Alexander Friedenstein, Mesenchymal Stem Cells, Shifting Paradigms and Euphemisms
Abstract
1. Friedenstein, Transitional Epithelium, and Diffusion Chambers
2. Evidence for a Bone Marrow Osteogenic Stem Cell
3. Fibroblast Colony-Forming Cells (FCFCs), Colony-Forming Unit-Fibroblasts (CFU-Fs), and Multi-Potency
4. Euphemisms and Paradigms
5. Mechanistic Link between Stem and Stromal Critical Quality Attributes
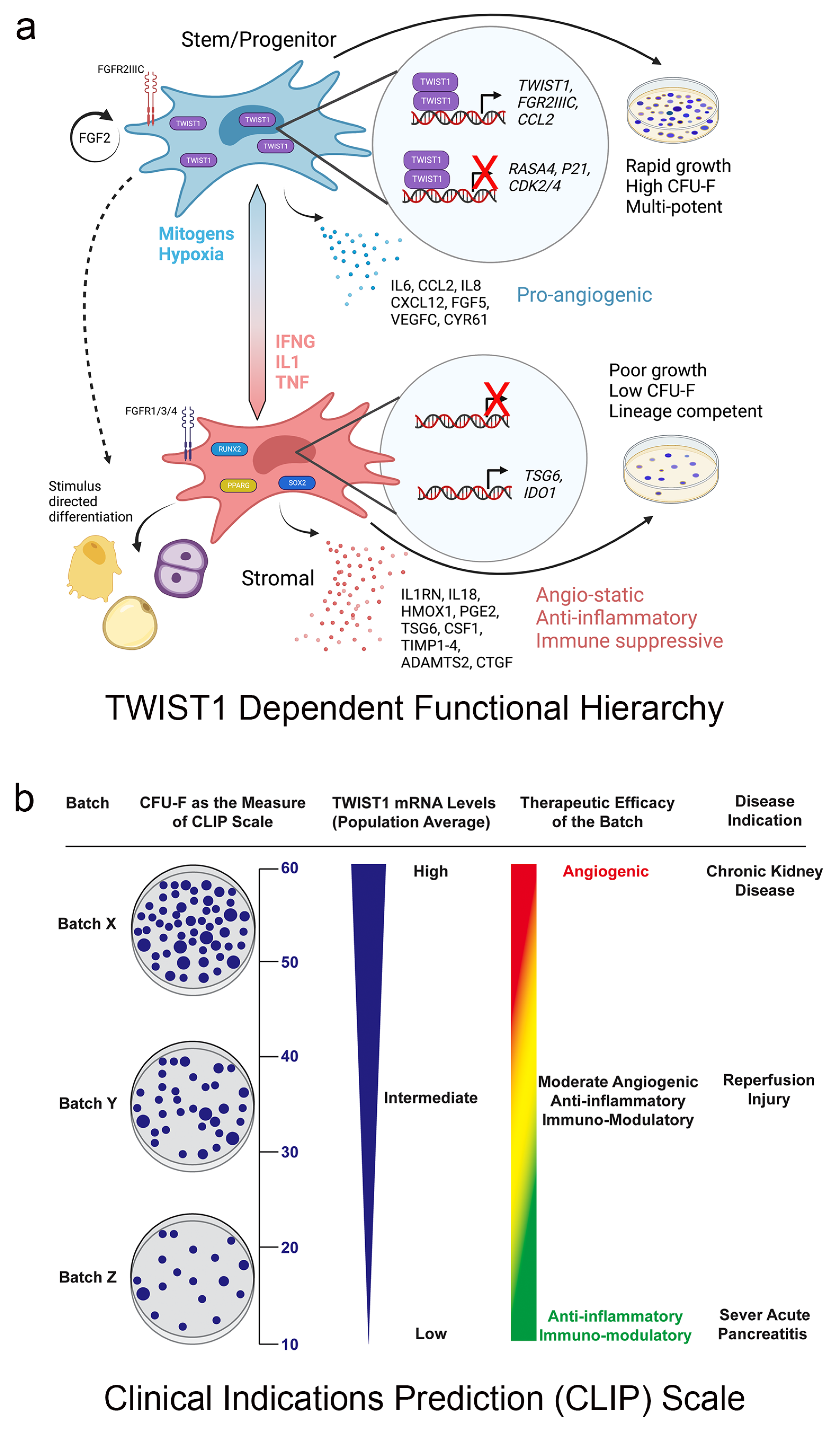
6. Future Perspective
Funding
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| CCL2 | C-C motif chemokine ligand 2 |
| CDKN1A | Cyclin dependent kinase inhibitor 1A |
| CFE-F | Colony-forming efficiency |
| CFU-F | Colony-forming unit-fibroblast |
| CLIP. | Clinical indications prediction scale |
| CSF1 | Colony stimulating factor 1 |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| EGF | Epidermal growth factor |
| FBS | Fetal bovine serum |
| FCFC | Fibroblast colony-forming cell |
| FGF | Fibroblast growth factor |
| FGFR2IIIC | Fibroblast growth factor receptor 2 IIIC |
| IFNG | Interferon gamma |
| hPL | Human platelet lysate |
| HSC | Hematopoietic stem cell |
| IDO1 | Indoleamine 2,3-dioxygenase 1 |
| ISCT | International Society of Cell & Gene Therapy |
| IL1RN | Interleukin 1 receptor antagonist |
| LepR | Leptin receptor |
| MSC | Mesenchymal stem cell |
| NGF | Nerve growth factor |
| PDGF | Platelet-derived growth factor |
| PGE2 | Prostaglandin E synthase 2 |
| PPARG | Peroxisome proliferator activated receptor Gamma |
| Rad | Radiation unit |
| RASA4 | RAS P21 protein activator 4 |
| RUNX2 | RUNX family transcription factor 2 |
| SCF | Stem cell factor |
| SDF-1α | Stromal cell-derived factor 1 alpha |
| SOX4 | SRY-Box transcription factor 4 |
| SSPC | Skeletal stem/progenitor cell |
| TGF-beta | Transforming growth factor beta |
| TSG6 | TNF-stimulated gene/protein 6 |
| TWIST1 | Twist family BHLH transcription factor 1 |
References
- Friedenstein, A.J. Osteogenetic Activity of Transplanted Transitional Epithelium. Acta Anat. 1961, 45, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J. Humoral Nature of Osteogenic Activity of Transitional Epithelium. Nature 1962, 194, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Rosin, A.; Freiberg, H.; Zajicek, G. The Fate of Rat Bone Marrow, Spleen and Periosteum Cultivated In Vivo in the Diffusion Chamber, with Special Reference to Bone Formation. Exp. Cell Res. 1963, 29, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Petrakova, K.V.; Tolmacheva, A.A.; Fridenshtein, A.Y. Osteogenesis Following Transplantation of Marrow in Diffusion Chambers. Bull. Exp. Biol. Med. 1963, 56, 1375–1378. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of Bone Marrow. Analysis of Precursor Cells for Osteogenic and Hematopoietic Tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.; Kuralesova, A.I. Osteogenic Precursor Cells of Bone Marrow in Radiation Chimeras. Transplantation 1971, 12, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal Cells Responsible for Transferring the Microenvironment of the Hemopoietic Tissues. Cloning In Vitro and Retransplantation In Vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone Marrow Osteogenic Stem Cells: In Vitro Cultivation and Transplantation in Diffusion Chambers. Cell Tissue Kinet. 1987, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, S.A.; Friedenstein, A.J.; Robey, P.G. Factors Required for Bone Marrow Stromal Fibroblast Colony Formation In Vitro. Br. J. Haematol. 1997, 97, 561–570. [Google Scholar] [CrossRef]
- Russell, K.C.; Phinney, D.G.; Lacey, M.R.; Barrilleaux, B.L.; Meyertholen, K.E.; O’Connor, K.C. In Vitro High-Capacity Assay to Quantify the Clonal Heterogeneity in Trilineage Potential of Mesenchymal Stem Cells Reveals a Complex Hierarchy of Lineage Commitment. Stem Cells 2010, 28, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Muraglia, A.; Cancedda, R.; Quarto, R. Clonal Mesenchymal Progenitors from Human Bone Marrow Differentiate in Vitro According to a Hierarchical Model. J. Cell Sci. 2000, 113 Pt 7, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Halleux, C.; Sottile, V.; Gasser, J.A.; Seuwen, K. Multi-Lineage Potential of Human Mesenchymal Stem Cells Following Clonal Expansion. J. Musculoskelet. Neuronal Interact. 2001, 2, 71–76. [Google Scholar] [PubMed]
- Okamoto, T.; Aoyama, T.; Nakayama, T.; Nakamata, T.; Hosaka, T.; Nishijo, K.; Nakamura, T.; Kiyono, T.; Toguchida, J. Clonal Heterogeneity in Differentiation Potential of Immortalized Human Mesenchymal Stem Cells. Biochem. Biophys. Res. Commun. 2002, 295, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.E.; Merriam, A.; Awadallah, A.; Yoo, J.U.; Johnstone, B.; Caplan, A.I. A Quadripotential Mesenchymal Progenitor Cell Isolated from the Marrow of an Adult Mouse. J. Bone Miner. Res. 1999, 14, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.C.; Lacey, M.R.; Gilliam, J.K.; Tucker, H.A.; Phinney, D.G.; O’Connor, K.C. Clonal Analysis of the Proliferation Potential of Human Bone Marrow Mesenchymal Stem Cells as a Function of Potency. Biotechnol. Bioeng. 2011, 108, 2716–2726. [Google Scholar] [CrossRef] [PubMed]
- Colter, D.C.; Class, R.; Digirolamo, C.M.; Prockop, D.J. Rapid Expansion of Recycling Stem Cells in Cultures of Plastic-Adherent Cells from Human Bone Marrow. Proc. Natl. Acad. Sci. USA 2000, 97, 3213–3218. [Google Scholar] [CrossRef] [PubMed]
- Colter, D.C.; Sekiya, I.; Prockop, D.J. Identification of a Subpopulation of Rapidly Self-Renewing and Multipotential Adult Stem Cells in Colonies of Human Marrow Stromal Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 7841–7845. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; My’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and Haematopoietic Stem Cells Form a Unique Bone Marrow Niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-Receptor-Expressing Mesenchymal Stromal Cells Represent the Main Source of Bone Formed by Adult Bone Marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Solidum, J.G.N.; Jeong, Y.; Heralde, F., 3rd; Park, D. Differential Regulation of Skeletal Stem/Progenitor Cells in Distinct Skeletal Compartments. Front. Physiol. 2023, 14, 1137063. [Google Scholar] [CrossRef] [PubMed]
- Comazzetto, S.; Shen, B.; Morrison, S.J. Niches That Regulate Stem Cells and Hematopoiesis in Adult Bone Marrow. Dev. Cell 2021, 56, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Muller-Sieburg, C.E. Stromal Cells in Long-Term Cultures: Keys to the Elucidation of Hematopoietic Development? Crit. Rev. Immunol. 1993, 13, 115–150. [Google Scholar] [PubMed]
- Dexter, T.M.; Moore, M.A.; Sheridan, A.P. Maintenance of Hemopoietic Stem Cells and Production of Differentiated Progeny in Allogeneic and Semiallogeneic Bone Marrow Chimeras in Vitro. J. Exp. Med. 1977, 145, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Dexter, T.M.; Coutinho, L.H.; Spooncer, E.; Heyworth, C.M.; Daniel, C.P.; Schiro, R.; Chang, J.; Allen, T.D. Stromal Cells in Haemopoiesis. Ciba Found. Symp. 1990, 148, 76–86, discussion 86–95. [Google Scholar] [PubMed]
- Morrison, S.J.; Uchida, N.; Weissman, I.L. The Biology of Hematopoietic Stem Cells. Annu. Rev. Cell Dev. Biol. 1995, 11, 35–71. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. The Mesengenic Process. Clin. Plast. Surg. 1994, 21, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Sipp, D.; Robey, P.G.; Turner, L. Clear up This Stem-Cell Mess. Nature 2018, 561, 455–457. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal Stem Cells as Trophic Mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The Msc: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.; Hill, K.; Michelson, C.; DuTreil, M.; Hughes, C.; Humphries, S.; Wilkinson, R.; Baddoo, M.; Bayly, E. Biological Activities Encoded by the Murine Mesenchymal Stem Cell Transcriptome Provide a Basis for Their Developmental Potential and Broad Therapeutic Efficacy. Stem Cells 2006, 24, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Kota, D.J.; Bazhanov, N.; Reger, R.L. Evolving Paradigms for Repair of Tissues by Adult Stem/Progenitor Cells (Mscs). J. Cell. Mol. Med. 2010, 14, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.F.; Seo, E.Y.; Chen, J.Y.; Lo, D.; McArdle, A.; Sinha, R.; Tevlin, R.; Seita, J.; Vincent-Tompkins, J.; Wearda, T.; et al. Identification and Specification of the Mouse Skeletal Stem Cell. Cell 2015, 160, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.S.; Murphy, M.P.; Marecic, O.; Lopez, M.; Brewer, R.E.; Koepke, L.S.; Manjunath, A.; Ranson, R.C.; Salhotra, A.; Weissman, I.L.; et al. Isolation and Functional Assessment of Mouse Skeletal Stem Cell Lineage. Nat. Protoc. 2018, 13, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ranson, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56.e21. [Google Scholar] [CrossRef] [PubMed]
- Sensebe, L.; Tarte, K.; Galipeau, J.; Krampera, M.; Martin, I.; Phinney, D.G.; Shi, Y. Limited Acquisition of Chromosomal Aberrations in Human Adult Mesenchymal Stromal Cells. Cell Stem Cell 2012, 10, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Ciccicioppo, R.; Galipeau, J.; Krampera, M.; Le Blanc, K.; Martin, I.; Moniz, K.; Nolta, J.; Phinney, D.G.; Shi, Y.; et al. Consensus International Council for Commonality in Blood Banking Automation-International Society for Cell & Gene Therapy Statement on Standard Nomenclature Abbreviations for the Tissue of Origin of Mesenchymal Stromal Cells. Cytotherapy 2021, 23, 1060–1063. [Google Scholar] [PubMed]
- Tsai, M.S.; Hwang, S.M.; Chen, K.D.; Lee, Y.S.; Hsu, L.W.; Chang, Y.J.; Wang, C.N.; Peng, H.H.; Chang, Y.L.; Chao, A.S.; et al. Functional Network Analysis of the Transcriptomes of Mesenchymal Stem Cells Derived from Amniotic Fluid, Amniotic Membrane, Cord Blood, and Bone Marrow. Stem Cells 2007, 25, 2511–2523. [Google Scholar] [CrossRef] [PubMed]
- Pelekanos, R.A.; Li, J.; Gongora, M.; Chandrakanthan, V.; Scown, J.; Suhaimi, N.; Brooke, G.; Christensen, M.E.; Doan, T.; Rice, A.M.; et al. Comprehensive Transcriptome and Immunophenotype Analysis of Renal and Cardiac Msc-Like Populations Supports Strong Congruence with Bone Marrow Msc Despite Maintenance of Distinct Identities. Stem Cell Res. 2012, 8, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Roson-Burgo, B.; Sanchez-Guijo, F.; Del Canizo, C.; De Las Rivas, J. Transcriptomic Portrait of Human Mesenchymal Stromal/Stem Cells Isolated from Bone Marrow and Placenta. BMC Genom. 2014, 15, 910. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.A.; Park, M.; Kim, Y.H.; Woo, S.Y.; Ryu, K.H. Rna Sequencing Reveals a Transcriptomic Portrait of Human Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Palatine Tonsils. Sci. Rep. 2017, 7, 17114. [Google Scholar] [CrossRef] [PubMed]
- Ménard, C.; Dulong, J.; Roulois, D.; Hebraud, B.; Verdiere, L.; Pangault, C.; Sibut, V.; Bezier, I.; Bescher, N.; Monvoison, C.; et al. Integrated Transcriptomic, Phenotypic, and Functional Study Reveals Tissue-Specific Immune Properties of Mesenchymal Stromal Cells. Stem Cells 2020, 38, 146–159. [Google Scholar] [CrossRef]
- Crigler, L.; Robey, R.; Asawachaicharn, A.; Gaupp, D.; Phinney, D. Human Mesenchymal Stem Cell Subpopulations Express a Variety of Neuro-Regulatory Molecules and Promote Neuronal Cell Survival and Neuritogenesis. Exp. Neurol. 2006, 198, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Kwee, B.J.; Lam, J.; Akue, A.; KuKuruga, M.A.; Zhang, K.; Gu, L.; Sung, K.E. Functional Heterogeneity of Ifn-Γ-Licensed Mesenchymal Stromal Cell Immunosuppressive Capacity on Biomaterials. Proc. Natl. Acad. Sci. USA 2021, 118, e2105972118. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, K.; Yue, J.; Meng, S.; Zhang, X. Deconstructing Transcriptional Variations and Their Effects on Immunomodulatory Function among Human Mesenchymal Stromal Cells. Stem Cell Res. Ther. 2021, 12, 53. [Google Scholar] [CrossRef]
- Maughon, T.S.; Shen, X.; Huang, D.; Michael, A.O.A.; Shockey, W.A.; Andrews, S.H.; McRae, J.M.; Platt, M.O.; Fernandez, F.M.; Edison, A.S.; et al. Metabolomics and Cytokine Profiling of Mesenchymal Stromal Cells Identify Markers Predictive of T-Cell Suppression. Cytotherapy 2022, 24, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Robb, K.P.; Audet, J.; Gandhi, R.; Viswanathan, S. Putative Critical Quality Attribute Matrix Identifies Mesenchymal Stromal Cells with Potent Immunomodulatory and Angiogenic “Fitness” Ranges in Response to Culture Process Parameters. Front. Immunol. 2022, 13, 972095. [Google Scholar] [CrossRef]
- Gao, X.; Murphy, M.M.; Peyer, J.G.; Ni, Y.; Yang, M.; Zhang, Y.; Guo, J.; Kara, N.; Embree, C.; Tasdogan, A.; et al. Leptin Receptor(+) Cells Promote Bone Marrow Innervation and Regeneration by Synthesizing Nerve Growth Factor. Nat. Cell Biol. 2023, 25, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Boregowda, S.V.; Krishnappa, V.; Haga, C.L.; Ortiz, L.A.; Phinney, D.G. A Clinical Indications Prediction Scale Based on Twist1 for Human Mesenchymal Stem Cells. EBioMedicine 2016, 4, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Boregowda, S.V.; Phinney, D.G. Quantifiable Metrics for Predicting Msc Therapeutic Efficacy. J. Stem Cell Res. Ther. 2016, 6, 365. [Google Scholar] [CrossRef] [PubMed]
- Haga, C.L.; Booker, C.N.; Carvalho, A.; Boregowda, S.V.; Phinney, D.G. Transcriptional Targets of Twist1 in Human Mesenchymal Stem/Stromal Cells Mechanistically Link Stem/Progenitor and Paracrine Functions. Stem Cells 2023, 41, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shao, Y.; Mei, Y.; Zhang, L.; Li, Q.; Li, D.; Shi, S.; Hong, Q.; Lin, H.; Chen, X. Novel Mechanism for Mesenchymal Stem Cells in Attenuating Peritoneal Adhesion: Accumulating in the Lung and Secreting Tumor Necrosis Factor A-Stimulating Gene-6. Stem Cell Res. Ther. 2012, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Hua, J.; Qian, D.; Gong, J.; Lin, S.; Xu, C.; Wei, G.; Meng, H.; Yang, T.; Zhou, B.; et al. Intravenous Hmscs Ameliorate Acute Pancreatitis in Mice Via Secretion of Tumor Necrosis Factor-A Stimulated Gene/Protein 6. Sci. Rep. 2016, 6, 38438. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-Inflammatory Protein Tsg-6 Secreted by Activated Mscs Attenuates Zymosan-Induced Mouse Peritonitis by Decreasing Tlr2/Nf-Κb Signaling in Resident Macrophages. Blood 2011, 118, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Genua, M.; Petti, L.; Anselmo, A.; Arena, V.; Cibella, J.; Zanotti, L.; D’Alessio, S.; Scaldaferri, F.; Luca, G.; et al. Mesenchymal Stem Cells Reduce Colitis in Mice Via Release of Tsg6, Independently of Their Localization to the Intestine. Gastroenterology 2015, 149, 163–176.e20. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Li, Q.; Ryu, M.O.; Ahn, J.O.; Bhang, D.H.; Jung, Y.C.; Youn, H.Y. Tsg-6 Secreted by Human Adipose Tissue-Derived Mesenchymal Stem Cells Ameliorates Dss-Induced Colitis by Inducing M2 Macrophage Polarization in Mice. Sci. Rep. 2017, 7, 5187. [Google Scholar] [CrossRef]
- Song, H.B.; Park, S.Y.; Ko, J.H.; Park, J.W.; Yoon, C.H.; Kim, D.H.; Kin, J.H.; Kim, M.K.; Lee, R.H.; Prockop, D.J.; et al. Mesenchymal Stromal Cells Inhibit Inflammatory Lymphangiogenesis in the Cornea by Suppressing Macrophage in a Tsg-6-Dependent Manner. Mol. Ther. 2018, 26, 162–172. [Google Scholar] [CrossRef]
- Kota, D.J.; Wiggins, L.L.; Yoon, N.; Lee, R.H. Tsg-6 Produced by Hmscs Delays the Onset of Autoimmune Diabetes by Suppressing Th1 Development and Enhancing Tolerogenicity. Diabetes 2013, 62, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Lee, R.H.; Yu, J.M.; Ko, J.H.; Lee, H.J.; Roddy, G.W.; Prockop, D.J. Intravenous Mesenchymal Stem Cells Prevented Rejection of Allogeneic Corneal Transplants by Aborting the Early Inflammatory Response. Mol. Ther. 2012, 20, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Boregowda, S.V.; Shigemoto-Kuroda, T.; Bai, E.; Haga, C.L.; Abbery, C.A.; Bayless, K.J.; Haskell, A.; Gregory, C.A.; Ortiz, L.A.; et al. TWIST1 and TSG6 Are Coordinately Regulated and Function as Potency Biomarkers in Human MSCs. Sci. Adv. 2023, 9, eadi2387. [Google Scholar] [CrossRef] [PubMed]
- Bister, N.; Pistono, C.; Huremagic, B.; Jolkkonen, J.; Giugno, R.; Malm, T. Hypoxia and Extracellular Vesicles: A Review on Methods, Vesicular Cargo and Functions. J. Extracell. Vesicles 2020, 10, e12002. [Google Scholar] [CrossRef] [PubMed]
- Boregowda, S.V.; Phinney, D.G. Therapeutic Applications of Mesenchymal Stem Cells Current Outlook. Biodrugs 2012, 26, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ratushnyy, A.; Ezdakova, M.; Buravkova, L. Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis. Int. J. Mol. Sci. 2020, 21, 1802. [Google Scholar] [CrossRef] [PubMed]
- Willer, H.; Spohn, G.; Morgenroth, K.; Thielemann, C.; Elvers-Hornung, S.; Bugert, P.; Delorme, B.; Giesen, M.; Schmitz-Rixen, T.; Seifreid, E.; et al. Pooled Human Bone Marrow-Derived Mesenchymal Stromal Cells with Defined Trophic Factors Cargo Promote Dermal Wound Healing in Diabetic Rats by Improved Vascularization and Dynamic Recruitment of M2-Like Macrophages. Front. Immunol. 2022, 13, 976511. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Liao, H.T. Platelet-Rich Plasma Enhances Adipose-Derived Stem Cell-Mediated Angiogenesis in a Mouse Ischemic Hindlimb Model. World J. Stem Cells 2018, 10, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Klinker, M.W.; Marklein, R.A.; Lo Surdo, J.L.; Wei, C.H.; Bauer, S.R. Morphological Features of Ifn-Γ-Stimulated Mesenchymal Stromal Cells Predict Overall Immunosuppressive Capacity. Proc. Natl. Acad. Sci. USA 2017, 114, E2598–E2607. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, E.R.; Udartseva, O.O.; Zhidkova, O.V.; Buravkov, S.V.; Ezdakova, M.I.; Buravkova, L.B. IFN-Gamma Priming of Adipose-Derived Stromal Cells at “Physiological” Hypoxia. J. Cell. Physiol. 2018, 233, 1535–1547. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, M.K.; Shin, M.S.; Lee, H.J.; Ko, J.H.; Wee, W.R.; Lee, J.H. The Anti-Inflammatory and Anti-Angiogenic Role of Mesenchymal Stem Cells in Corneal Wound Healing Following Chemical Injury. Stem Cells 2008, 26, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, C.; Ceresa, D.; Lesage, R.; Villa, F.; Reverberi, D.; Balbi, C.; Santamaria, S.; Cortese, K.; Malatesta, P.; Geris, L.; et al. Dissecting the Effects of Preconditioning with Inflammatory Cytokines and Hypoxia on the Angiogenic Potential of Mesenchymal Stromal Cell (Msc)-Derived Soluble Proteins and Extracellular Vesicles (Evs). Biomaterials 2021, 269, 120633. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazik, H.; Spaggiari, G.M.; Chiossone, L.; Moretta, L. Mesenchymal Stem Cells Expanded in Human Platelet Lysate Display a Decreased Inhibitory Capacity on T- and Nk-Cell Proliferation and Function. Eur. J. Immunol. 2011, 41, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- Von Bonin, M.; Stolzel, F.; Goedecke, A.; Richter, K.; Wuschek, N.; Holig, K.; Platzbecker, U.; Illmer, T.; Schaich, M.; Schetelig, J.; et al. Treatment of Refractory Acute Gvhd with Third-Party Msc Expanded in Platelet Lysate-Containing Medium. Bone Marrow Transplant. 2009, 43, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, G.; Introna, M.; Dander, E.; Rovelli, A.; Balduzzi, A.; Bonanomi, S.; Salvade, A.; Capelli, C.; Belotti, D.; Gaipa, G.; et al. Platelet-Lysate-Expanded Mesenchymal Stromal Cells as a Salvage Therapy for Severe Resistant Graft-Versus-Host Disease in a Pediatric Population. Biol. Blood Marrow Transplant. 2010, 16, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Mckinnirey, F.; Herbert, B.; Vesey, G.; Mccracken, S. Immune Modulation Via Adipose Derived Mesenchymal Stem Cells Is Driven by Donor Sex in Vitro. Sci. Rep. 2021, 11, 12454. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Lee, R.H.; Boregowda, S.V. Revisiting the Mesenchymal “Stem vs. Stromal” Cell Dichotomy and Its Implications for Development of Improved Potency Metrics. Stem Cells 2023, 41, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Renesme, L.; Cobey, K.D.; Le, M.; Lalu, M.M.; Thebaud, B. Establishment of a Consensus Definition for Mesenchymal Stromal Cells (Msc) and Reporting Guidelines for Clinical Trials of Msc Therapy: A Modified Delphi Study Protocol. BMJ Open 2021, 11, e054740. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phinney, D.G. Alexander Friedenstein, Mesenchymal Stem Cells, Shifting Paradigms and Euphemisms. Bioengineering 2024, 11, 534. https://doi.org/10.3390/bioengineering11060534
Phinney DG. Alexander Friedenstein, Mesenchymal Stem Cells, Shifting Paradigms and Euphemisms. Bioengineering. 2024; 11(6):534. https://doi.org/10.3390/bioengineering11060534
Chicago/Turabian StylePhinney, Donald G. 2024. "Alexander Friedenstein, Mesenchymal Stem Cells, Shifting Paradigms and Euphemisms" Bioengineering 11, no. 6: 534. https://doi.org/10.3390/bioengineering11060534
APA StylePhinney, D. G. (2024). Alexander Friedenstein, Mesenchymal Stem Cells, Shifting Paradigms and Euphemisms. Bioengineering, 11(6), 534. https://doi.org/10.3390/bioengineering11060534








