Integrating Deep Learning with Electronic Health Records for Early Glaucoma Detection: A Multi-Dimensional Machine Learning Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants, Feature Selection, and Dataset Preparation
2.2. Training Models
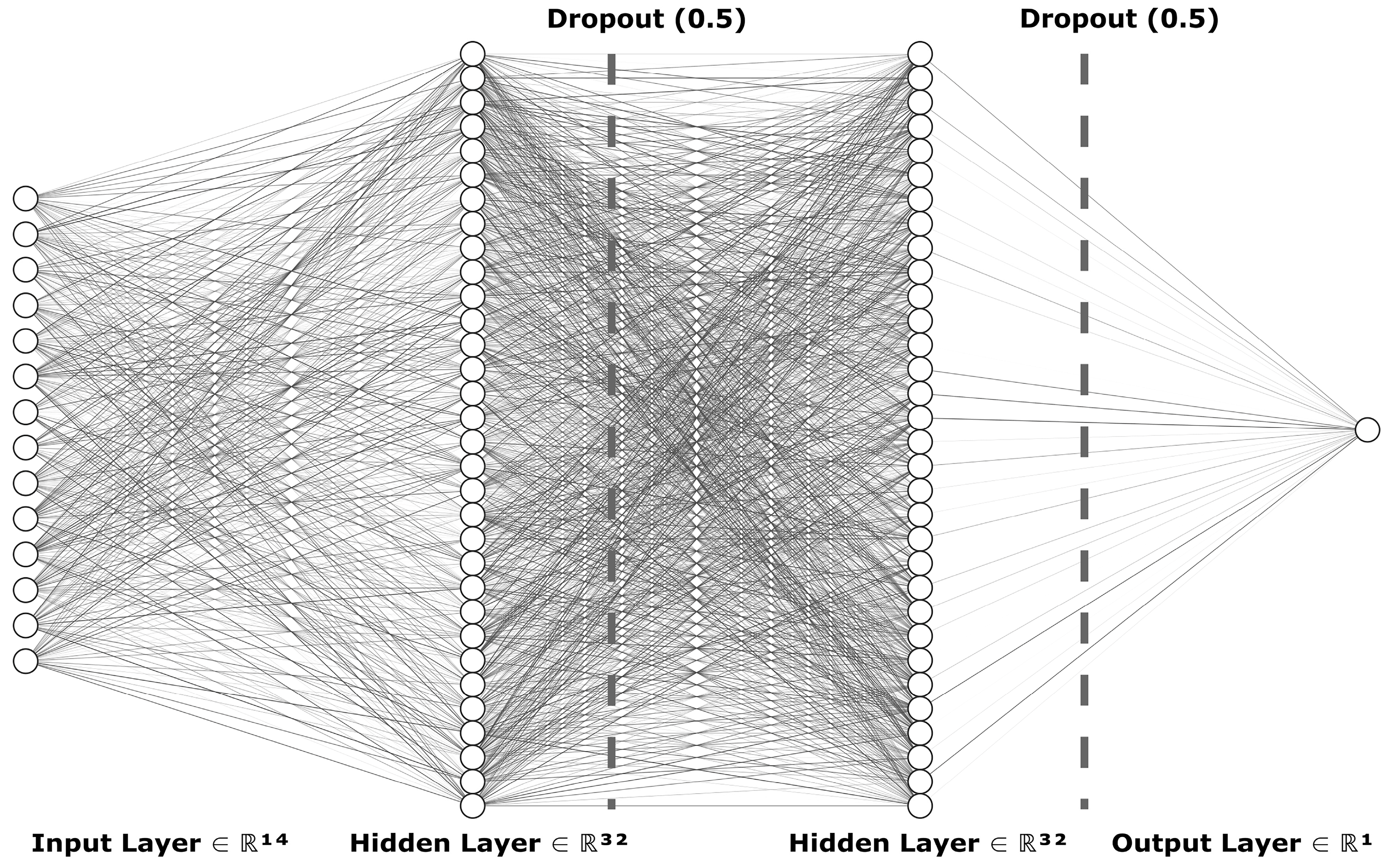
2.2.1. Sequential Model from Keras Library of TensorFlow
2.2.2. Random Forest and Gradient Boosting
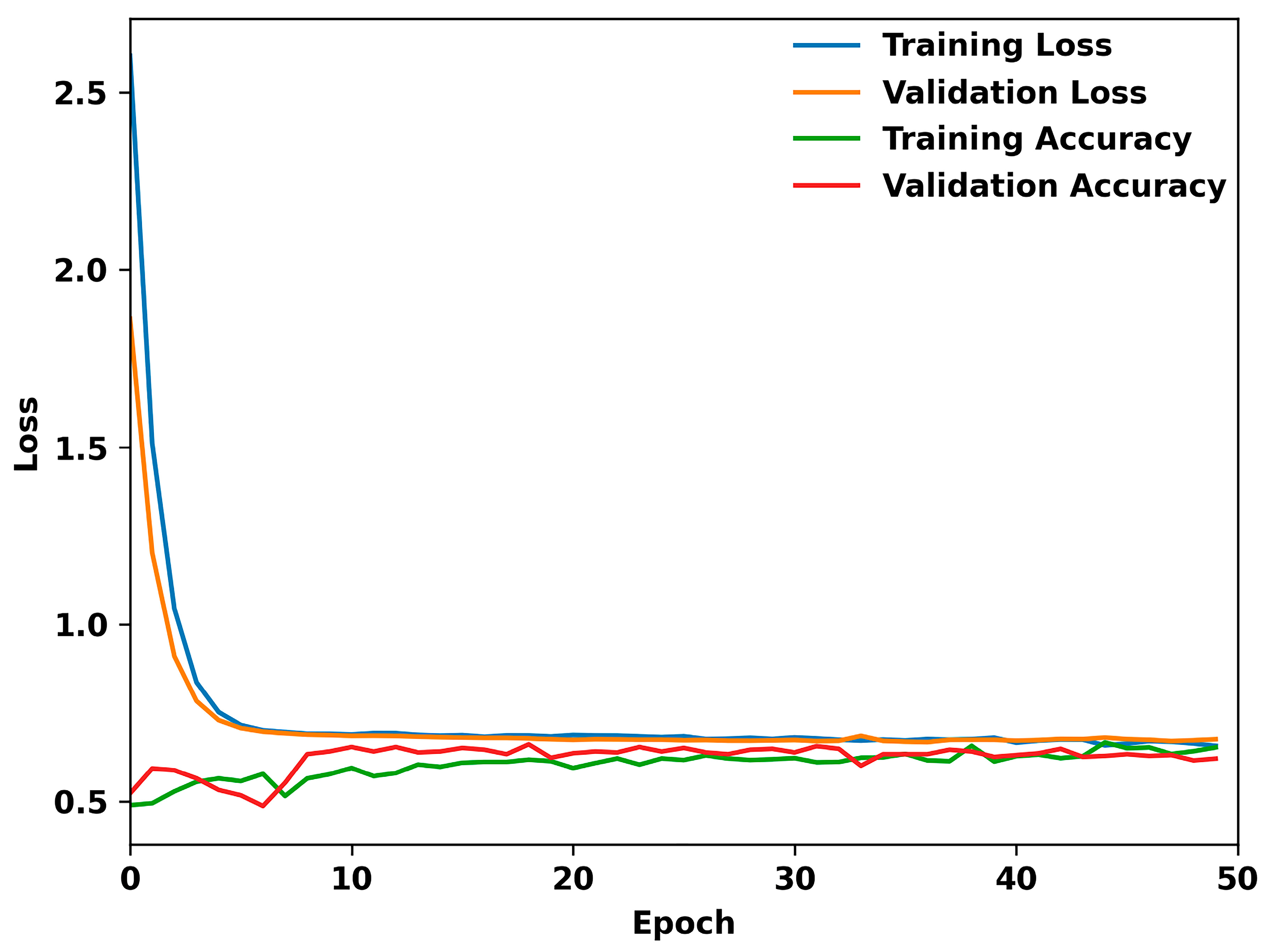
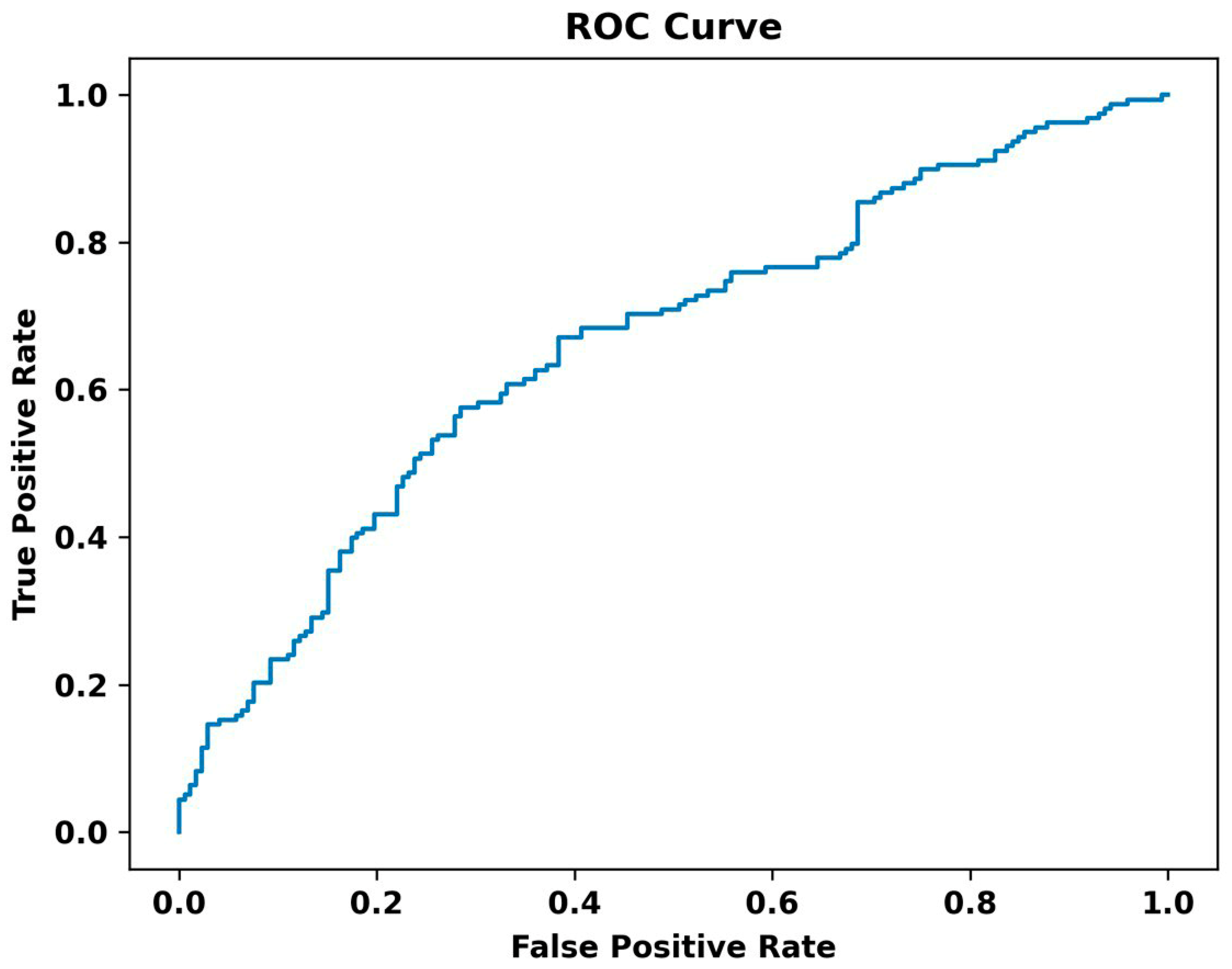
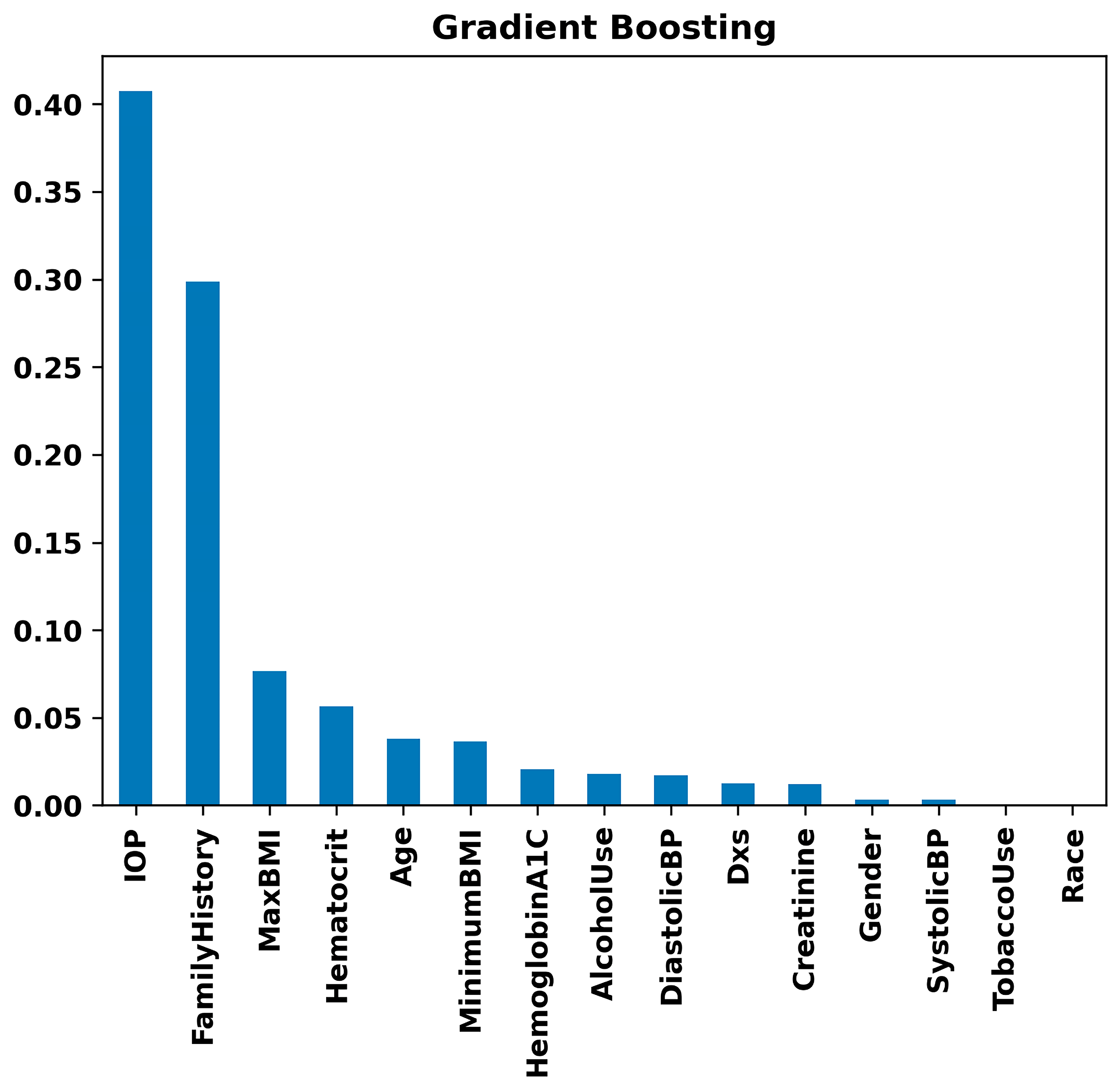
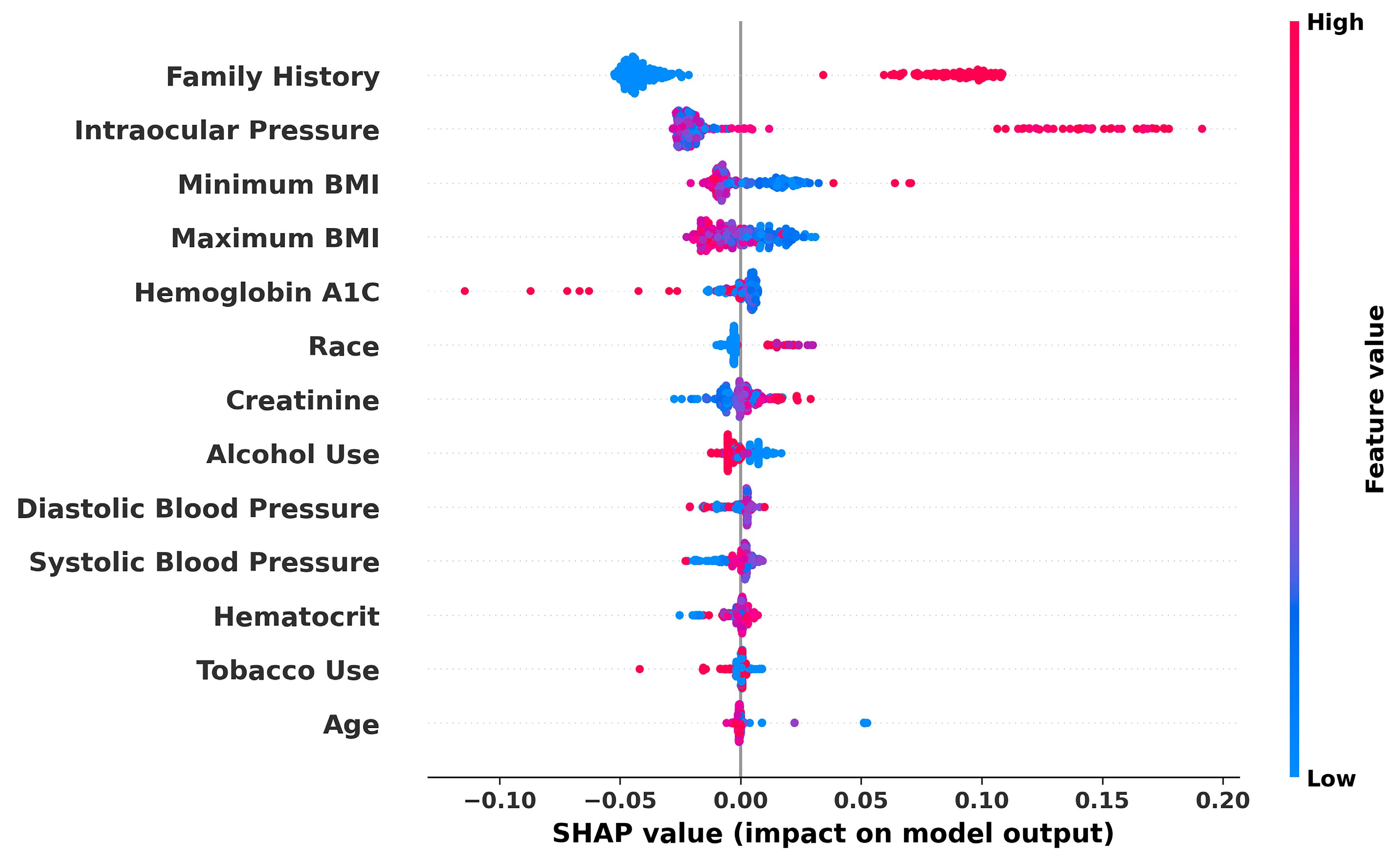
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitazawa, Y.; Horie, T. Diurnal variation of intraocular pressure in primary open-angle glaucoma. Am. J. Ophthalmol. 1975, 79, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Tielsch, J.M.; Katz, J.; Quigley, H.A.; Gottsch, J.D.; Javitt, J.; Singh, K. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch. Ophthalmol. 1991, 109, 1090–1095. [Google Scholar] [CrossRef]
- Van Buskirk, E.M.; Cioffi, G.A. Glaucomatous optic neuropathy. Am. J. Ophthalmol. 1992, 113, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Open-angle glaucoma. N. Engl. J. Med. 1993, 328, 1097–1106. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Fautsch, M.P.; Johnson, D.H.; Group, S.A.P.R.I.W. Aqueous humor outflow: What do we know? Where will it lead us? Investig. Ophthalmol. Vis. Sci. 2006, 47, 4181–4187. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H.; Fingert, J.H.; Kuehn, M.H.; Alward, W.L. Primary open-angle glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef]
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef]
- Stamer, W.D.; Acott, T.S. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 2012, 23, 135–143. [Google Scholar] [CrossRef]
- Casson, R.J.; Chidlow, G.; Wood, J.P.; Crowston, J.G.; Goldberg, I. Definition of glaucoma: Clinical and experimental concepts. Clin. Exp. Ophthalmol. 2012, 40, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Tamm, E. Glaucoma opinion: A unifying concept for trabecular meshwork pathology in primary open angle glaucoma. Int. Glaucoma Rev. 2013, 15, 45–47. [Google Scholar]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Leung, C.K.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary open-angle glaucoma. Nat. Rev. Dis. Primers 2016, 2, 16067. [Google Scholar] [CrossRef] [PubMed]
- Carreon, T.; van der Merwe, E.; Fellman, R.L.; Johnstone, M.; Bhattacharya, S.K. Aqueous outflow—A continuum from trabecular meshwork to episcleral veins. Prog. Retin. Eye Res. 2017, 57, 108–133. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Malihi, M.; Moura Filho, E.R.; Hodge, D.O.; Sit, A.J. Long-term trends in glaucoma-related blindness in Olmsted County, Minnesota. Ophthalmology 2014, 121, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Rylander, N.R.; Vold, S.D. Cost analysis of glaucoma medications. Am. J. Ophthalmol. 2008, 145, 106–113. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, K.J.; Oh, S. Development of machine learning models for diagnosis of glaucoma. PLoS ONE 2017, 12, e0177726. [Google Scholar] [CrossRef]
- Asaoka, R.; Murata, H.; Hirasawa, K.; Fujino, Y.; Matsuura, M.; Miki, A.; Kanamoto, T.; Ikeda, Y.; Mori, K.; Iwase, A.; et al. Using Deep Learning and Transfer Learning to Accurately Diagnose Early-Onset Glaucoma From Macular Optical Coherence Tomography Images. Am. J. Ophthalmol. 2019, 198, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.; Shanmugam, K.P.; Shyu, C.-R. Application of Machine Learning Predictive Models for Early Detection of Glaucoma Using Real World Data. Appl. Sci. 2023, 13, 2445. [Google Scholar] [CrossRef]
- Ozdek, S.C.; Onol, M.; Gürelik, G.; Hasanreisoglu, B. Scanning laser polarimetry in normal subjects and patients with myopia. Br. J. Ophthalmol. 2000, 84, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Tay, E.; Seah, S.K.; Chan, S.P.; Lim, A.T.; Chew, S.J.; Foster, P.J.; Aung, T. Optic disk ovality as an index of tilt and its relationship to myopia and perimetry. Am. J. Ophthalmol. 2005, 139, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Rouland, J.-F.; Berdeaux, G.; Lafuma, A. The economic burden of glaucoma and ocular hypertension: Implications for patient management: A review. Drugs Aging 2005, 22, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Jammal, A.A.; Medeiros, F.A. A Review of Deep Learning for Screening, Diagnosis, and Detection of Glaucoma Progression. Transl. Vis. Sci. Technol. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Burr, J.M.; Mowatt, G.; Hernández, R.; Siddiqui, M.A.; Cook, J.; Lourenco, T.; Ramsay, C.; Vale, L.; Fraser, C.; Azuara-Blanco, A.; et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: A systematic review and economic evaluation. Health Technol. Assess. 2007, 11, iii–iv, ix–x, 1–190. [Google Scholar] [CrossRef] [PubMed]
- Shuldiner, S.R.; Boland, M.V.; Ramulu, P.Y.; De Moraes, C.G.; Elze, T.; Myers, J.; Pasquale, L.; Wellik, S.; Yohannan, J. Predicting eyes at risk for rapid glaucoma progression based on an initial visual field test using machine learning. PLoS ONE 2021, 16, e0249856. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Hong, S. Artificial neural network approach for differentiating open-angle glaucoma from glaucoma suspect without a visual field test. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3957–3966. [Google Scholar]
- Medeiros, F.A.; Lisboa, R.; Weinreb, R.N.; Liebmann, J.M.; Girkin, C.; Zangwill, L.M. Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology 2013, 120, 736–744. [Google Scholar] [CrossRef]
- Lisboa, R.; Leite, M.T.; Zangwill, L.M.; Tafreshi, A.; Weinreb, R.N.; Medeiros, F.A. Diagnosing preperimetric glaucoma with spectral domain optical coherence tomography. Ophthalmology 2012, 119, 2261–2269. [Google Scholar] [CrossRef]
- Na, J.H.; Lee, K.; Lee, J.R.; Baek, S.; Yoo, S.J.; Kook, M.S. Detection of macular ganglion cell loss in preperimetric glaucoma patients with localized retinal nerve fibre defects by spectral-domain optical coherence tomography. Clin. Exp. Ophthalmol. 2013, 41, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Mantravadi, A.V.; Vadhar, N. Glaucoma. Prim. Care Clin. Off. Pract. 2015, 42, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Harasymowycz, P.; Birt, C.; Gooi, P.; Heckler, L.; Hutnik, C.; Jinapriya, D.; Shuba, L.; Yan, D.; Day, R. Medical management of glaucoma in the 21st century from a Canadian perspective. J. Ophthalmol. 2016, 2016, 6509809. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Sommer, A. Risk factors for primary open angle glaucoma. Am. J. Prev. Med. 1988, 4, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Deokule, S.; Weinreb, R.N. Relationships among systemic blood pressure, intraocular pressure, and open-angle glaucoma. Can. J. Ophthalmol. 2008, 43, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Dielemans, I.; Vingerling, J.R.; Algra, D.; Hofman, A.; Grobbee, D.E.; de Jong, P.T. Primary open-angle glaucoma, intraocular pressure, and systemic blood pressure in the general elderly population: The Rotterdam Study. Ophthalmology 1995, 102, 54–60. [Google Scholar] [CrossRef]
- Bonomi, L.; Marchini, G.; Marraffa, M.; Bernardi, P.; Morbio, R.; Varotto, A. Vascular risk factors for primary open angle glaucoma: The Egna-Neumarkt Study. Ophthalmology 2000, 107, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S.L.; Marks, C.; Kuo, T.T.; Ohno-Machado, L.; Weinreb, R.N. Machine Learning-Based Predictive Modeling of Surgical Intervention in Glaucoma Using Systemic Data From Electronic Health Records. Am. J. Ophthalmol. 2019, 208, 30–40. [Google Scholar] [CrossRef]
- Lin, W.C.; Chen, A.; Song, X.; Weiskopf, N.G.; Chiang, M.F.; Hribar, M.R. Prediction of multiclass surgical outcomes in glaucoma using multimodal deep learning based on free-text operative notes and structured EHR data. J. Am. Med. Inform. Assoc. 2024, 31, 456–464. [Google Scholar] [CrossRef]
- Wang, S.Y.; Tseng, B.; Hernandez-Boussard, T. Deep Learning Approaches for Predicting Glaucoma Progression Using Electronic Health Records and Natural Language Processing. Ophthalmol. Sci. 2022, 2, 100127. [Google Scholar] [CrossRef]
- Norouzifard, M.; Nemati, A.; GholamHosseini, H.; Klette, R.; Nouri-Mahdavi, K.; Yousefi, S. Automated glaucoma diagnosis using deep and transfer learning: Proposal of a system for clinical testing. In Proceedings of the 2018 International Conference on Image and Vision Computing New Zealand (IVCNZ), Auckland, New Zealand, 19–21 November 2018; pp. 1–6. [Google Scholar]
- Chai, Y.; Bian, Y.; Liu, H.; Li, J.; Xu, J. Glaucoma diagnosis in the Chinese context: An uncertainty information-centric Bayesian deep learning model. Inf. Process. Manag. 2021, 58, 102454. [Google Scholar] [CrossRef]
- Goldbaum, M.H.; Sample, P.A.; White, H.; Côlt, B.; Raphaelian, P.; Fechtner, R.D.; Weinreb, R.N. Interpretation of automated perimetry for glaucoma by neural network. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3362–3373. [Google Scholar]
- Ahn, J.M.; Kim, S.; Ahn, K.S.; Cho, S.H.; Lee, K.B.; Kim, U.S. A deep learning model for the detection of both advanced and early glaucoma using fundus photography. PLoS ONE 2018, 13, e0207982. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Goldbaum, M.; Yousefi, S. Predicting Glaucoma before Onset Using Deep Learning. Ophthalmol. Glaucoma 2020, 3, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ran, E.A.; Tan, T.F.; Ramachandran, R.; Li, F.; Cheung, C.; Yousefi, S.; Tham, C.C.Y.; Ting, D.S.W.; Zhang, X.; et al. Applications of Artificial Intelligence and Deep Learning in Glaucoma. Asia-Pac. J. Ophthalmol. 2023, 12, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Oguz, C.; Aydin, T.; Yaganoglu, M. A CNN-based hybrid model to detect glaucoma disease. Multimed. Tools Appl. 2024, 83, 17921–17939. [Google Scholar] [CrossRef]
- Chan, K.; Lee, T.W.; Sample, P.A.; Goldbaum, M.H.; Weinreb, R.N.; Sejnowski, T.J. Comparison of machine learning and traditional classifiers in glaucoma diagnosis. IEEE Trans. Biomed. Eng. 2002, 49, 963–974. [Google Scholar] [CrossRef]
- Kucur, Ş.S.; Holló, G.; Sznitman, R. A deep learning approach to automatic detection of early glaucoma from visual fields. PLoS ONE 2018, 13, e0206081. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Kiwaki, T.; Zheng, Y.; Sugiura, H.; Asaoka, R.; Murata, H.; Lemij, H.; Yamanishi, K. Detection of Longitudinal Visual Field Progression in Glaucoma Using Machine Learning. Am. J. Ophthalmol. 2018, 193, 71–79. [Google Scholar] [CrossRef]
- Dixit, A.; Yohannan, J.; Boland, M.V. Assessing Glaucoma Progression Using Machine Learning Trained on Longitudinal Visual Field and Clinical Data. Ophthalmology 2021, 128, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Burgansky-Eliash, Z.; Wollstein, G.; Chu, T.; Ramsey, J.D.; Glymour, C.; Noecker, R.J.; Ishikawa, H.; Schuman, J.S. Optical Coherence Tomography Machine Learning Classifiers for Glaucoma Detection: A Preliminary Study. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4147–4152. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.R.; Vidotti, V.G.; Cremasco, F.; Dias, M.; Gomi, E.S.; Costa, V.P. Sensitivity and specificity of machine learning classifiers for glaucoma diagnosis using Spectral Domain OCT and standard automated perimetry. Arq. Bras. Oftalmol. 2013, 76, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chua, J.; Schwarzhans, F.; Husain, R.; Girard, M.J.A.; Majithia, S.; Tham, Y.-C.; Cheng, C.-Y.; Aung, T.; Fischer, G.; et al. Assessing the external validity of machine learning-based detection of glaucoma. Sci. Rep. 2023, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bradley, C.; Herbert, P.; Hou, K.; Ramulu, P.; Breininger, K.; Unberath, M.; Yohannan, J. Deep learning-based identification of eyes at risk for glaucoma surgery. Sci. Rep. 2024, 14, 599. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Nijkamp, E.; Wu, Y.N. Deep learning with tensorflow: A review. J. Educ. Behav. Stat. 2020, 45, 227–248. [Google Scholar] [CrossRef]
- Jackins, V.; Vimal, S.; Kaliappan, M.; Lee, M.Y. AI-based smart prediction of clinical disease using random forest classifier and Naive Bayes. J. Supercomput. 2021, 77, 5198–5219. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, S.; Kim, S.; Kang, B.B. AI-HydRa: Advanced hybrid approach using random forest and deep learning for malware classification. Inf. Sci. 2021, 546, 420–435. [Google Scholar] [CrossRef]
- Konstantinov, A.V.; Utkin, L.V. Interpretable machine learning with an ensemble of gradient boosting machines. Knowl.-Based Syst. 2021, 222, 106993. [Google Scholar] [CrossRef]
- Bentéjac, C.; Csörgő, A.; Martínez-Muñoz, G. A comparative analysis of gradient boosting algorithms. Artif. Intell. Rev. 2021, 54, 1937–1967. [Google Scholar] [CrossRef]
- Pileggi, C.; Papadopoli, R.; De Sarro, C.; Nobile, C.G.A.; Pavia, M. Obesity, Blood Pressure, and Intraocular Pressure: A Cross-Sectional Study in Italian Children. Obes. Facts 2021, 14, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.-Y.; Mackey, D.A. Glaucoma–risk factors and current challenges in the diagnosis of a leading cause of visual impairment. Maturitas 2022, 163, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Angriani, N.; Akib, M.N.; Mulyadi, F.E.; Irmandha, S.; Hadi, S.; Syamsu, R.F. Glaucoma risk factors. Green Med. J. 2022, 4, 1–8. [Google Scholar] [CrossRef]
- Coster, D.; Rafie, A.; Savion-Gaiger, N.; Rachmiel, R.; Kurtz, S.; Berliner, S.; Shapira, I.; Zeltser, D.; Rogowski, O.; Shenhar-Tsarfaty, S.; et al. The effect of body mass index reduction on intraocular pressure in a large prospective cohort of apparently healthy individuals in Israel. PLoS ONE 2023, 18, e0285759. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.; Stümpfig, D.; Schötzau, A.; Ackermann-Liebrich, U.; Flammer, J. Body mass index in glaucoma. J. Glaucoma 1999, 8, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ling, J.; Chen, Y.; Wu, Y.; Lu, P. The Association between Adiposity and the Risk of Glaucoma: A Meta-Analysis. J. Ophthalmol. 2017, 2017, 9787450. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Pasquale, L.R.; Singh, K.; Lin, S.C. The Association Between Body Mass Index and Open-angle Glaucoma in a South Korean Population-based Sample. J. Glaucoma 2018, 27, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Mowbray, F.; Zargoush, M.; Jones, A.; de Wit, K.; Costa, A. Predicting hospital admission for older emergency department patients: Insights from machine learning. Int. J. Med. Inform. 2020, 140, 104163. [Google Scholar] [CrossRef] [PubMed]
- McMonnies, C.W. Glaucoma history and risk factors. J. Optom. 2017, 10, 71–78. [Google Scholar] [CrossRef]
- Ryskulova, A.; Turczyn, K.; Makuc, D.M.; Cotch, M.F.; Klein, R.J.; Janiszewski, R. Self-reported age-related eye diseases and visual impairment in the United States: Results of the 2002 national health interview survey. Am. J. Public Health 2008, 98, 454–461. [Google Scholar] [CrossRef]
- Varma, R.; Ying-Lai, M.; Francis, B.A.; Nguyen, B.B.; Deneen, J.; Wilson, M.R.; Azen, S.P. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: The Los Angeles Latino Eye Study. Ophthalmology 2004, 111, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D. Prevalence of Open-Angle Glaucoma Among Adults in the United States. Arch. Ophthalmol. 2004, 122, 532–538, Erratum in Arch. Ophthalmol. 2011, 129, 1224. [Google Scholar]
- Khachatryan, N.; Pistilli, M.; Maguire, M.G.; Salowe, R.J.; Fertig, R.M.; Moore, T.; Gudiseva, H.V.; Chavali, V.R.; Collins, D.W.; Daniel, E. Primary open-angle African American glaucoma genetics (POAAGG) study: Gender and risk of POAG in African Americans. PLoS ONE 2019, 14, e0218804. [Google Scholar] [CrossRef] [PubMed]
- Charlson, E.S.; Sankar, P.S.; Miller-Ellis, E.; Regina, M.; Fertig, R.; Salinas, J.; Pistilli, M.; Salowe, R.J.; Rhodes, A.L.; Merritt III, W.T. The primary open-angle african american glaucoma genetics study: Baseline demographics. Ophthalmology 2015, 122, 711–720. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Salowe, R.J.; Fertig, R.; Salinas, J.; Pistilli, M.; Sankar, P.S.; Miller-Ellis, E.; Lehman, A.; Murphy, W.H.; Homsher, M. Family history in the primary open-angle African American glaucoma genetics study cohort. Am. J. Ophthalmol. 2018, 192, 239–247. [Google Scholar] [CrossRef]
- Collins, D.W.; Gudiseva, H.V.; Chavali, V.R.; Trachtman, B.; Ramakrishnan, M.; Merritt, W.T., III; Pistilli, M.; Rossi, R.A.; Blachon, S.; Sankar, P.S. The MT-CO1 V83I polymorphism is a risk factor for primary open-angle glaucoma in African American men. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, W.; Ryu, I.H.; Kim, J.K.; Kim, H.; Yoo, T.K. Association between metformin use and the risk of developing open-angle glaucoma among patients with diabetes: A retrospective cohort study and meta-analysis. Int. Ophthalmol. 2024, 44, 6. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K. Actions are needed to develop artificial intelligence for glaucoma diagnosis and treatment. J. Med. Artif. Intell. 2023, 6, 3. [Google Scholar] [CrossRef]
- Mehta, P.; Petersen, C.A.; Wen, J.C.; Banitt, M.R.; Chen, P.P.; Bojikian, K.D.; Egan, C.; Lee, S.-I.; Balazinska, M.; Lee, A.Y.; et al. Automated Detection of Glaucoma With Interpretable Machine Learning Using Clinical Data and Multimodal Retinal Images. Am. J. Ophthalmol. 2021, 231, 154–169. [Google Scholar] [CrossRef]

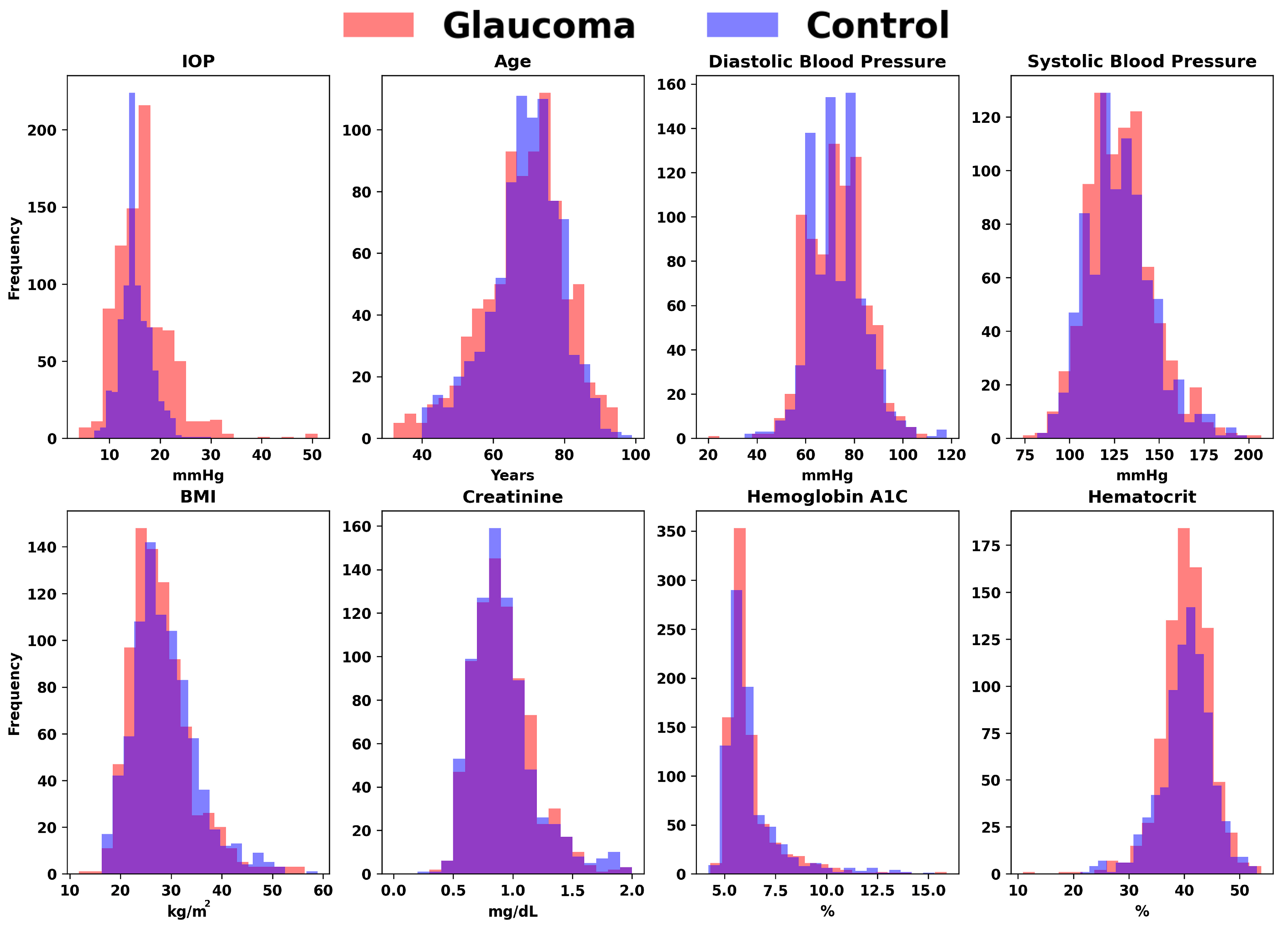




| Parameters | Control | Glaucoma |
|---|---|---|
| IOP (mm Hg) | 15.18 | 16.68 |
| Age (year-old) | 69.25 | 68.79 |
| Diastolic blood pressure (mm Hg) | 73.2 | 72.8 |
| Systolic blood pressure (mm Hg) | 128.2 | 128.49 |
| Body mass index (kg/m2) | 28.69 | 27.89 |
| Creatinine (mg/dL) | 0.99 | 1.04 |
| Hemoglobin A1C (%) | 6.27 | 6.21 |
| Hematocrit (%) | 40.28 | 40.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi, A.; Stanik, A.; Kozitza, C.; Chen, A. Integrating Deep Learning with Electronic Health Records for Early Glaucoma Detection: A Multi-Dimensional Machine Learning Approach. Bioengineering 2024, 11, 577. https://doi.org/10.3390/bioengineering11060577
Karimi A, Stanik A, Kozitza C, Chen A. Integrating Deep Learning with Electronic Health Records for Early Glaucoma Detection: A Multi-Dimensional Machine Learning Approach. Bioengineering. 2024; 11(6):577. https://doi.org/10.3390/bioengineering11060577
Chicago/Turabian StyleKarimi, Alireza, Ansel Stanik, Cooper Kozitza, and Aiyin Chen. 2024. "Integrating Deep Learning with Electronic Health Records for Early Glaucoma Detection: A Multi-Dimensional Machine Learning Approach" Bioengineering 11, no. 6: 577. https://doi.org/10.3390/bioengineering11060577
APA StyleKarimi, A., Stanik, A., Kozitza, C., & Chen, A. (2024). Integrating Deep Learning with Electronic Health Records for Early Glaucoma Detection: A Multi-Dimensional Machine Learning Approach. Bioengineering, 11(6), 577. https://doi.org/10.3390/bioengineering11060577







