Predictive Refined Computational Modeling of ACL Tear Injury Patterns
Abstract
:1. Introduction
2. Methods
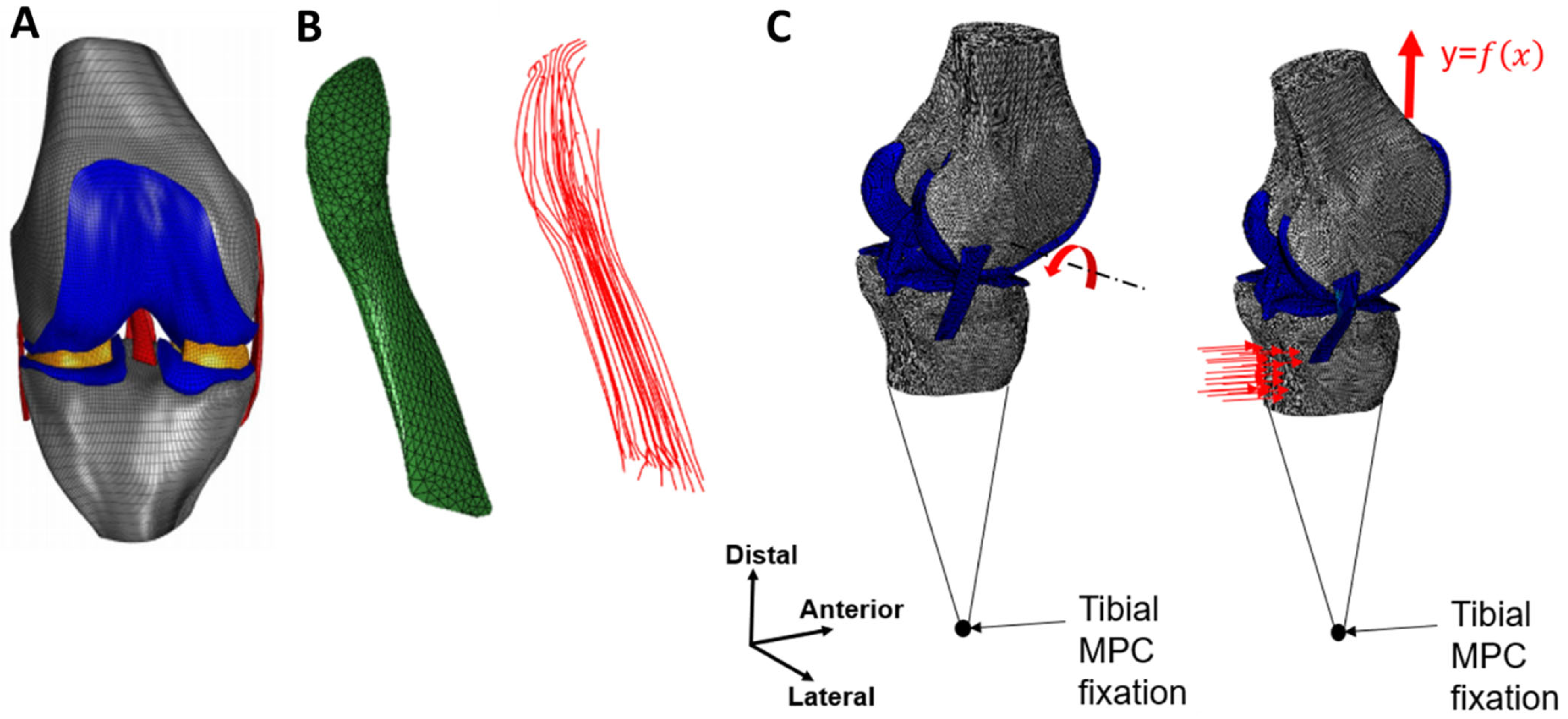
2.1. Model Geometry
2.2. Composite Representation of the ACL
2.3. Boundary Conditions
2.4. Material Properties
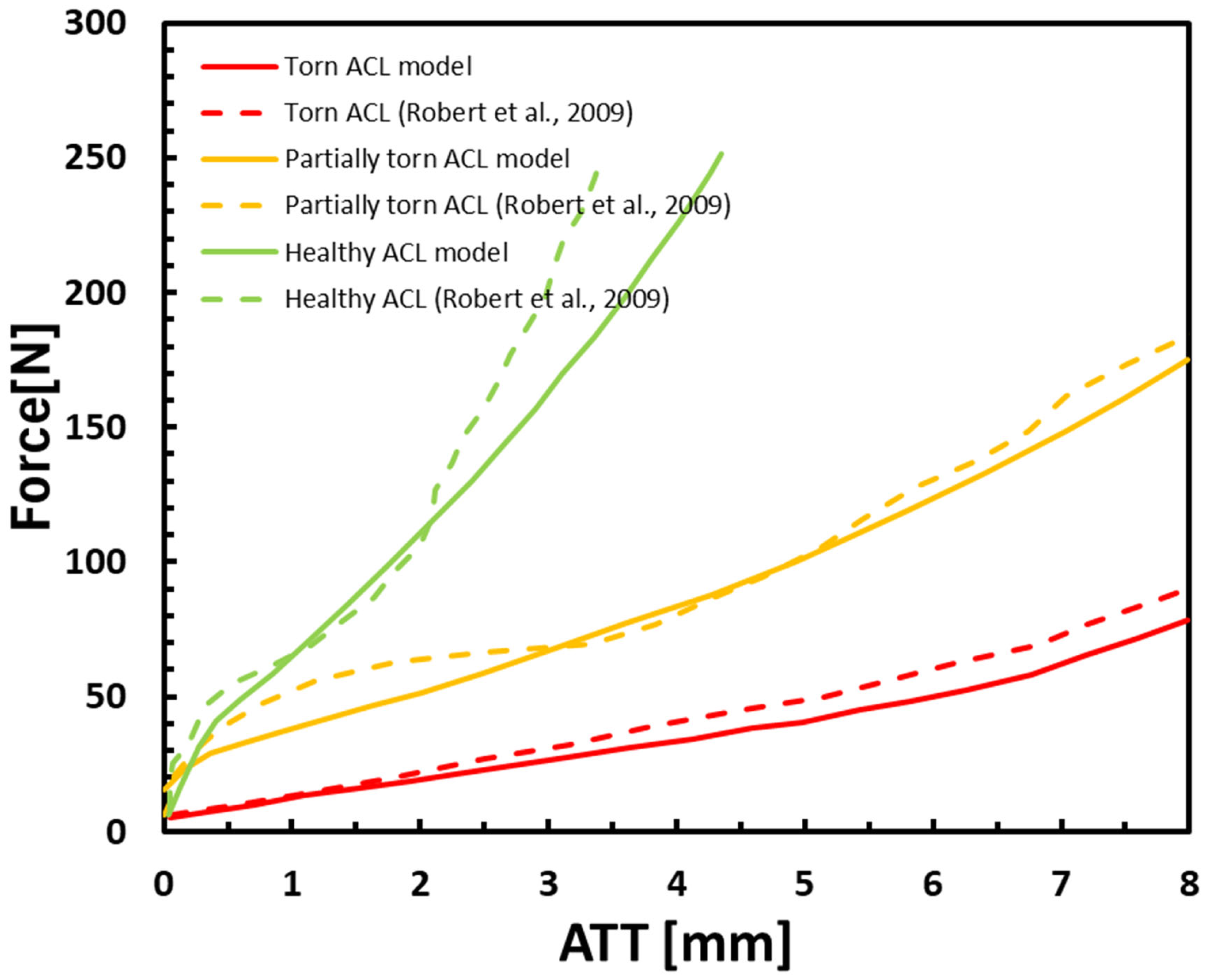
2.5. Lachman Test Model Validation
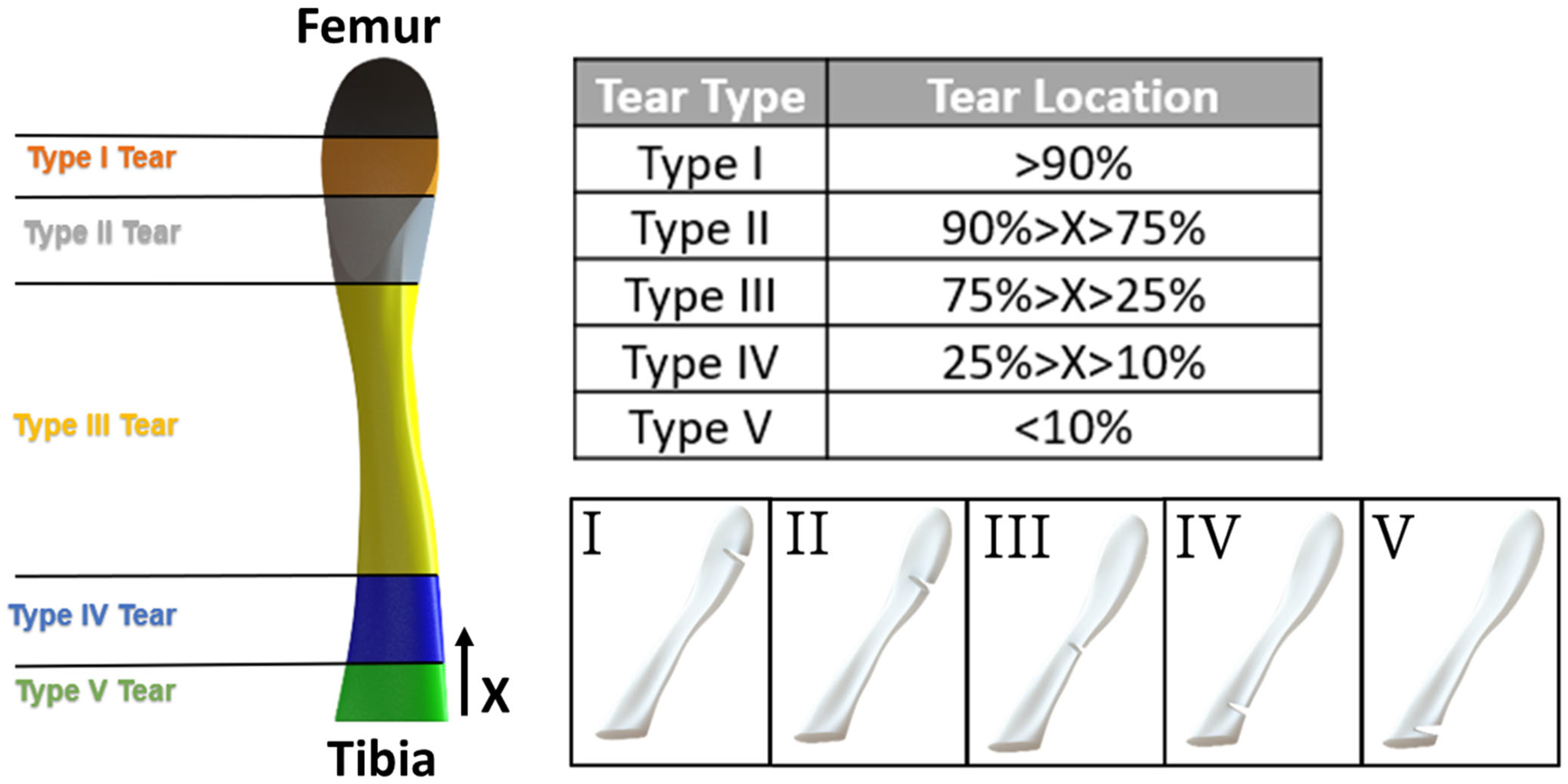
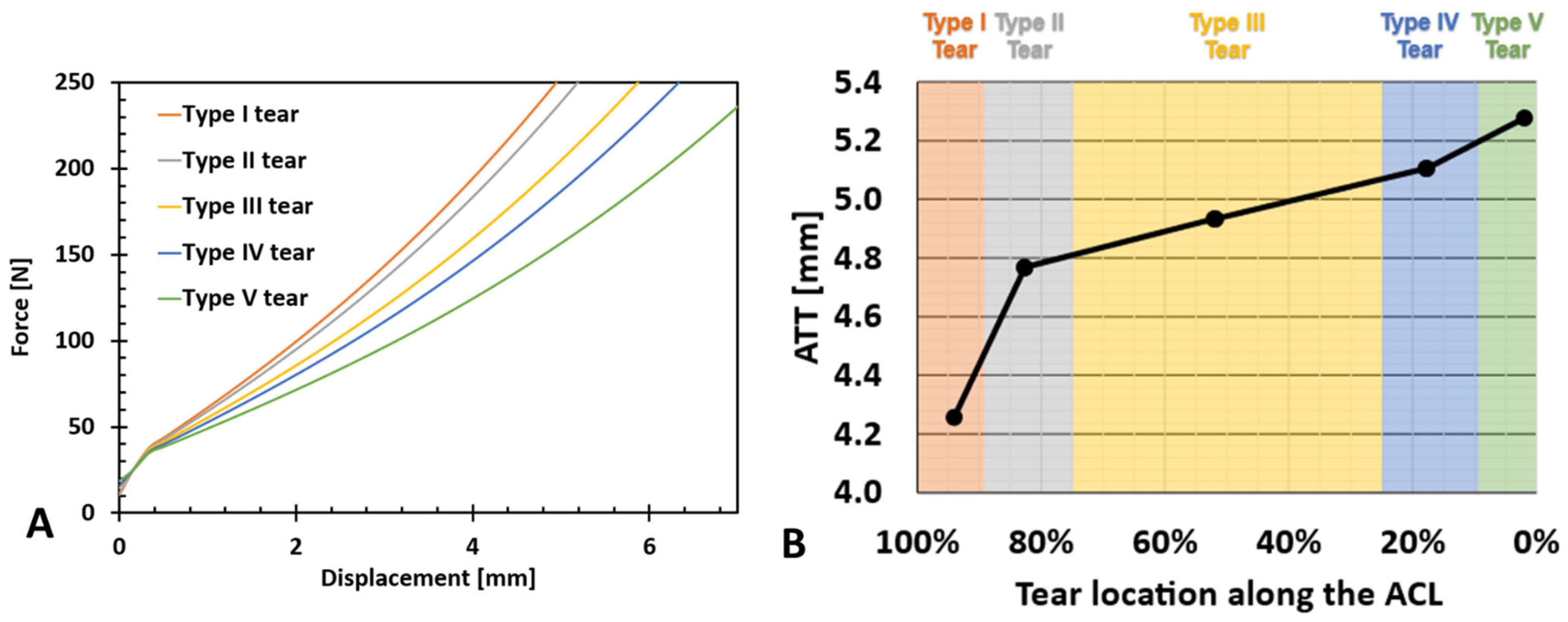
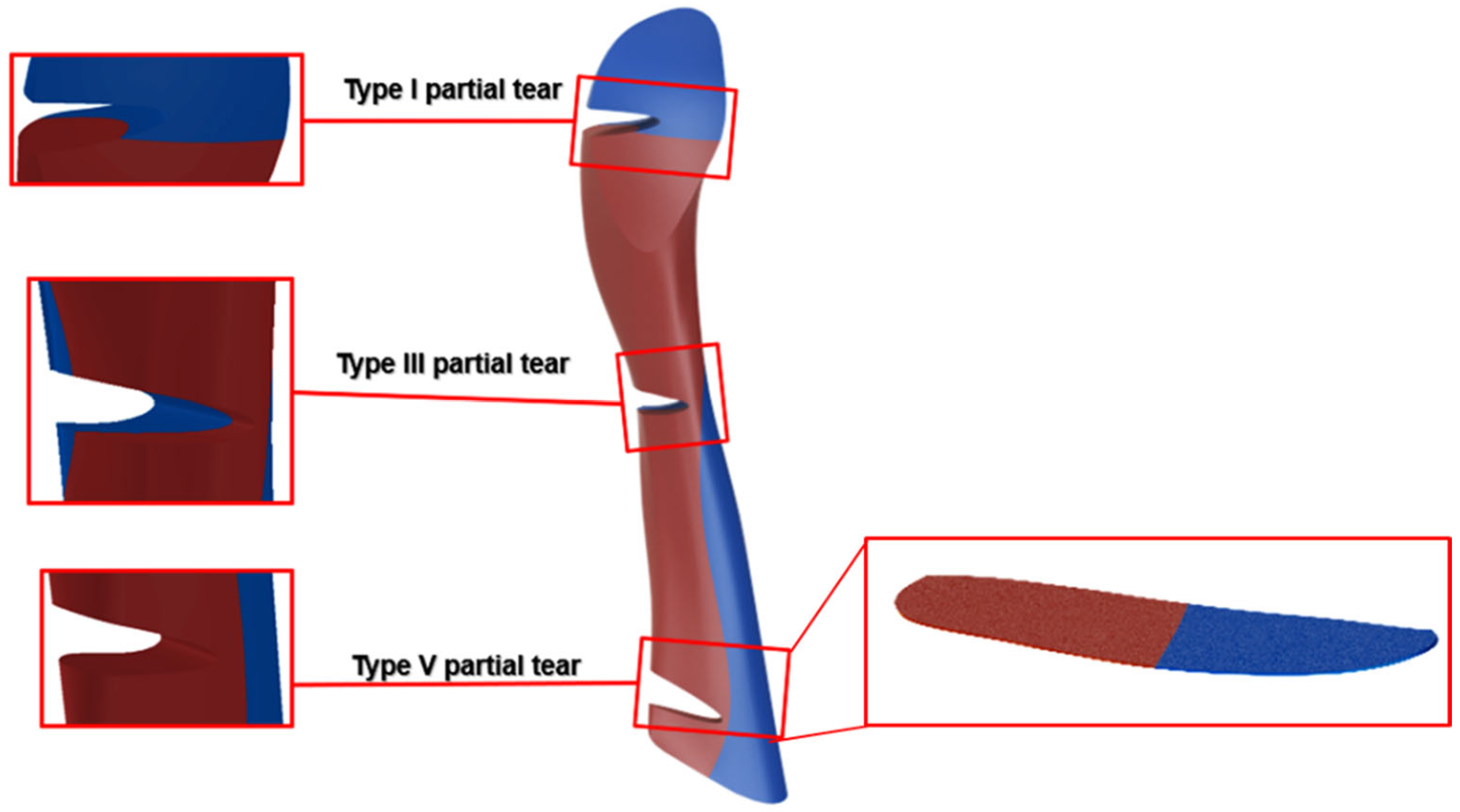
2.6. Tear Type and Size Model Generation
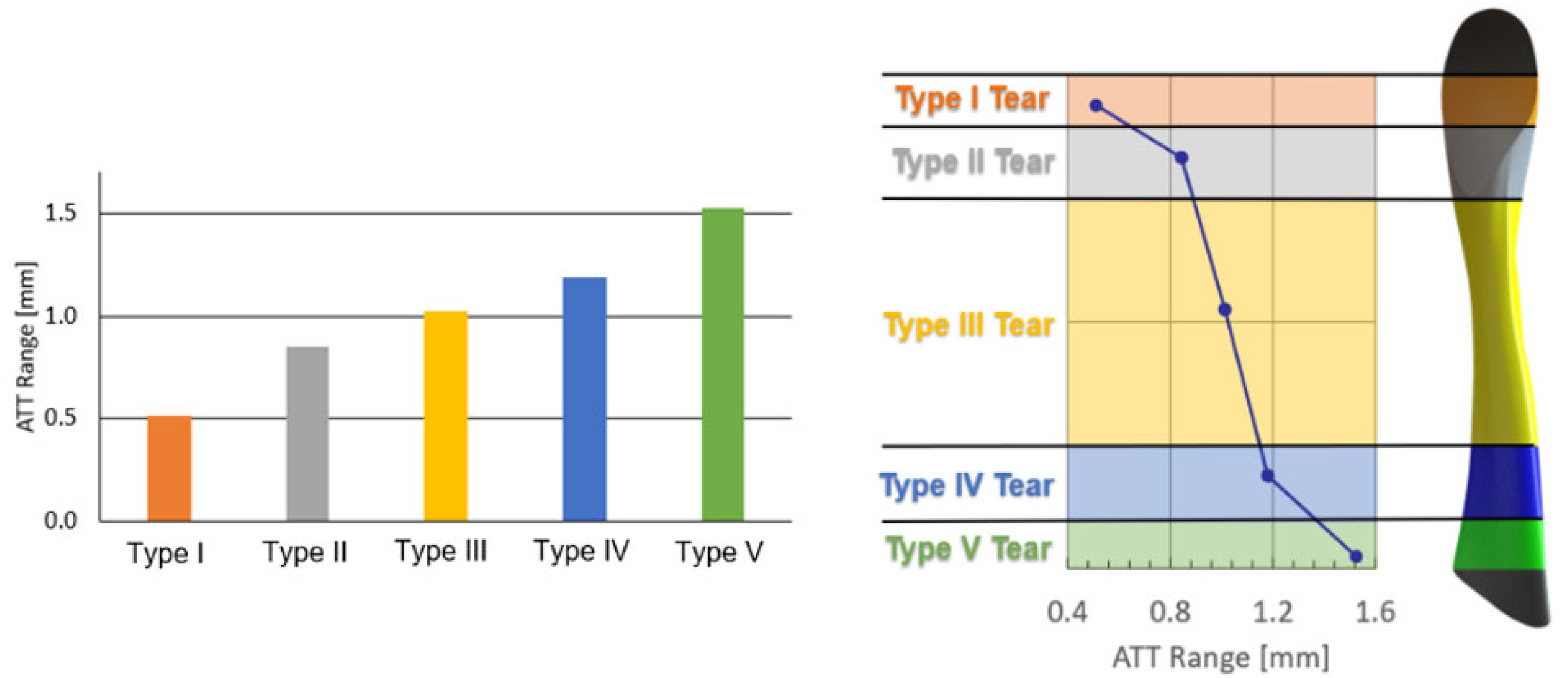
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escamilla, R.F.; MacLeod, T.D.; Wilk, K.E.; Paulos, L.; Andrews, J.R. Anterior Cruciate Ligament Strain and Tensile Forces for Weight-Bearing and Non-Weight-Bearing Exercises: A Guide to Exercise Selection. J. Orthop. Sports Phys. Ther. 2012, 42, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Hootman, J.M.; Albohm, M.J. Anterior Cruciate Ligament Injury Prevention and Primary Prevention of Knee Osteoarthritis. J. Athl. Train. 2012, 47, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Hewett, T.E.; Ford, K.R.; Hoogenboom, B.J.; Myer, G.D. Understanding and Preventing Acl Injuries: Current Biomechanical and Epidemiologic Considerations—Update 2010. N. Am. J. Sports Phys. Ther. 2010, 5, 234–251. [Google Scholar] [PubMed]
- Hewett, T.E.; Myer, G.D.; Ford, K.R.; Paterno, M.V.; Quatman, C.E. Mechanisms, Prediction, and Prevention of ACL Injuries: Cut Risk with Three Sharpened and Validated Tools. J. Orthop. Res. 2016, 34, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhou, H.; Quan, W.; Gusztav, F.; Wang, M.; Baker, J.S.; Gu, Y. Accurately and Effectively Predict the ACL Force: Utilizing Biomechanical Landing Pattern before and after-Fatigue. Comput. Methods Programs Biomed. 2023, 241, 107761. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, X.; Cen, X.; Baker, J.S.; Gu, Y. Single-Leg Landings Following a Volleyball Spike May Increase the Risk of Anterior Cruciate Ligament Injury More than Landing on Both-Legs. Appl. Sci. 2021, 11, 130. [Google Scholar] [CrossRef]
- Musahl, V.; Karlsson, J. Anterior Cruciate Ligament Tear. N. Engl. J. Med. 2019, 380, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Dargel, J.; Gotter, M.; Mader, K.; Pennig, D.; Koebke, J.; Schmidt-Wiethoff, R. Biomechanics of the Anterior Cruciate Ligament and Implications for Surgical Reconstruction. Strateg. Trauma Limb Reconstr. 2007, 2, 1–12. [Google Scholar] [CrossRef]
- Beasley, L.S.; Weiland, D.E.; Vidal, A.F.; Chhabra, A.; Herzka, A.S.; Feng, M.T.; West, R.V. Anterior Cruciate Ligament Reconstruction: A Literature Review of the Anatomy, Biomechanics, Surgical Considerations, and Clinical Outcomes. Oper. Tech. Orthop. 2005, 15, 5–19. [Google Scholar] [CrossRef]
- Mallett, K.F.; Arruda, E.M. Digital Image Correlation-Aided Mechanical Characterization of the Anteromedial and Posterolateral Bundles of the Anterior Cruciate Ligament. Acta Biomater. 2017, 56, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Van der List, J.P.; Mintz, D.N.; DiFelice, G.S. The Location of Anterior Cruciate Ligament Tears: A Prevalence Study Using Magnetic Resonance Imaging. Orthop. J. Sport. Med. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Logan, M.C.; Williams, A.; Lavelle, J.; Gedroyc, W.; Freeman, M. What Really Happens during the Lachman Test? A Dynamic MRI Analysis of Tibiofemoral Motion. Am. J. Sports Med. 2004, 32, 369–375. [Google Scholar] [CrossRef]
- Panisset, J.C.; Ntagiopoulos, P.G.; Saggin, P.R.; Dejour, D. A Comparison of TelosTM Stress Radiography versus RolimeterTM in the Diagnosis of Different Patterns of Anterior Cruciate Ligament Tears. Orthop. Traumatol. Surg. Res. 2012, 98, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Balasch, H.; Schiller, M.; Friebel, H.; Hoffmann, F. Evaluation of Anterior Knee Joint Instability with the Rolimeter A Test in Comparison with Manual Assessment and Measuring with the KT-1000 Arthrometer. Knee Surg. Sports Traumatol. Arthrosc. 1999, 7, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.M.; Malcom, L.L.; Losse, G.; Stone, M.L.; Sachs, R.; Burks, R. Instrumented Measurement of Anterior Laxity of the Knee. J. Bone Joint Surg. Am. 1985, 67, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Pouderoux, T.; Muller, B.; Robert, H. Joint Laxity and Graft Compliance Increase during the First Year Following ACL Reconstruction with Short Hamstring Tendon Grafts. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Rahemi, H.; Farahmand, F.; Rezaeian, T.; Parnianpour, M. Computer Simulation of Knee Arthrometry to Study the Effects of Partial ACL Injury and Tibiofemoral Contact. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society EMBS’08, Vancouver, BC, Canada, 20–25 August 2008; pp. 895–898. [Google Scholar] [CrossRef]
- Robert, H.; Nouveau, S.; Gageot, S.; Gagnière, B. A New Knee Arthrometer, the GNRB®: Experience in ACL Complete and Partial Tears. Orthop. Traumatol. Surg. Res. 2009, 95, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Trad, Z.; Barkaoui, A.; Chafra, M.; Tavares, J.M.R.S. FEM Analysis of the Human Knee Joint; SpringerBriefs in Applied Sciences and Technology; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-74157-4. [Google Scholar]
- Erdemir, A. Open Knee: Open Source Modeling & Simulation to Enable Scientific Discovery and Clinical Care in Knee Biomechanics. J. Knee Surg. 2016, 29, 107. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Mochizuki, T.; Sekiya, I.; Yamaguchi, K.; Akita, K.; Muneta, T. Anatomy of Normal Human Anterior Cruciate Ligament Attachments Evaluated by Divided Small Bundles. Am. J. Sports Med. 2009, 37, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.H.; Carlin, G.J.; Vogrin, T.M.; Woo, S.L.Y.; Harner, C.D. Quantitative Analysis of Collagen Fibrils of Human Cruciate and Meniscofemoral Ligaments. Clin. Orthop. Relat. Res. 1998, 357, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ogden, R.W. Large Deformation Isotropic Elasticity—On the Correlation of Theory and Experiment for Incompressible Rubberlike Solids. Proc. R. Soc. A Math. Phys. Eng. Sci. 1972, 326, 565–584. [Google Scholar] [CrossRef]
- Peña, E.; Calvo, B.; Martínez, M.A.; Doblaré, M. A Three-Dimensional Finite Element Analysis of the Combined Behavior of Ligaments and Menisci in the Healthy Human Knee Joint. J. Biomech. 2006, 39, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Kay, M.D.; Stouffer, D.C. Comparison of Material Properties in Fascicle-Bone Units from Human Patellar Tendon and Knee Ligaments. J. Biomech. 1986, 19, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, M.; Wertheimer, S.; Wade, K.R.; Galbusera, F.; Benayahu, D.; Wilke, H.-J.; Haj-Ali, R. Towards Intervertebral Disc Engineering: Bio-Mimetics of Form and Function of the Annulus Fibrosus Lamellae. J. Mech. Behav. Biomed. Mater. 2019, 94, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Lay, A.N.; Dickerson, D.A.; Nauman, E.A.; Livesay, G.A.; Dee, K.C. Mechanical Characterization of Collagen Fibers and Scaffolds for Tissue Engineering. Biomaterials 2003, 24, 3805–3813. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, M.; Varssano, D.; Eliasy, R.; Benayahu, Y.; Benayahu, D.; Haj-Ali, R. Mechanical Flexure Behavior of Bio-Inspired Collagen-Reinforced Thin Composites. Compos. Struct. 2016, 153, 392–400. [Google Scholar] [CrossRef]
- Sharabi, M.; Benayahu, D.; Benayahu, Y.; Isaacs, J.; Haj-Ali, R. Laminated Collagen-Fiber Bio-Composites for Soft-Tissue Bio-Mimetics. Compos. Sci. Technol. 2015, 117, 268–276. [Google Scholar] [CrossRef]
- Mordechai, H.S.; Aharonov, A.; Sharon, S.E.; Bonshtein, I.; Simon, C.; Sivan, S.S.; Sharabi, M. Toward a Mechanically Biocompatible Intervertebral Disc: Engineering of Combined Biomimetic Annulus Fibrosus and Nucleus Pulposus Analogs. J. Biomed. Mater. Res. Part A 2023, 111, 618–633. [Google Scholar] [CrossRef]
- Van der List, J.P.; DiFelice, G.S. Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality. Am. J. Orthop. 2016, 45, E393–E405. [Google Scholar] [PubMed]
- Al Khatib, F.; Gouissem, A.; Eilaghi, A.; Adouni, M. The Effect of Enzymatic Crosslink Degradation on the Mechanics of the Anterior Cruciate Ligament: A Hybrid Multi-Domain Model. Appl. Sci. 2021, 11, 8580. [Google Scholar] [CrossRef]
- Peters, A.E.; Akhtar, R.; Comerford, E.J.; Bates, K.T. Tissue Material Properties and Computational Modelling of the Human Tibiofemoral Joint: A Critical Review. PeerJ 2018, 2018, e4298. [Google Scholar] [CrossRef]
- Chandrashekar, N.; Mansouri, H.; Slauterbeck, J.; Hashemi, J. Sex-Based Differences in the Tensile Properties of the Human Anterior Cruciate Ligament. J. Biomech. 2006, 39, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Readioff, R.; Geraghty, B.; Comerford, E.; Elsheikh, A. A Full-Field 3D Digital Image Correlation and Modelling Technique to Characterise Anterior Cruciate Ligament Mechanics Ex Vivo. Acta Biomater. 2020, 113, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Kondo, E.; Takeda, R.; Akita, K.; Yasuda, K.; Amis, A.A. The Role of Fibers in the Femoral Attachment of the Anterior Cruciate Ligament in Resisting Tibial Displacement. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Zantop, T. Anatomy of the Anterior Cruciate Ligament with Regard to Its Two Bundles. Clin. Orthop. Relat. Res. 2007, 454, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Bosco, F.; Giustra, F.; Crivellaro, M.; Giai Via, R.; Lavia, A.D.; Capella, M.; Sabatini, L.; Risitano, S.; Rovere, G.; Massè, A.; et al. Is Augmentation the Best Solution in Partial Anterior Cruciate Ligament Tears? A Literature Systematic Review and Meta-Analysis. J. Orthop. 2023, 36, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Barber-Westin, S.; Noyes, F.R. One in 5 Athletes Sustain Reinjury Upon Return to High-Risk Sports After ACL Reconstruction: A Systematic Review in 1239 Athletes Younger Than 20 Years. Sports Health 2020, 12, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Erard, J.; Cance, N.; Shatrov, J.; Fournier, G.; Gunst, S.; Ciolli, G.; Porcelli, P.; Lustig, S.; Servien, E. Delaying ACL Reconstruction Is Associated with Increased Rates of Medial Meniscal Tear. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 4458–4466. [Google Scholar] [CrossRef]
- Sharabi, M. Structural Mechanisms in Soft Fibrous Tissues: A Review. Front. Mater. 2022, 8, 573. [Google Scholar] [CrossRef]



| Part Name | No. of Elements | Element Type | Material Properties | Reference | |
|---|---|---|---|---|---|
| Femoral Cartilage | 17,226 | C3D8H | Linear elastic E = 5 MPa, υ = 0.46 | [24] | |
| Tibial Cartilage | 8847 | C3D8H | Linear elastic E = 5 Mpa, υ = 0.46 | [24] | |
| LCL | 6656 | C3D8H | Hyperelastic Ogden (1st order) | [25] | |
| MCL | 5120 | C3D8H | Hyperelastic Ogden (1st order) | [25] | |
| PCL | 5248 | C3D8H | Hyperelastic Ogden (1st order) | [25] | |
| ACL I | 4096 | C3D4H | Hyperelastic Ogden (3rd order) | [25] | |
| Composite ACL | Fibers | 824 | T3D2H | Hyperelastic Ogden (3rd order) | Based on [26,27] |
| Matrix | 7412 | C3D4H | Hyperelastic Ogden (1st order) | [26] | |
| Part Name | N | i | [MPa] | [MPa−1] | ||
|---|---|---|---|---|---|---|
| LCL | 1st order | 1 | 51.01 | 19.40 | 0 | |
| MCL | 1st order | 1 | 37.62 | 24.40 | 0 | |
| PCL | 1st order | 1 | 50.63 | 16.80 | 0 | |
| ACL Homogeneous (for stress concentration) | 1st order | 1 | 84.70 | 20.48 | 0 | |
| Composite ACL | Collagen Fibers | 3rd order | 1 | −15,890.38 | −7.12 | 0 |
| 2 | 8450.90 | −3.11 | 0 | |||
| 3 | 7455.22 | −11.38 | 0 | |||
| Matrix | 1st order | 1 | 0.0473 | 25 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharabi, M.; Agron, R.; Dolev, A.; Haj-Ali, R.; Yassin, M. Predictive Refined Computational Modeling of ACL Tear Injury Patterns. Bioengineering 2024, 11, 413. https://doi.org/10.3390/bioengineering11050413
Sharabi M, Agron R, Dolev A, Haj-Ali R, Yassin M. Predictive Refined Computational Modeling of ACL Tear Injury Patterns. Bioengineering. 2024; 11(5):413. https://doi.org/10.3390/bioengineering11050413
Chicago/Turabian StyleSharabi, Mirit, Raz Agron, Amir Dolev, Rami Haj-Ali, and Mustafa Yassin. 2024. "Predictive Refined Computational Modeling of ACL Tear Injury Patterns" Bioengineering 11, no. 5: 413. https://doi.org/10.3390/bioengineering11050413
APA StyleSharabi, M., Agron, R., Dolev, A., Haj-Ali, R., & Yassin, M. (2024). Predictive Refined Computational Modeling of ACL Tear Injury Patterns. Bioengineering, 11(5), 413. https://doi.org/10.3390/bioengineering11050413






