A.; Andronowski, J.M.; Ashique, A.M.; King, G.A.; Arnason, T.; Swekla, K.J.; Pivonka, P.; Cooper, D.M.L. Cortical Bone Porosity in Rabbit Models of Osteoporosis. J. Bone Miner. Res. 2020, 35, 2211–2228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tang, J.; Zhou, D.; Weng, Y.; Qin, W.; Liu, C.; Lv, S.; Wang, W.; Zhao, X. Electrospun Icariin-Loaded Core-Shell Collagen, Polycaprolactone, Hydroxyapatite Composite Scaffolds for the Repair of Rabbit Tibia Bone Defects. Int. J. Nanomed. 2020, 15, 3039–3056. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Aramburú, J.; Pérez-Díaz, L.; do Prado, T.D.; Dedavid, B.A.; Mazon, P.N.; De Aza, P. Can changes in implant macrogeometry accelerate the osseointegration process? An in vivo experimental biomechanical and histological evaluations. PLoS ONE 2020, 15, e0233304. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Shi, X.Y.; Yu, W.T.; Wei, X.W.; Cheng, L.L.; Qiu, X.; Li, B.R.; Fan, D.C.; Li, J.L.; Zhang, X.Z.; et al. In vivo biocompatibility evaluation of Zn-0.05Mg-(0, 0.5, 1wt%)Ag implants in New Zealand rabbits. Mater. Sci. Eng. C 2021, 119, 111435. [Google Scholar] [CrossRef] [PubMed]
- Öz, U.C.; Toptaş, M.; Küçüktürkmen, B.; Devrim, B.; Saka, O.M.; Deveci, M.S.; Bilgili, H.; Ünsal, E.; Bozkır, A. Guided bone regeneration by the development of alendronate sodium loaded in-situ gel and membrane formulations. Eur. J. Pharm. Sci. 2020, 155, 105561. [Google Scholar] [CrossRef] [PubMed]
- Trento, G.; Carvalho, P.H.; Reis, E.N.R.; Spin-Neto, R.; Bassi, A.P.F.; Pereira-Filho, V.A. Bone formation around two titanium implant surfaces placed in bone defects with and without a bone substitute material: A histological, histomorphometric, and micro-computed tomography evaluation. Clin. Implant. Dent. Relat. Res. 2020, 22, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.; Mardas, N.; Spratt, D.; Hassan, I.A.; Walters, N.J.; Beltrán, V.; Donos, N. The Effect of Microcosm Biofilm Decontamination on Surface Topography, Chemistry, and Biocompatibility Dynamics of Implant Titanium Surfaces. Int. J. Mol. Sci. 2022, 23, 10033. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.; Griffiths, P. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Orhan, K. Micro-Computed Tomography (micro-CT) in Medicine and Engineering; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Sanz-Esporrin, J.; Blanco, J.; Sanz-Casado, J.V.; Muñoz, F.; Sanz, M. The adjunctive effect of rhBMP-2 on the regeneration of peri-implant bone defects after experimental peri-implantitis. Clin. Oral Implant. Res. 2019, 30, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Seong, W.J.; Kotsakis, G.; Huh, J.-K.; Jeong, S.C.; Nam, K.Y.; Kim, J.R.; Heo, Y.C.; Kim, H.-C.; Zhang, L.; Evans, M.D. Clinical and microbiologic investigation of an expedited peri-implantitis dog model: An animal study. BMC Oral Health 2019, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Jowsey, J. Studies of Haversian systems in man and some animals. J. Anat. 1966, 100, 857–864. [Google Scholar] [PubMed]
- Sanz-Esporrín, J.; Carral, C.; Blanco, J.; Sanz-Casado, J.V.; Muñoz, F.; Sanz, M. Differences in the progression of experimental peri-implantitis depending on the implant to abutment connection. Clin. Oral Investig. 2021, 25, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Voor, M.J.; Yang, S.; Burden, R.L.; Waddell, S.W. In vivo micro-CT scanning of a rabbit distal femur: Repeatability and reproducibility. J. Biomech. 2008, 41, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.H.L.; Gaêta-Araujo, H.; Brasil, D.M.; Madlum, D.V.; Freitas, D.Q.; Haiter-Neto, F.; Oliveira-Santos, C. Impact of micro-computed tomography reconstruction protocols on bone microarchitecture analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 411–417. [Google Scholar] [CrossRef]
- Adams, G.J.; Cook, R.B.; Hutchinson, J.R.; Zioupos, P. Microarchitecture and morphology of bone tissue over a wide range of BV/TV assessed by micro-computed tomography and three different threshold backgrounds. Med. Eng. Phys. 2022, 106, 103828. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.-K.; Li, L.; Qin, L.; Wang, X.-L.; Lai, Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bozo, I.Y.; Drobyshev, A.Y.; Redko, N.A.; Komlev, V.S.; Isaev, A.A.; Deev, R.V. Bringing a Gene-Activated Bone Substitute into Clinical Practice: From Bench to Bedside. Front. Bioeng. Biotechnol. 2021, 9, 599300. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Abboud, M.; Ramirez-Fernández, M.P.; Maté-Sánchez, J.E.; Negri, B.; Won, A.; Romanos, G. Porous titanium granules in critical size defects of rabbit tibia with or without membranes. Int. J. Oral Sci. 2014, 6, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, L.; Pawłowska, M.; Radzki, R.P.; Bieńko, M.; Polkowska, I.; Belcarz, A.; Karpiński, M.; Słowik, T.; Matuszewski, Ł.; Ślósarczyk, A.; et al. Effect of a carbonated HAP/Β-glucan composite bone substitute on healing of drilled bone voids in the proximal tibial metaphysis of rabbits. Mater. Sci. Eng. C 2015, 53, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Seman, C.N.Z.C.; Zakaria, Z.; Sharifudin, M.A.; Ahmad, A.C.; Awang, M.S.; Yusof, N.M.; Buyong, Z. Model of A Critical Size Defect in the New Zealand White Rabbit’s Tibia. Int. Med. J. Malays. 2018, 17. [Google Scholar] [CrossRef]
- Aaboe, M.; Pinholt, E.M.; Hjørting-Hansen, E. Unicortical critical size defect of rabbit tibia is larger than 8 mm. J. Craniofacial Surg. 1994, 5, 201–203. [Google Scholar] [CrossRef]
- Da Silva Brum, I.; Elias, C.N.; De Carvalho, J.J.; Pires, J.L.S.; Pereira, M.; De Biasi, R. Properties of a bovine collagen type I membrane for guided bone regeneration applications. e-Polymers 2021, 21, 210–221. [Google Scholar] [CrossRef]
- Itsumi, Y.; Sasaki, J.; Tsuboi, R.; Yamaguchi, S.; Kitagawa, H.; Imazato, S. Development of layered PLGA membranes for periodontal tissue regeneration. Dent. Mater. 2018, 34, 538–550. [Google Scholar] [CrossRef]
- Alqahtani, A. Guided Tissue and Bone Regeneration Membranes: A review of biomaterials and techniques for periodontal treatments. Polymers 2023, 15, 3355. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier membranes for tissue regeneration in dentistry. Biomater. Investig. Dent. 2021, 8, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, S.; Shi, B.; Wang, Y.; Chen, Y.; Wang, X.; Lee, E.; Jiang, H.B. Advances in modification methods based on biodegradable membranes in Guided Bone/Tissue Regeneration: A review. Polymers 2022, 14, 871. [Google Scholar] [CrossRef]
- Waletzko-Hellwig, J.; Pohl, C.; Loeffler, H.; Dau, M.; Schlosser, M.; Bader, R.; Klinder, A. In-vitro analysis of resorption processes following high hydrostatic pressure treatment of human trabecular bone. Mater. Des. 2023, 225, 111539. [Google Scholar] [CrossRef]
- Williams, J.A.; Windmill, J.F.C.; Tanner, K.E.; Riddell, J.S.; Coupaud, S. Global and site-specific analysis of bone in a rat model of spinal cord injury-induced osteoporosis. Bone Rep. 2020, 12, 100233. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Martin, K.L. Handbook of Histology Methods for Bone and Cartilage; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003; ISSN 978-1-61737-277-3. [Google Scholar]
- Zenzes, M.; Zaslansky, P. Micro-CT data of early physiological cancellous bone formation in the lumbar spine of female C57BL/6 mice. Sci. Data 2021, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.A.; Zaghloul, M.; Mahmoud, W.; Helal, M.E.; Grawish, M.E. Gelfoam haemostatic agent with or without autologous bone marrow-derived stem cells for the regeneration of critical-size mandibular defects in the rabbit. Int. J. Oral Maxillofac. Surg. 2018, 47, 1488–1494. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panes, C.; Valdivia-Gandur, I.; Veuthey, C.; Sousa, V.; del Sol, M.; Beltrán, V. Micro-Computed Tomography Analysis of Peri-Implant Bone Defects Exposed to a Peri-Implantitis Microcosm, with and without Bone Substitute, in a Rabbit Model: A Pilot Study. Bioengineering 2024, 11, 397. https://doi.org/10.3390/bioengineering11040397
Panes C, Valdivia-Gandur I, Veuthey C, Sousa V, del Sol M, Beltrán V. Micro-Computed Tomography Analysis of Peri-Implant Bone Defects Exposed to a Peri-Implantitis Microcosm, with and without Bone Substitute, in a Rabbit Model: A Pilot Study. Bioengineering. 2024; 11(4):397. https://doi.org/10.3390/bioengineering11040397
Chicago/Turabian StylePanes, Camila, Iván Valdivia-Gandur, Carlos Veuthey, Vanessa Sousa, Mariano del Sol, and Víctor Beltrán. 2024. "Micro-Computed Tomography Analysis of Peri-Implant Bone Defects Exposed to a Peri-Implantitis Microcosm, with and without Bone Substitute, in a Rabbit Model: A Pilot Study" Bioengineering 11, no. 4: 397. https://doi.org/10.3390/bioengineering11040397
APA StylePanes, C., Valdivia-Gandur, I., Veuthey, C., Sousa, V., del Sol, M., & Beltrán, V. (2024). Micro-Computed Tomography Analysis of Peri-Implant Bone Defects Exposed to a Peri-Implantitis Microcosm, with and without Bone Substitute, in a Rabbit Model: A Pilot Study. Bioengineering, 11(4), 397. https://doi.org/10.3390/bioengineering11040397






