Role of Mesenchymal Stem/Stromal Cells (MSCs) and MSC-Derived Extracellular Vesicles (EVs) in Prevention of Telomere Length Shortening, Cellular Senescence, and Accelerated Biological Aging
Abstract
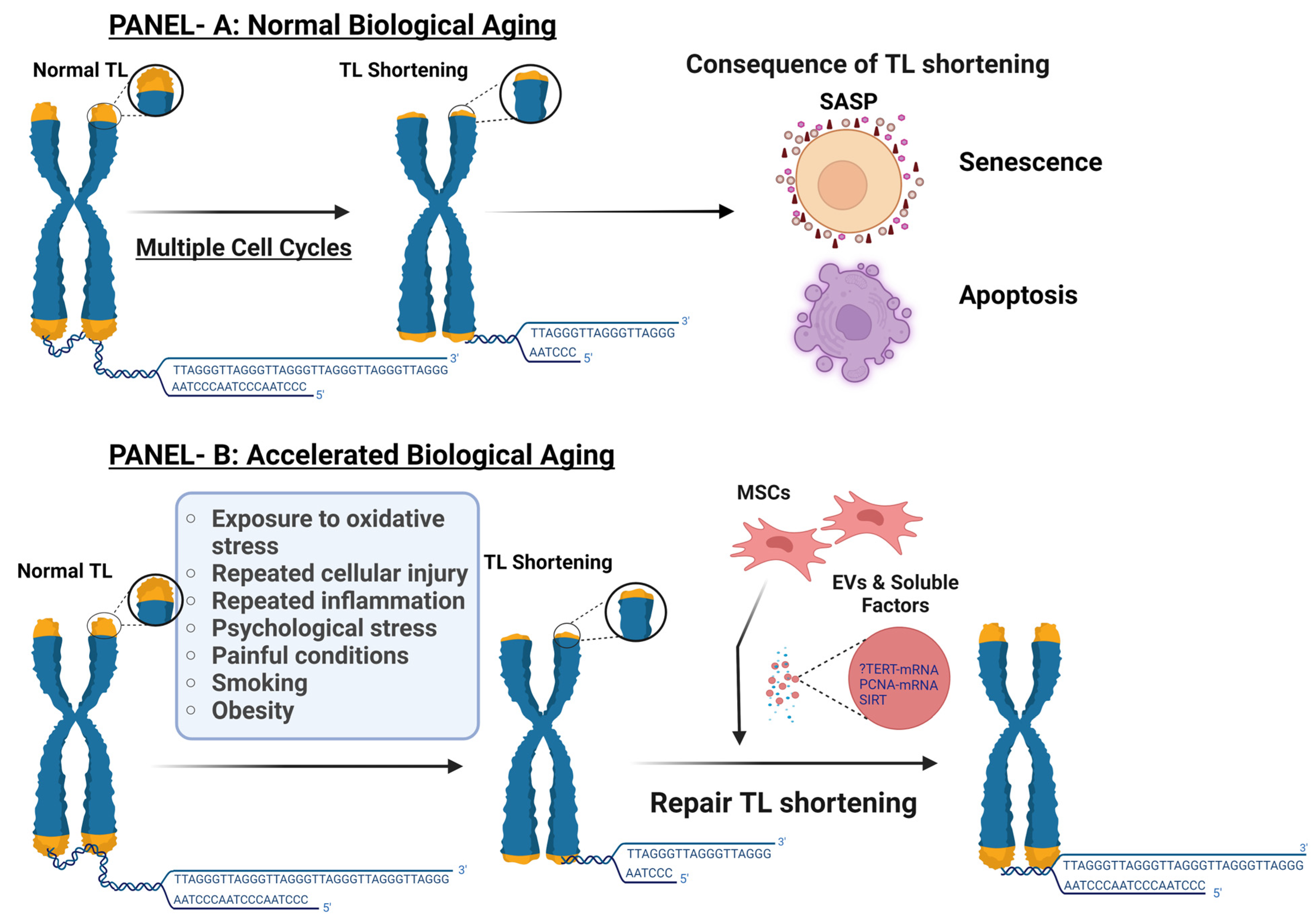
1. Introduction
1.1. Telomere Length, Cellular Senescence, and Normal Biological Age
1.2. Accelerated Biological Aging
1.3. Telomerase Role in Maintenance of TL
2. Factors Contributing to TS and Accelerated Biological Aging
3. Role of Stem Cells in TS, Cellular Senescence, and Advanced Biological Age
4. Mesenchymal/Stromal Stem Cells (MSCs)
5. Cellular Senescence and Functional Decline of MSCs with Aging
6. Role of Gestational Tissue-Derived MSCs in the Prevention of TS, Cellular Senescence, and Accelerated Biological Aging
7. Role of Aged MSCs in Pathogenesis of Diseases
8. Strategies to Prevent MSCs’ Senescence and Improve Their Clinical Application
9. Antiaging Therapeutic Application of MSCs
10. Paracrine Effect of MSCs by Extracellular Vesicles (EVs)
10.1. Role of MSCs Derived Extracellular Vesicles (EVs) in TL Shortening, Cellular Senescence, and Biological Age
10.2. Antiaging Effect of MSCs-Derived EVs
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Liu, X.; Ding, X.; Wang, F.; Geng, X. Telomere and its role in the aging pathways: Telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology 2019, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Oeseburg, H.; de Boer, R.A.; van Gilst, W.H.; van der Harst, P. Telomere biology in healthy aging and disease. Pflügers Arch. 2010, 459, 259–268. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Smith, E.M.; Pendlebury, D.F.; Nandakumar, J. Structural biology of telomeres and telomerase. Cell. Mol. Life Sci. 2020, 77, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. The limited in vitro Lifetime of Human Diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef]
- Lazzerini-Denchi, E.; Sfeir, A. Stop pulling my strings-what telomeres taught us about the DNA damage response. Nat. Rev. Mol. Cell Biol. 2016, 17, 364–378. [Google Scholar] [CrossRef]
- Shahini, A.; Rajabian, N.; Choudhury, D.; Shahini, S.; Vydiam, K.; Nguyen, T.; Kulczyk, J.; Santarelli, T.; Ikhapoh, I.; Zhang, Y.; et al. Ameliorating the hallmarks of cellular senescence in skeletal muscle myogenic progenitors in vitro and in vivo. Sci. Adv. 2021, 7, eabe5671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cianflone, E.; Torella, M.; Biamonte, F.; De Angelis, A.; Urbanek, K.; Costanzo, F.S.; Rota, M.; Ellison-Hughes, G.M.; Torella, D. Targeting Cardiac Stem Cell Senescence to Treat Cardiac Aging and Disease. Cells 2020, 9, 1558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghosh, M.; Hill, C.L.; Alsudayri, A.; Lallier, S.W.; Hayes, D., Jr.; Wijeratne, S.; Tan, Z.H.; Chiang, T.; Mahoney, J.E.; Carraro, G.; et al. Repeated injury promotes tracheobronchial tissue stem cell attrition. Stem Cells Transl. Med. 2021, 10, 1696–1713. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Lansdorp, P.M. Telomeres and aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Artandi, S.E.; Chang, S.; Lee, S.L.; Alson, S.; Gottlieb, G.J.; Chin, L.; DePinho, R.A. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 2000, 406, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gonzalez De Los Santos, F.; Zhao, Y.; Wu, Z.; Rinke, A.E.; Kim, K.K.; Phan, S.H. Telomerase reverse transcriptase ameliorates lung fibrosis by protecting alveolar epithelial cells against senescence. J. Biol. Chem. 2019, 294, 8861–8871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mangaonkar, A.A.; Ferrer, A.; Pinto EVairo, F.; Cousin, M.A.; Kuisle, R.J.; Klee, E.W.; Kennedy, C.C.; Peters, S.G.; Scott, J.P.; Utz, J.P.; et al. Clinical Correlates and Treatment Outcomes for Patients With Short Telomere Syndromes. Mayo Clin. Proc. 2018, 93, 834–839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armstrong, L.; Saretzki, G.; Peters, H.; Wappler, I.; Evans, J.; Hole, N.; von Zglinicki, T.; Lako, M. Overexpression of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic lineage. Stem Cells. 2005, 23, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chan, S.L.; Fu, W.; Mendoza, M.; Mattson, M.P. TERT suppresses apoptotis at a premitochondrial step by a mechanism requiring reverse transcriptase activity and 14-3-3 protein-binding ability. FASEB Journal Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 767–769. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, A.; Pantell, M.S.; Puterman, E.; Dhabhar, F.S.; Blackburn, E.H.; Yaffe, K.; Cawthon, R.M.; Opresko, P.L.; Hsueh, W.C.; Satterfield, S.; et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the health, aging and body composition study. PLoS ONE 2011, 6, e19687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.P.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef]
- Ahmed, S.; Passos, J.F.; Birket, M.J.; Beckmann, T.; Brings, S.; Peters, H.; Birch-Machin, M.A.; von Zglinicki, T.; Saretzki, G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 2008, 121 Pt. 7, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, P.; Zheng, Q.; Gao, G.; Yuan, J.; Wang, P.; Huang, J.; Xie, L.; Lu, X.; Tong, T.; et al. Mitochondrial Trafficking and Processing of Telomerase RNA TERC. Cell Rep. 2018, 24, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Müller, F.L.; Guo, M.; Cooper, M.; Kotton, D.; Fabian, A.J.; Walkey, C.; et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011, 470, 359–365, Erratum in Nature 2011, 475, 254. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef] [PubMed]
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Rhee, D.B.; Lu, J.; Bohr, C.T.; Zhou, F.; Vallabhaneni, H.; de Souza-Pinto, N.C.; Liu, Y. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet. 2010, 6, e1000951. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valdes, A.M.; Andrew, T.; Gardner, J.P.; Kimura, M.; Oelsner, E.; Cherkas, L.F.; Aviv, A.; Spector, T.D. Obesity, cigarette smoking, and telomere length in women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef] [PubMed]
- von Zglinicki, T.; Saretzki, G.; Döcke, W.; Lotze, C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: A model for senescence? Exp. Cell Res. 1995, 220, 186–193. [Google Scholar] [CrossRef]
- Bar-Or, D.; Thomas, G.W.; Rael, L.T.; Lau, E.P.; Winkler, J.V. Asp-Ala-His-Lys (DAHK) inhibits copper-induced oxidative DNA double strand breaks and telomere shortening. Biochem. Biophys. Res. Commun. 2001, 282, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Bernet, J.D.; Doles, J.D.; Hall, J.K.; Kelly Tanaka, K.; Carter, T.A.; Olwin, B.B. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 2014, 20, 265–271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Leading Edge Review Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qu, J.; Zhang, W.; Izpisua Belmonte, J.C.; Liu, G.H. A stem cell aging framework, from mechanisms to interventions. Cell Rep. 2022, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sareen, N.; Sequiera, G.L.; Chaudhary, R.; Abu-El-Rub, E.; Chowdhury, S.R.; Sharma, V.; Surendran, A.; Moudgil, M.; Fernyhough, P.; Ravandi, A.; et al. Early passaging of mesenchymal stem cells does not instigate significant modifications in their immunological behavior. Stem Cell Res. Ther. 2018, 9, 121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, G.; Li, J.; Purkayastha, S.; Tang, Y.; Zhang, H.; Yin, Y.; Li, B.; Liu, G.; Cai, D. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 2013, 497, 211–216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, J.Y.; De Vivo, I.; Lin, X.; Fang, S.C.; Christiani, D.C. The relationship between inflammatory biomarkers and telomere length in an occupational prospective cohort study. PLoS ONE 2014, 9, e87348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Stojadinovic, O.; Pastar, I.; Nusbaum, A.G.; Vukelic, S.; Krzyzanowska, A.; Tomic-Canic, M. Deregulation of epidermal stem cell niche contributes to pathogenesis of nonhealing venous ulcers. Wound Repair. Regen. 2014, 22, 220–227. [Google Scholar] [CrossRef]
- Secunda, R.; Vennila, R.; Mohanashankar, A.M.; Rajasundari, M.; Jeswanth, S.; Surendran, R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: A comparative study. Cytotechnology 2015, 67, 793–807. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, L.; Chen, Q.; Wang, F.; Li, Q.; Zeng, Q.; Huang, J.; Luo, M.; Li, W.; Zheng, Y.; et al. Hypoxia with Wharton’s jelly mesenchymal stem cell coculture maintains stemness of umbilical cord blood-derived CD34+ cells. Stem Cell Res. Ther. 2018, 13, 158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magin, A.S.; Körfer, N.R.; Partenheimer, H.; Lange, C.; Zander, A.; Noll, T. Primary cells as feeder cells for coculture expansion of human hematopoietic stem cells from umbilical cord blood—A comparative study. Stem Cells Dev. 2009, 18, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Denham, M.; Conley, B.; Olsson, F.; Cole, T.J.; Mollard, R. Stem cells: An overview. Curr. Protoc. Cell Biol. 2005, 23, 23-1. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Bongso, A.; Fong, C.Y. The Therapeutic Potential, Challenges and Future Clinical Directions of Stem Cells from the Wharton’s Jelly of the Human Umbilical Cord. Stem Cell Rev. Rep. 2013, 9, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Noratto, G.E.; Luo, G.; Denoeud, C.; Padrona, M.; Moya, A.; Bensidhoum, M.; Bizios, R.; Potier, E.; Logeart-Avramoglou, D.; Petite, H. Understanding and leveraging cell metabolism to enhance mesenchymal stem cell transplantation survival in tissue engineering and regenerative medicine applications. Stem Cells 2020, 38, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Kundrotas, G.; Gasperskaja, E.; Slapsyte, G.; Gudleviciene, Z.; Krasko, J.; Stumbryte, A.; Liudkeviciene, R. Identity, proliferation capacity, genomic stability and novel senescence markers of mesenchymal stem cells isolated from low volume of human bone marrow. Oncotarget 2016, 7, 10788. [Google Scholar] [CrossRef]
- Chen, G.; Yue, A.; Ruan, Z.; Yin, Y.; Wang, R.; Ren, Y.; Zhu, L. Comparison of biological characteristics of mesenchymal stem cells derived from maternal-origin placenta and Wharton’s jelly. Stem Cell Res. Ther. 2015, 6, 228. [Google Scholar] [CrossRef]
- Deuse, T.; Stubbendorff, M.; Tang-Quan, K.; Phillips, N.; Kay, M.A.; Eiermann, T.; Phan, T.T.; Volk, H.D.; Reichenspurner, H.; Robbins, R.C.; et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011, 20, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Soncini, M. Placenta as a Source of Stem Cells and as a Key Organ for Fetomaternal Tolerance. In Regenerative Medicine Using Pregnancy-Specific Biological Substances; Springer: London, UK, 2011. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Jin, J.; Chen, L.; Zhu, J.; Huang, W.; Zhao, J.; Qian, H.; Zhang, X. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 2006, 30, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Teleb, R.S.; Abdul-Hafez, A.; Othman, A.; Ahmed, A.E.-A.; Elsaid, A.A.; Arif, H.; Zarea, A.A.; Abdulmageed, M.; Mohamed, H.; Ibrahim, S.A.; et al. Cord Blood Plasma and Placental Mesenchymal Stem Cells-Derived Exosomes Increase Ex Vivo Expansion of Human Cord Blood Hematopoietic Stem Cells While Maintaining Their Stemness. Cells 2023, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Buhring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise Review: Isolation and Characterization of Cells from Human Term Placenta: Outcome of the First International Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Soncini, M.; Vertua, E.; Gibelli, L.; Zorzi, F.; Denegri, M.; Albertini, A.; Wengler, G.S.; Parolini, O. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 2007, 1, 296–305. [Google Scholar] [CrossRef]
- Troyer, D.L.; Weiss, M.L. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 2008, 26, 591–599. [Google Scholar] [CrossRef]
- Anker, P.S.I.T.; Scherjon, S.A.; der Keur, C.K.; de Groot-Swings, G.M.J.S.; Claas, F.H.J.; Fibbe, W.E.; Kanhai, H.H. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Igura, K.; Zhang, X.; Takahashi, K.; Mitsuru, A.; Yamaguchi, S.; Takahashi, T.A. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy 2004, 6, 543–553. [Google Scholar] [CrossRef]
- Duscher, D.; Rennert, R.C.; Januszyk, M.; Anghel, E.; Maan, Z.N.; Whittam, A.J.; Perez, M.G.; Kosaraju, R.; Hu, M.S.; Walmsley, G.G.; et al. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci. Rep. 2014, 4, 7144. [Google Scholar] [CrossRef]
- Wang, S.S.; Ren, J. Aging as an essential modifier for the efficacy in mesenchymal stem cell therapy through an inositol phosphate 6 kinase-inositol pyrophosphate 7-dependent mechanism. Stem Cell Res. Ther. 2014, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, J.C.; Tome, M.; Fernandez, M.E.; Delgado, M.; Campisi, J.; Bernad, A.; Gonzalez, M.A. Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Stem Cells 2014, 32, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Yujia, W.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl. Med. 2022, 11, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Tormos, A.M.; Taléns-Visconti, R.; Nebreda, A.R.; Sastre, J. P38 MAPK: A dual role in hepatocyte proliferation through reactive oxygen species. Free Radic. Res. 2013, 47, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial dysfunction and cell senescence: Deciphering a complex relationship. FEBS Lett. 2019, 593, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- D’Adda Di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef]
- Shibata, K.R.; Aoyama, T.; Shima, Y.; Fukiage, K.; Otsuka, S.; Furu, M.; Kohno, Y.; Ito, K.; Fujibayashi, S.; Neo, M.; et al. Expression of the p16INK4A Gene Is Associated Closely with Senescence of Human Mesenchymal Stem Cells and Is Potentially Silenced by DNA Methylation during In Vitro Expansion. Stem Cells 2007, 25, 2371–2382. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, S.; Seo, M.S.; Park, S.B.; Kurtz, A.; Kang, S.K.; Kang, K.S. Histone deacetylase controls adult stem cell aging by balancing the expression of polycomb genes and jumonji domain containing 3. Cell. Mol. Life Sci. 2010, 67, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Starr, M.E.; Evers, B.M.; Saito, H. Age-associated increase in cytokine production during systemic inflammation: Adipose tissue as a major source of IL-6. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2009, 64, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Stout, M.B.; Justice, J.N.; Nicklas, B.J.; Kirkland, J.L. Physiological aging: Links among adipose tissue dysfunction, diabetes, and frailty. Physiology 2017, 32, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, H.; Tan, Y. Wnt/β-Catenin signaling induces the aging of Mesenchymal stem cells through the DNA damage response and the P53/P21 pathway. PLoS ONE 2011, 6, e21397. [Google Scholar] [CrossRef] [PubMed]
- Baht, G.S.; Silkstone, D.; Vi, L.; Nadesan, P.; Amani, Y.; Whetstone, H.; Wei, Q.; Alman, B.A. Exposure to a youthful circulaton rejuvenates bone repair through modulation of β-catenin. Nat. Commun. 2015, 6, 7131. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev. Rep. 2018, 14, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhang, Y.; Yu, H.; Li, X. Role of Hyperglycemia in the Senescence of Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 665412. [Google Scholar] [CrossRef]
- Al-Azab, M.; Safi, M.; Idiiatullina, E.; Al-Shaebi, F.; Zaky, M.Y. Aging of mesenchymal stem cell: Machinery, markers, and strategies of fighting. Cell. Mol. Biol. Lett. 2022, 27, 69. [Google Scholar] [CrossRef]
- Garcia, S.; Bernad, A.; Martín, M.C.; Cigudosa, J.C.; Garcia-Castro, J.; de la Fuente, R. Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp. Cell Res. 2010, 316, 1648–1650. [Google Scholar] [CrossRef]
- Rodriguez, R.; Rubio, R.; Masip, M.; Catalina, P.; Nieto, A.; de la Cueva, T.; Arriero, M.; Martin, N.S.; de la Cueva, E.; Balomenos, D.; et al. Loss of p53 induces tumorigenesis in p21-deficient mesenchymal stem cells. Neoplasia 2009, 11, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.; Naaldijk, Y.; Leovsky, C.; Johnson, A.A.; Rudolph, L.; Jaeger, C.; Arnold, K.; Stolzing, A. Distribution pattern following systemic mesenchymal stem cell injection depends on the age of the recipient and neuronal health. Stem Cell Res. Ther. 2017, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Serakinci, N.; Graakjaer, J.; Kolvraa, S. Telomere stability and telomerase in mesenchymal stem cells. Biochimie 2008, 90, 33–40. [Google Scholar] [CrossRef]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.; Jensen, T.G.; Kassem, M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002, 20, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Takeuchi, K.; Kohara, A.; Satoh, M.; Shioda, S.; Ozawa, Y.; Mizusawa, H. Chromosomal instability in human mesenchymal stem cells immortalized with human papilloma virus E6, E7, and hTERT genes. Cell. Dev. Biol. Anim. 2007, 43, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Trachana, V.; Petrakis, S.; Fotiadis, Z.; Siska, E.K.; Balis, V.; Gonos, E.S.; Kaloyianni, M.; Koliakos, G. Human mesenchymal stem cells with enhanced telomerase activity acquire resistance against oxidative stress-induced genomic damage. Cytotherapy 2017, 19, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Ogawa, K.; Ikei, T.; Udono, M.; Fujiki, T.; Katakura, Y. SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene. Biochem. Biophys. Res. Commun. 2012, 417, 630–634. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Zhu, W.; Chen, H.; Hu, X.; Jiang, Z.; Xu, Y.; Wang, L.; Zhou, Y.; Chen, P.; et al. SIRT1 ameliorates age-related senescence of mesenchymal stem cells via modulating telomere shelterin. Front. Aging Neurosci. 2014, 6, 103. [Google Scholar] [CrossRef]
- Phermthai, T.; Pokathikorn, P.; Wichitwiengrat, S.; Thongbopit, S.; Tungprasertpol, K.; Julavijitphong, S. P53 mutation and epigenetic imprinted IGF2/H19 gene analysis in mesenchymal stem cells derived from amniotic fluid, amnion, endometrium, and Wharton’s jelly. Stem Cells Dev. 2017, 26, 1344–1354. [Google Scholar] [CrossRef]
- Pipes, B.L.; Tsang, T.; Peng, S.X.; Fiederlein, R.; Graham, M.; Harris, D.T. Telomere length changes after umbilical cord blood transplant. Transfusion 2006, 46, 1038–1043. [Google Scholar] [CrossRef]
- Xie, C.; Jin, J.; Lv, X.; Tao, J.; Wang, R.; Miao, D. Anti-aging effect of transplanted amniotic membrane mesenchymal stem cells in a premature aging model of bmi-1 deficiency. Sci. Rep. 2015, 5, 13975. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.A.; Walker, N.J.; Napoli, E.; Borjesson, D.L. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue. Stem Cells Dev. 2012, 21, 273–283. [Google Scholar] [CrossRef]
- Hu, N.; Gao, Y.; Jayasuriya, C.T.; Liu, W.; Du, H.; Ding, J.; Feng, M.; Chen, Q. Chondrogenic induction of human osteoarthritic cartilage-derived mesenchymal stem cells activates mineralization and hypertrophic and osteogenic gene expression through a mechanomiR. Arthritis Res. Ther. 2019, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Cárdenes, N.; Álvarez, D.; Sellarés, J.; Peng, Y.; Corey, C.; Wecht, S.; Nouraie, S.M.; Shanker, S.; Sembrat, J.; Bueno, M.; et al. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res. Ther. 2018, 9, 257. [Google Scholar] [CrossRef]
- Lewis-McDougall, F.C.; Ruchaya, P.J.; Domenjo-Vila, E.; Shin Teoh, T.; Prata, L.; Cottle, B.J.; Clark, J.E.; Punjabi, P.P.; Awad, W.; Torella, D.; et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 2019, 18, e12931. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, A.; Dodia, R.M.; Orogo, A.M.; Zambrano, C.M.; Najor, R.H.; Gustafsson, Å.B.; Heller Brown, J.; Purcell, N.H. Decline in cellular function of aged mouse c-kit+ cardiac progenitor cells. J. Physiol. 2017, 595, 6249–6262. [Google Scholar] [CrossRef]
- Yang, M.; Teng, S.; Ma, C.; Yu, Y.; Wang, P.; Yi, C. Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology 2018, 70, 1301–1313. [Google Scholar] [CrossRef]
- Denu, R.A. SIRT3 Enhances Mesenchymal Stem Cell Longevity and Differentiation. Oxid. Med. Cell. Longev. 2017, 2017, 5841716. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; He, H.; Fan, B.; Deng, R.; Hong, Y.; Liang, X.; Zhao, H.; Li, X.; Zhang, F. Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging 2019, 11, 12641–12660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okada, M.; Kim, H.W.; Matsu-Ura, K.; Wang, Y.G.; Xu, M.; Ashraf, M. Abrogation of Age-Induced MicroRNA-195 Rejuvenates the Senescent Mesenchymal Stem Cells by Reactivating Telomerase. Stem Cells 2016, 34, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ding, Y.; Lin, F.; Zhang, Y.; Zhou, X.; Meng, Q.; Lu, X.; Jiang, G.; Zhu, H.; Chen, Y.; et al. Overexpression of ERBB4 rejuvenates aged mesenchymal stem cells and enhances angiogenesis via PI3K/AKT and MAPK/ERK pathways. FASEB J. 2019, 33, 4559–4570. [Google Scholar] [CrossRef] [PubMed]
- Korotkov, A.; Seluanov, A.; Gorbunova, V. Sirtuin 6: Linking longevity with genome and epigenome stability Genomic and epigenomic instability. Trends Cell Biol. 2021, 31, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- De Gioia, R.; Biella, F.; Citterio, G.; Rizzo, F.; Abati, E.; Nizzardo, M.; Bresolin, N.; Comi, G.P.; Corti, S. Neural stem cell transplantation for neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 3103. [Google Scholar] [CrossRef] [PubMed]
- Dell’ Amico, C.; Tata, A.; Pellegrino, E.; Onorati, M.; Conti, L. Genome editing in stem cells for genetic neurodisorders. Prog. Mol. Biol. Transl. Sci. 2021, 182, 403–438. [Google Scholar] [CrossRef]
- Navarro Negredo, P.; Yeo, R.W.; Brunet, A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem. Cell 2020, 27, 202–223. [Google Scholar] [CrossRef] [PubMed]
- Amor., C.; Feucht., J.; Leibold, J.; Ho, Y.J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhou, H.; Zhu, Y.; Sun, Q.; Ji, Y.; Xue, A.; Wang, Y.; Chen, W.; Yu, X.; Wang, L.; et al. Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020, 30, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wu, W.; Xu, X.; Liao, L.; Zheng, F.; Messinger, S.; Sun, X.; Chen, J.; Yang, S.; Cai, J.; et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA 2012, 307, 1169–1177. [Google Scholar] [CrossRef]
- Mayer, L.; Pandak, W.M.; Melmed, G.Y.; Hanauer, S.B.; Johnson, K.; Payne, D.; Faleck, H.; Hariri, R.J.; Fischkoff, S.A. Safety and tolerability of human placenta-derived cells (PDA001) in treatment-resistant crohn’s disease: A phase 1 study. Inflamm. Bowel Dis. 2013, 19, 754–760. [Google Scholar] [CrossRef]
- Lublin, F.D.; Bowen, J.D.; Huddlestone, J.; Kremenchutzky, M.; Carpenter, A.; Corboy, J.R.; Freedman, M.S.; Krupp, L.; Paulo, C.; Hariri, R.J.; et al. Human placenta-derived cells(pda-001) for the treatment of adults with multiple sclerosis: Arandomized, placebo-controlled, multiple-dose study. Mult. Scler. Relat. Disord. 2014, 3, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Edinger, J.W.; Fischkoff, S.A.; Francki, A.; Jankovic, V.; Liang, B.; Martin, P.; Ray, C.; Zhang, X. Treatment of Sarcoidosis Using Placental Stem Cells. U.S. Patent 8562973B2, 22 October 2013. [Google Scholar]
- Kranz, A.; Wagner, D.C.; Kamprad, M.; Scholz, M.; Schmidt, U.R.; Nitzsche, F.; Aberman, Z.; Emmrich, F.; Riegelsberger, U.M.; Boltze, J. Transplantation of placenta-derived mesenchymal stromal cells upon experimental stroke in rats. Brain Res. 2010, 1315, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, E.; David, A.L. Placental stem cells. Best. Pract. Res. Clin. Obstet. Gynaecol. 2016, 31, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Evangelista, M.; Parolini, O. Human term placental cells: Phenotype, properties and new avenues in regenerative medicine. Int. J. Mol. Cell. Med. 2012, 1, 64. [Google Scholar] [PubMed]
- Xia, W.; Hou, M. Mesenchymal stem cells confer resistance to doxorubicin-induced cardiac senescence by inhibiting microRNA-34a. Oncol. Lett. 2018, 15, 10037–10046. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Khan, Y.S. Histology, Extracellular Vesicles. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Omar, S.A.; Abdul-Hafez, A.; Ibrahim, S.; Pillai, N.; Abdulmageed, M.; Thiruvenkataramani, R.P.; Mohamed, T.; Madhukar, B.V.; Uhal, B.D. Stem-Cell Therapy for Bronchopulmonary Dysplasia (BPD) in Newborns. Cells 2022, 11, 1275. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Qin, C.; Wang, J.; Hu, Y.; Zheng, G.; Qiu, G.; Ge, M.; Tao, H.; Shu, Q.; Xu, J. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging 2019, 11, 7996–8014. [Google Scholar] [CrossRef] [PubMed]
- Romero-García, N.; Huete-Acevedo, J.; Mas-Bargues, C.; Sanz-Ros, J.; Dromant, M.; Borrás, C. The Double-Edged Role of Extracellular Vesicles in the Hallmarks of Aging. Biomolecules 2023, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Radeghieri, A.; Savio, G.; Zendrini, A.; Di Noto, G.; Salvi, A.; Bergese, P.; Piovani, G. Cultured human amniocytes express hTERT, which is distributed between nucleus and cytoplasm and is secreted in extracellular vesicles. Biochem. Biophys. Res. Commun. 2017, 483, 706–711. [Google Scholar] [CrossRef]
- Burgos-Ravanal, R.; Campos, A.; Díaz-Vesga, M.C.; González, M.F.; León, D.; Lobos-González, L.; Leyton, L.; Kogan, M.J.; Quest, A.F.G. Extracellular vesicles as mediators of cancer disease and as nanosystems in theranostic applications. Cancers 2021, 13, 3324. [Google Scholar] [CrossRef]
- Lei, Q.; Gao, F.; Liu, T.; Ren, W.; Chen, L.; Cao, Y.; Chen, W.; Guo, S.; Zhang, Q.; Chen, W.; et al. Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow-derived mesenchymal stem cells and slow age-related degeneration. Sci. Transl. Med. 2021, 13, eaaz8697. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Young, A.; Autexier, C. PCNA, a focus on replication stress and the alternative lengthening of telomeres pathway. DNA Repair. 2021, 100, 103055. [Google Scholar] [CrossRef] [PubMed]
- Lanna, A.; Vaz, B.; D’Ambra, C.; Valvo, S.; Vuotto, C.; Chiurchiù, V.; Devine, O.; Sanchez, M.; Borsellino, G.; Akbar, A.N.; et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat. Cell Biol. 2022, 24, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- Dorronsoro, A.; Santiago, F.E.; Grassi, D.; Zhang, T.; Lai, R.C.; McGowan, S.J.; Angelini, L.; Lavasani, M.; Corbo, L.; Lu, A.; et al. Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell 2021, 20, e13337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Liu, S.; Lim, M.; Zhao, S.; Cui, K.; Zhang, K.; Wang, L.; Ji, Q.; Han, Z.; et al. Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells. Theranostics 2019, 9, 6976–6990. [Google Scholar] [CrossRef]
- Oh, M.; Lee, J.; Kim, Y.J.; Rhee, W.J.; Park, J.H. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arellano, M.Y.G.; VanHeest, M.; Emmadi, S.; Abdul-Hafez, A.; Ibrahim, S.A.; Thiruvenkataramani, R.P.; Teleb, R.S.; Omar, H.; Kesaraju, T.; Mohamed, T.; et al. Role of Mesenchymal Stem/Stromal Cells (MSCs) and MSC-Derived Extracellular Vesicles (EVs) in Prevention of Telomere Length Shortening, Cellular Senescence, and Accelerated Biological Aging. Bioengineering 2024, 11, 524. https://doi.org/10.3390/bioengineering11060524
Arellano MYG, VanHeest M, Emmadi S, Abdul-Hafez A, Ibrahim SA, Thiruvenkataramani RP, Teleb RS, Omar H, Kesaraju T, Mohamed T, et al. Role of Mesenchymal Stem/Stromal Cells (MSCs) and MSC-Derived Extracellular Vesicles (EVs) in Prevention of Telomere Length Shortening, Cellular Senescence, and Accelerated Biological Aging. Bioengineering. 2024; 11(6):524. https://doi.org/10.3390/bioengineering11060524
Chicago/Turabian StyleArellano, Myrna Y. Gonzalez, Matthew VanHeest, Sravya Emmadi, Amal Abdul-Hafez, Sherif Abdelfattah Ibrahim, Ranga P. Thiruvenkataramani, Rasha S. Teleb, Hady Omar, Tulasi Kesaraju, Tarek Mohamed, and et al. 2024. "Role of Mesenchymal Stem/Stromal Cells (MSCs) and MSC-Derived Extracellular Vesicles (EVs) in Prevention of Telomere Length Shortening, Cellular Senescence, and Accelerated Biological Aging" Bioengineering 11, no. 6: 524. https://doi.org/10.3390/bioengineering11060524
APA StyleArellano, M. Y. G., VanHeest, M., Emmadi, S., Abdul-Hafez, A., Ibrahim, S. A., Thiruvenkataramani, R. P., Teleb, R. S., Omar, H., Kesaraju, T., Mohamed, T., Madhukar, B. V., & Omar, S. A. (2024). Role of Mesenchymal Stem/Stromal Cells (MSCs) and MSC-Derived Extracellular Vesicles (EVs) in Prevention of Telomere Length Shortening, Cellular Senescence, and Accelerated Biological Aging. Bioengineering, 11(6), 524. https://doi.org/10.3390/bioengineering11060524









