Quantification of Visceral Fat at the L5 Vertebral Body Level in Patients with Crohn’s Disease Using T2-Weighted MRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
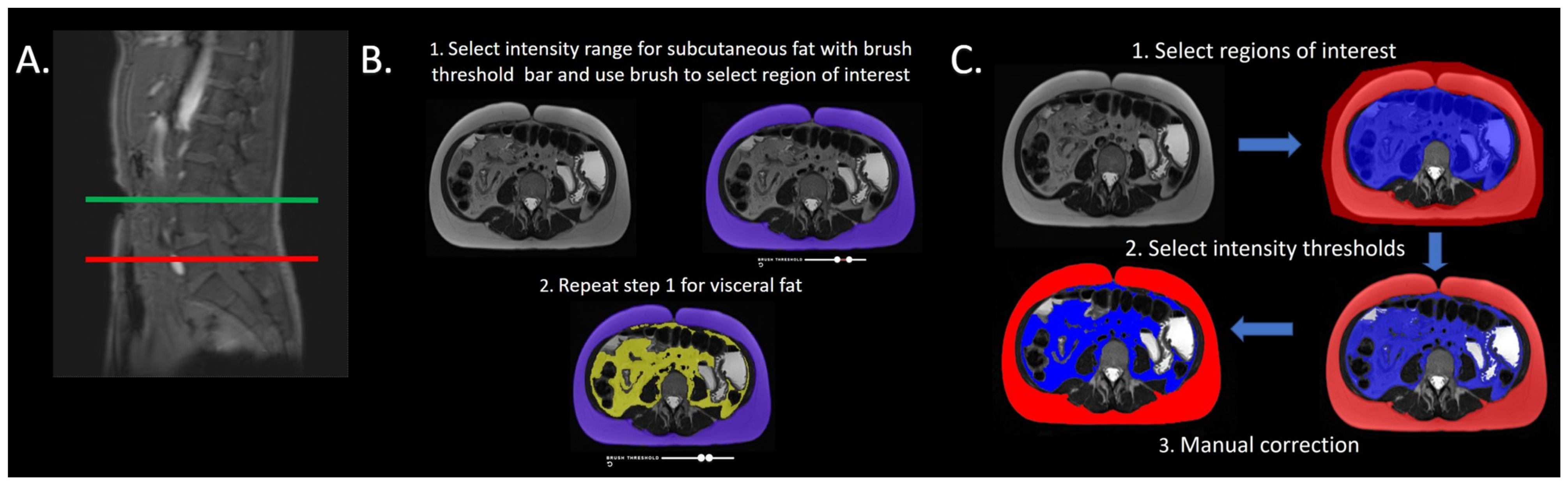
2.2. Adipose Tissue Quantification
2.3. Platform 1 Image Analysis
2.4. Platform 2 Image Analysis
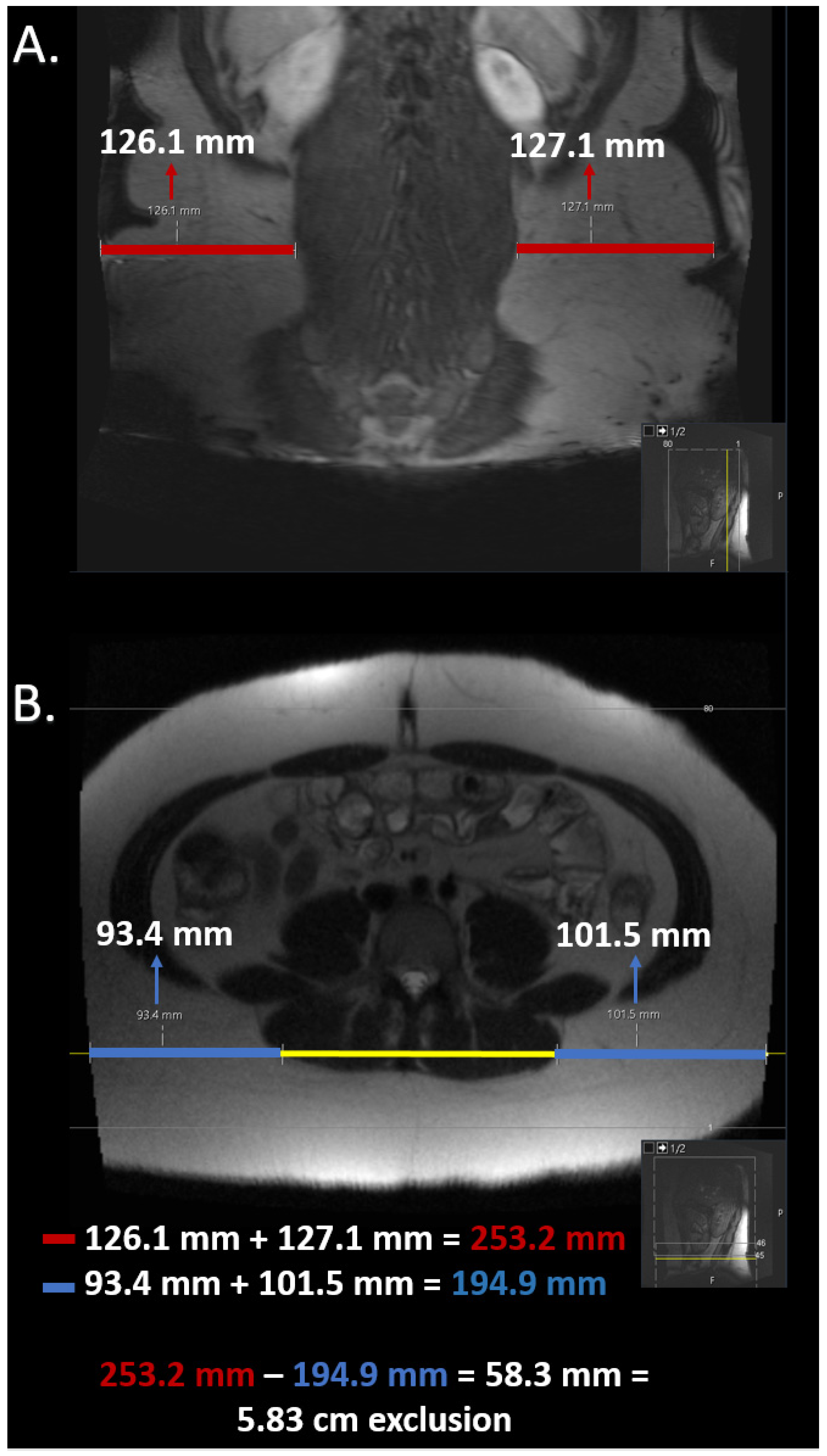
2.5. Estimation of Field of View Restriction
2.6. Statistical Analysis
3. Results
3.1. Study Population Characteristics
3.2. Subcutaneous and Visceral Fat
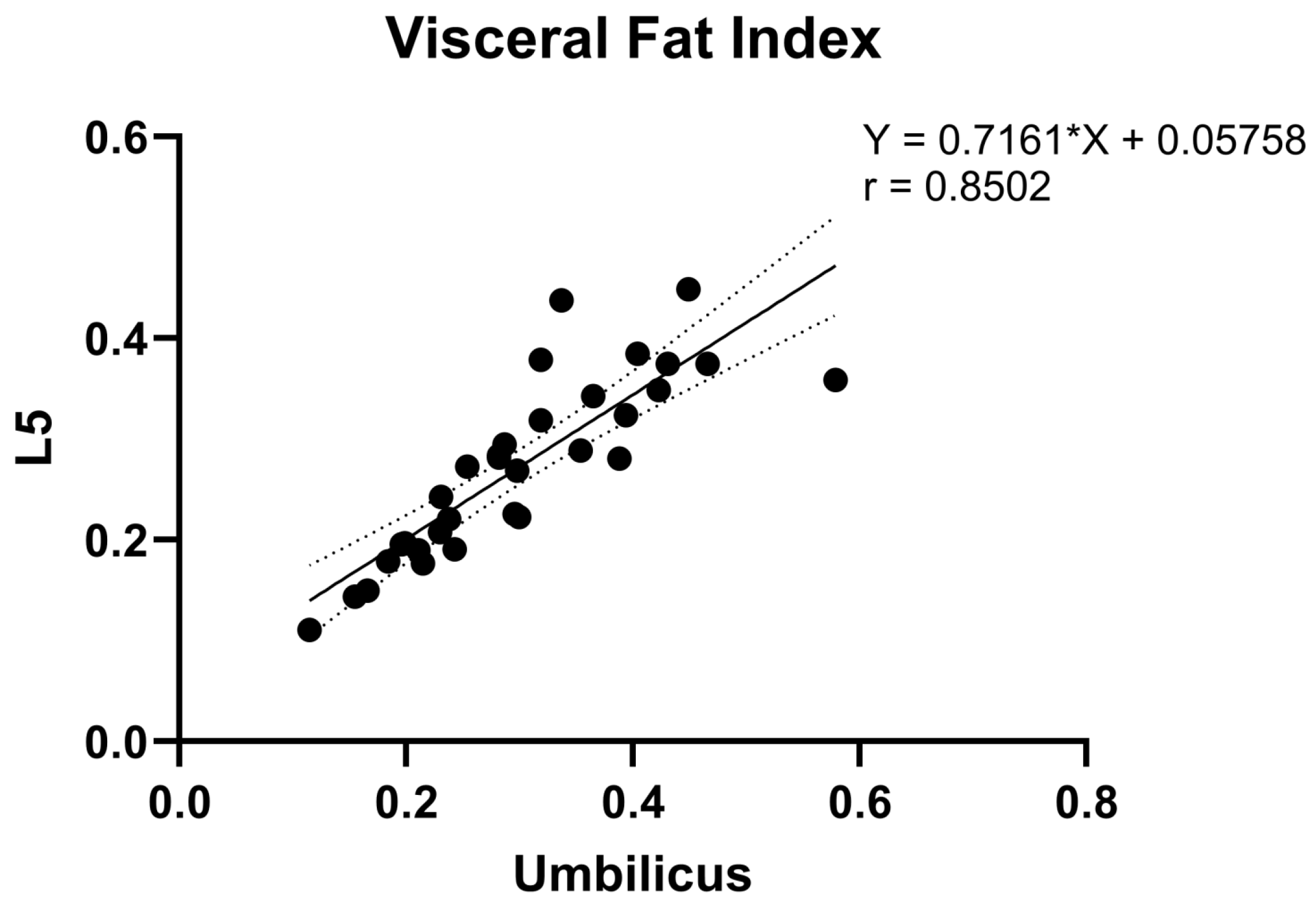
3.3. Correlation Analysis of Fat Measurements between Umbilicus and L5
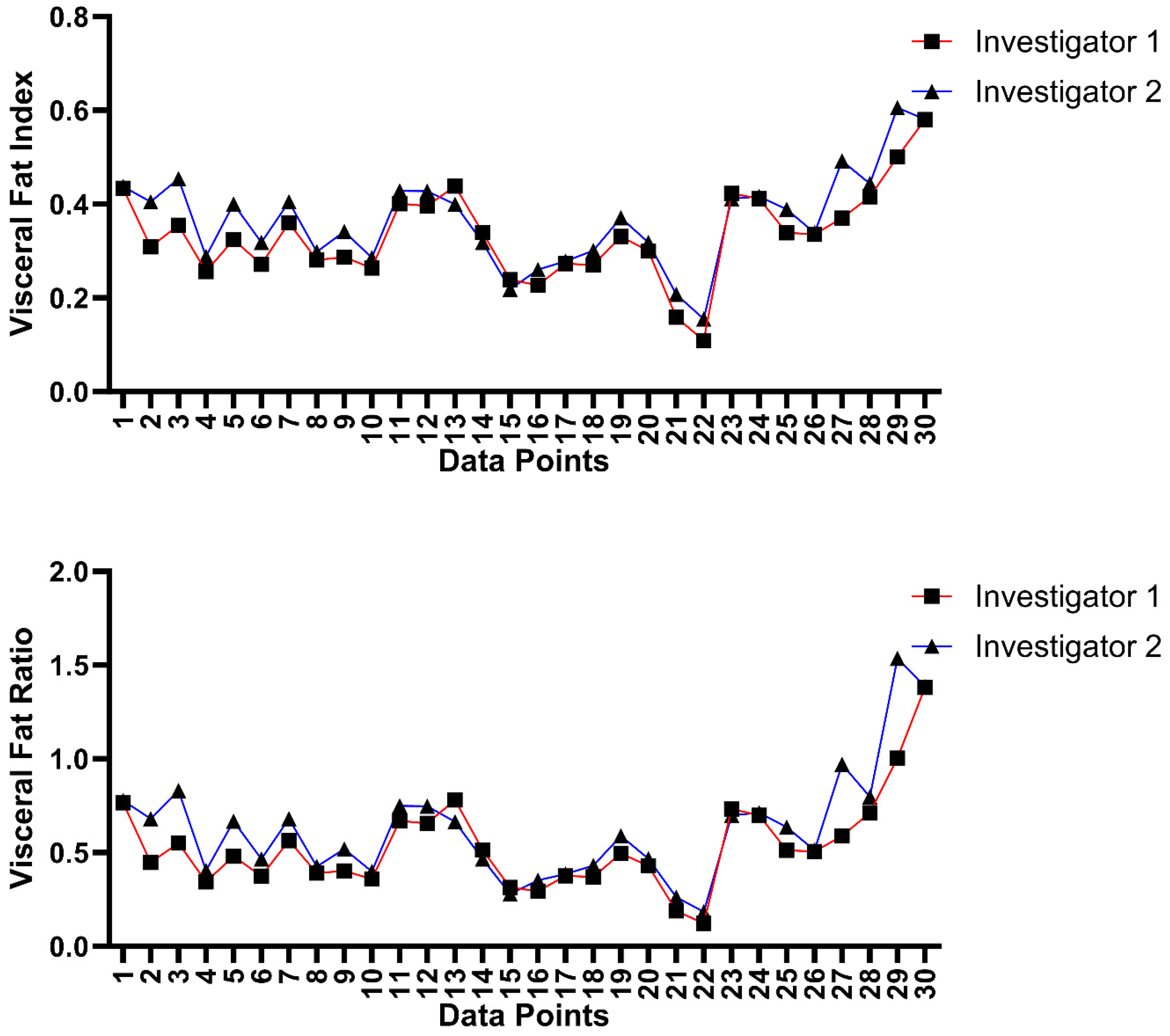
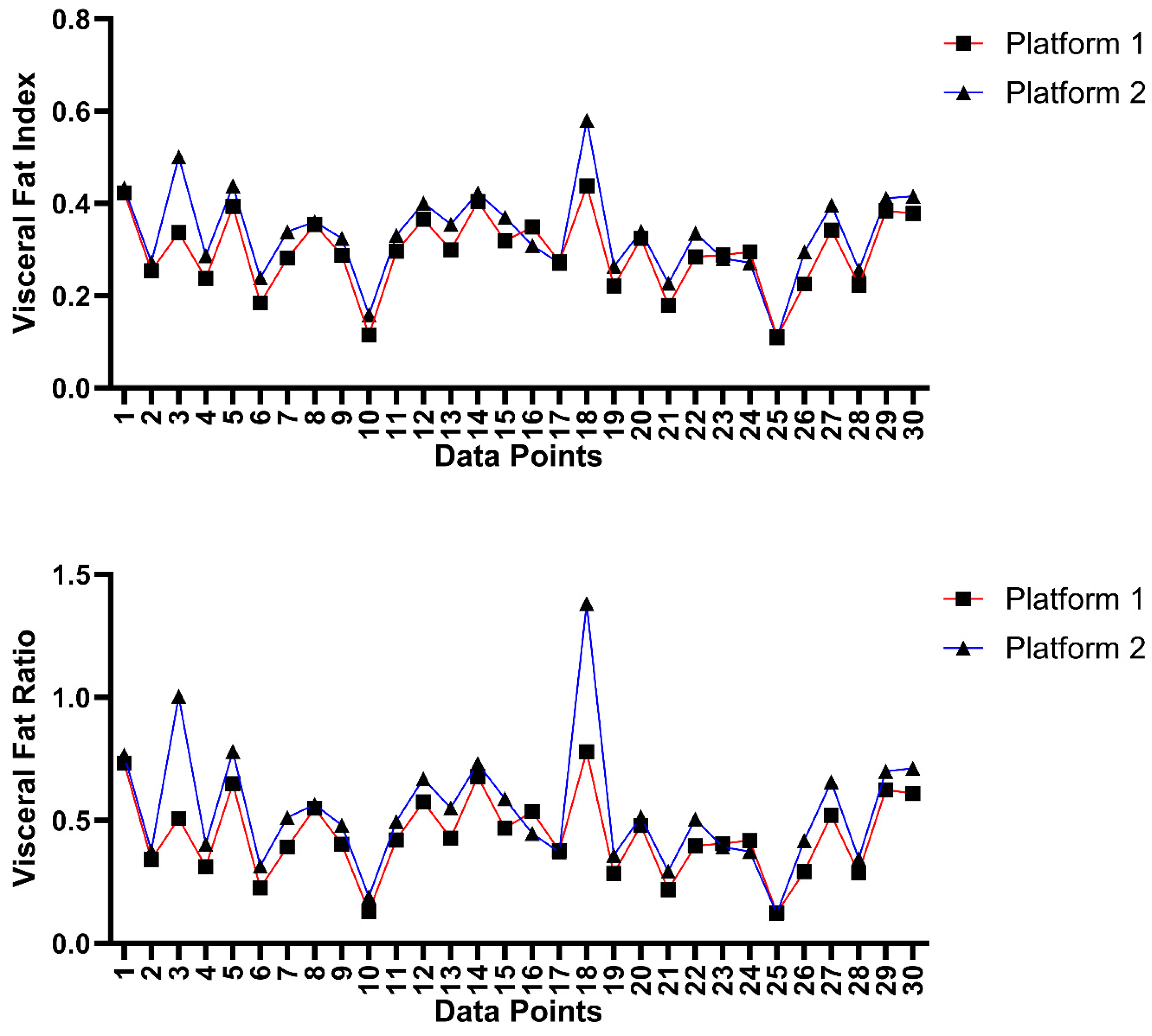
3.4. Inter-Platform and Inter-Rater Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goossens, G.H. The metabolic phenotype in obesity: Fat mass, body fat distribution, and adipose tissue function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, adipose tissue and vascular dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Yarur, A.J.; Bruss, A.; Moosreiner, A.; Beniwal-Patel, P.; Nunez, L.; Berens, B.; Colombel, J.F.; Targan, S.R.; Fox, C.; Melmed, G.Y. Higher intra-abdominal visceral adipose tissue mass is associated with lower rates of clinical and endoscopic remission in patients with inflammatory bowel diseases initiating biologic therapy: Results of the constellation study. Gastroenterology 2023, 165, 963–975.e5. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.; Connell, J. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, B.J.; Kim, M.; Krisky, C.; Szumowski, J.; Rooney, W.; Purnell, J. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br. J. Radiol. 2012, 85, e826–e830. [Google Scholar] [CrossRef] [PubMed]
- Zaffina, C.; Wyttenbach, R.; Pagnamenta, A.; Grasso, R.F.; Biroli, M.; Del Grande, F.; Rizzo, S. Body composition assessment: Comparison of quantitative values between magnetic resonance imaging and computed tomography. Quant. Imaging Med. Surg. 2022, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.D.; Korfiatis, P.; Kline, T.L.; Philbrick, K.A.; Kostandy, P.; Sakinis, T.; Sugimoto, M.; Takahashi, N.; Erickson, B.J. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology 2019, 290, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Küstner, T.; Hepp, T.; Fischer, M.; Schwartz, M.; Fritsche, A.; Häring, H.-U.; Nikolaou, K.; Bamberg, F.; Yang, B.; Schick, F. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol. Artif. Intell. 2020, 2, e200010. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Chen, J.; Shen, W. Segmentation and quantification of adipose tissue by magnetic resonance imaging. Magn. Reson. Mater. Phys. Biol. Med. 2016, 29, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Estrada, S.; Lu, R.; Conjeti, S.; Orozco-Ruiz, X.; Panos-Willuhn, J.; Breteler, M.M.; Reuter, M. FatSegNet: A fully automated deep learning pipeline for adipose tissue segmentation on abdominal dixon MRI. Magn. Reson. Med. 2020, 83, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Estrada, S.; van Gils, R.; Su, R.; Jaddoe, V.W.; Oei, E.H.; Klein, S. Automated Deep Learning–Based Segmentation of Abdominal Adipose Tissue on Dixon MRI in Adolescents: A Prospective Population-Based Study. Am. J. Roentgenol. 2023, 222, e2329570. [Google Scholar] [CrossRef] [PubMed]
- Marunowski, K.; Świętoń, D.; Bzyl, W.; Grzywińska, M.; Kaszubowski, M.; Bandosz, P.; Khrichenko, D.; Piskunowicz, M. MRI-derived subcutaneous and visceral adipose tissue reference values for children aged 6 to under 18 years. Front. Nutr. 2021, 8, 757274. [Google Scholar] [CrossRef] [PubMed]
- Dalah, E.Z.; Hasan, H.A.; Madkour, M.I.; Obaideen, A.; Faris, M.A.-I.E. Assessing visceral and subcutaneous adiposity using segmented T2-MRI and multi-frequency segmental bioelectrical impedance: A sex-based comparative study. Acta Bio Med. Atenei Parm. 2021, 92, e2021078. [Google Scholar]
- Shen, W.; Punyanitya, M.; Chen, J.; Gallagher, D.; Albu, J.; Pi-Sunyer, X.; Lewis, C.; Grunfeld, C.; Heymsfield, S.; Heshka, S. Visceral adipose tissue: Relationships between single slice areas at different locations and obesity-related health risks. Int. J. Obes. 2007, 31, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Greer, M.-L.C.; Taylor, S.A. Perianal imaging in Crohn disease: Current status with a focus on MRI, from the AJR special series on imaging of inflammation. Am. J. Roentgenol. 2022, 218, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Mullie, L.; Afilalo, J. CoreSlicer: A web toolkit for analytic morphomics. BMC Med Imaging 2019, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Holleran, S.; Ramakrishnan, R. Cufunctions, a Package to Facilitate Statistical Analyses in R; Columbia University Medical Center: New York, NY, USA, 2021. [Google Scholar]
- Bobak, C.A.; Barr, P.J.; O’Malley, A.J. Estimation of an inter-rater intra-class correlation coefficient that overcomes common assumption violations in the assessment of health measurement scales. BMC Med. Res. Methodol. 2018, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, L.; Geisler, C.; Pourhassan, M.; Braun, W.; Glüer, C.-C.; Bosy-Westphal, A.; Müller, M.J. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am. J. Clin. Nutr. 2015, 102, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Church, T.S.; Blair, S.N.; Ross, R. Measurement site and the association between visceral and abdominal subcutaneous adipose tissue with metabolic risk in women. Obesity 2010, 18, 1336–1340. [Google Scholar] [CrossRef]
- Sottier, D.; Petit, J.M.; Guiu, S.; Hamza, S.; Benhamiche, H.; Hillon, P.; Cercueil, J.P.; Krausé, D.; Guiu, B. Quantification of the visceral and subcutaneous fat by computed tomography: Interobserver correlation of a single slice technique. Diagn. Interv. Imaging 2013, 94, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Maislin, G.; Ahmed, M.M.; Gooneratne, N.; Thorne-Fitzgerald, M.; Kim, C.; Teff, K.; Arnardottir, E.S.; Benediktsdottir, B.; Einarsdottir, H.; Juliusson, S. Single slice vs. volumetric MR assessment of visceral adipose tissue: Reliability and validity among the overweight and obese. Obesity 2012, 20, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Kvist, H.; Chowdhury, B.; Sjöström, L.; Tylen, U.; Cederblad, A. Adipose tissue volume determination in males by computed tomography and 40K. Int. J. Obes. 1988, 12, 249–266. [Google Scholar] [PubMed]
- Tokunaga, K.; Matsuzawa, Y.; Ishikawa, K.; Tarui, S. A novel technique for the determination of body fat by computed tomography. Int. J. Obes. 1983, 7, 437–445. [Google Scholar] [PubMed]
- Ryo, M.; Kishida, K.; Nakamura, T.; Yoshizumi, T.; Funahashi, T.; Shimomura, I. Clinical significance of visceral adiposity assessed by computed tomography: A Japanese perspective. World J. Radiol. 2014, 6, 409. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhou, Z.; Hao, L.; Li, Y.; Hu, X.; Hu, D.; Luo, Y.; Wang, Y.; Shen, Y.; Li, Z. The relationship between perianal fistula activity and abdominal adipose tissue in Crohn’s disease: An observational study. Insights Imaging 2022, 13, 156. [Google Scholar] [CrossRef]
- Pescatori, L.C.; Savarino, E.; Mauri, G.; Silvestri, E.; Cariati, M.; Sardanelli, F.; Sconfienza, L.M. Quantification of visceral adipose tissue by computed tomography and magnetic resonance imaging: Reproducibility and accuracy. Radiol. Bras. 2019, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Haugen, C.; Lysne, V.; Haldorsen, I.; Tjora, E.; Gudbrandsen, O.A.; Sagen, J.V.; Dankel, S.N.; Mellgren, G. MRI adipose tissue segmentation and quantification in R (RAdipoSeg). Diabetol. Metab. Syndr. 2022, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Chen, J.; Gantz, M.; Velasquez, G.; Punyanitya, M.; Heymsfield, S.B. A single MRI slice does not accurately predict visceral and subcutaneous adipose tissue changes during weight loss. Obesity 2012, 20, 2458–2463. [Google Scholar] [CrossRef]
- Kullberg, J.; Angelhed, J.E.; Lönn, L.; Brandberg, J.; Ahlström, H.; Frimmel, H.; Johansson, L. Whole-body T1 mapping improves the definition of adipose tissue: Consequences for automated image analysis. J. Magn. Reason. Imaging 2006, 24, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, C.; Peng, H.; Qi, Y.; Wan, Q.; Zhou, H.; Qu, S.; Liang, D.; Liu, X.; Zheng, H.; et al. Automatic segmentation of whole-body adipose tissue from magnetic resonance fat fraction images based on machine learning. Magma 2022, 35, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Michel, S.; Linder, N.; Eggebrecht, T.; Schaudinn, A.; Blüher, M.; Dietrich, A.; Denecke, T. Abdominal subcutaneous fat quantification in obese patients from limited field-of-view MRI data. Sci. Rep. 2020, 10, 19039. [Google Scholar] [CrossRef] [PubMed]



| Baseline Characteristics of Patients on Study (n = 32) | |
|---|---|
| Age (years) | |
| Mean ± SD | 38.2 ± 14.1 |
| Sex | |
| Female | 18 (56.2%) |
| Male | 14 (43.8%) |
| Weight (kg) | |
| Mean ± SD | 73.2 ± 16 |
| Height (m) | |
| Mean ± SD | 1.72 ± 0.108 |
| BMI | |
| Mean ± SD | 24.8 ± 3.95 |
| Parameter (n = 32) | Umbilicus | L5 | p-Value |
|---|---|---|---|
| Subcutaneous Fat Area (SFA), mean (±SD) | 239 ± 96.3 cm2 | 262 ± 97.3 cm2 | 0.349 |
| Visceral Fat Area (VFA), mean (±SD) | 106 ± 68.2 cm2 | 95.4 ± 45.8 cm2 | 0.8932 † |
| Visceral Fat Index (VFI), mean (±SD) | 0.3 ± 0.105 | 0.273 ± 0.0881 | 0.256 |
| Visceral Fat Ratio (VFR), mean (±SD) | 0.465 ± 0.256 | 0.395 ± 0.177 | 0.2767 † |
| Parameter (N = 15) | Umbilicus Reader 1 | L5 Reader 1 | Umbilicus Reader 2 | L5 Reader 2 | p-Value between Umbilicus and L5 Levels |
|---|---|---|---|---|---|
| Subcutaneous Fat Volume, mean (±SD) | 120 ± 54.4 cm3 | 131 ± 56.7 cm3 | 119 ± 52.7 cm3 | 127 ± 55.3 cm3 | 0.5 |
| Visceral Fat Volume, mean (±SD) | 61.7 ± 27.7 cm3 | 55.6 ± 19.5 cm3 | 71.3 ± 25.9 cm3 | 60.6 ± 17.2 cm3 | 0.2805 † |
| Total Fat Volume, mean (±SD) | 182 ± 72 | 187 ± 64.6 | 191 ± 68.4 | 188 ± 64 | 0.95 |
| Visceral Fat Ratio, mean (±SD) | 0.562 ± 0.207 | 0.507 ± 0.289 | 0.683 ± 0.306 | 0.562 ± 0.282 | 0.06 † |
| Visceral Fat Index, mean (±SD) | 0.349 ± 0.0869 | 0.318 ± 0.107 | 0.389 ± 0.101 | 0.344 ± 0.099 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garuba, F.; Ganapathy, A.; McKinley, S.; Jani, K.H.; Lovato, A.; Viswanath, S.E.; McHenry, S.; Deepak, P.; Ballard, D.H. Quantification of Visceral Fat at the L5 Vertebral Body Level in Patients with Crohn’s Disease Using T2-Weighted MRI. Bioengineering 2024, 11, 528. https://doi.org/10.3390/bioengineering11060528
Garuba F, Ganapathy A, McKinley S, Jani KH, Lovato A, Viswanath SE, McHenry S, Deepak P, Ballard DH. Quantification of Visceral Fat at the L5 Vertebral Body Level in Patients with Crohn’s Disease Using T2-Weighted MRI. Bioengineering. 2024; 11(6):528. https://doi.org/10.3390/bioengineering11060528
Chicago/Turabian StyleGaruba, Favour, Aravinda Ganapathy, Spencer McKinley, Karan H. Jani, Adriene Lovato, Satish E. Viswanath, Scott McHenry, Parakkal Deepak, and David H. Ballard. 2024. "Quantification of Visceral Fat at the L5 Vertebral Body Level in Patients with Crohn’s Disease Using T2-Weighted MRI" Bioengineering 11, no. 6: 528. https://doi.org/10.3390/bioengineering11060528
APA StyleGaruba, F., Ganapathy, A., McKinley, S., Jani, K. H., Lovato, A., Viswanath, S. E., McHenry, S., Deepak, P., & Ballard, D. H. (2024). Quantification of Visceral Fat at the L5 Vertebral Body Level in Patients with Crohn’s Disease Using T2-Weighted MRI. Bioengineering, 11(6), 528. https://doi.org/10.3390/bioengineering11060528






