Simplified Models to Assess the Mechanical Performance Parameters of Stents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mechanical Performance Parameters of Stents
- Percent surface area: The area over which a stent contacts a vessel may affect the biological response of the vessel. The amount of open, non-contact area may influence tissue prolapse or ingrowth. The percentage of area is calculated as 100·(area in contact with vessel/full cylindrical surface area).
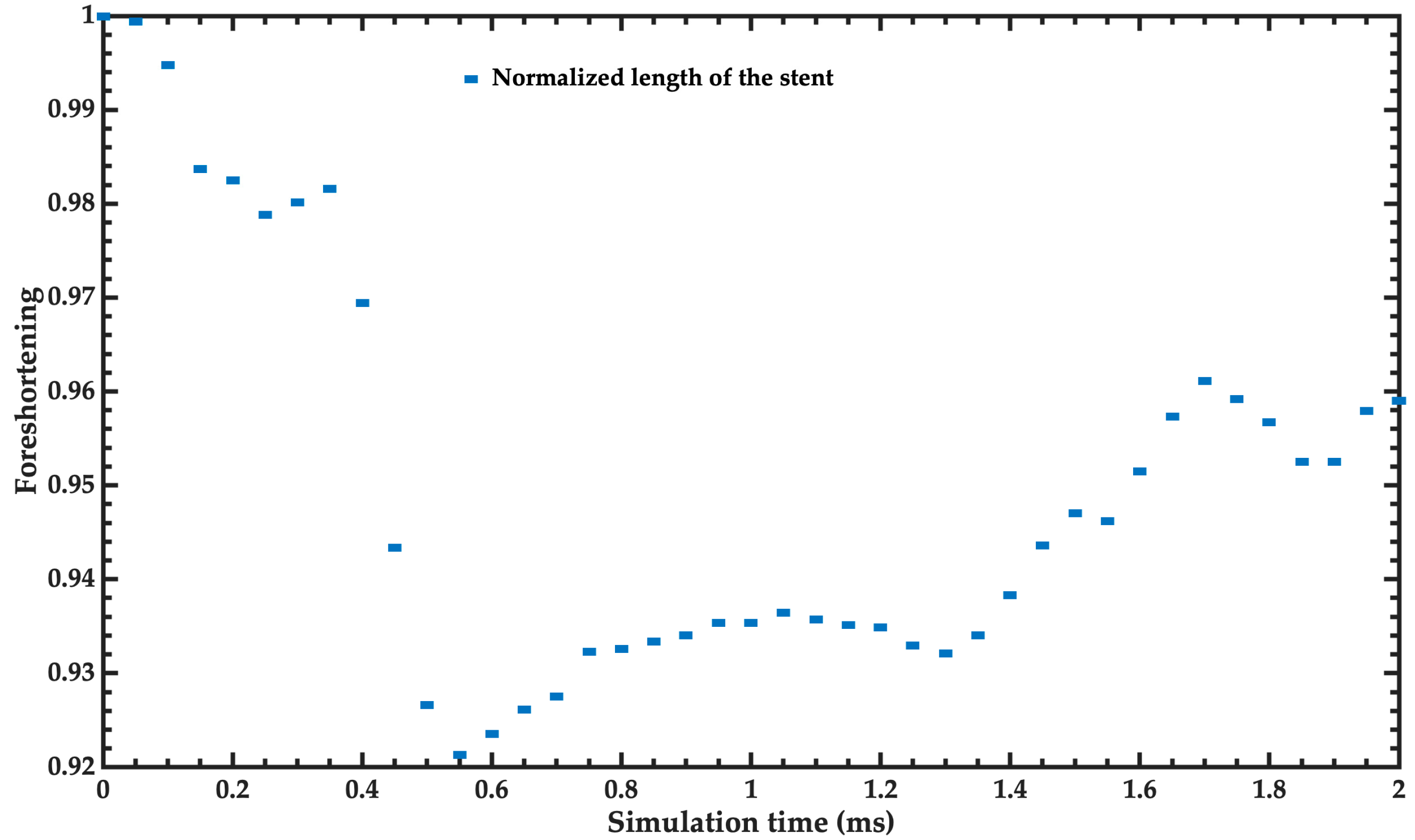
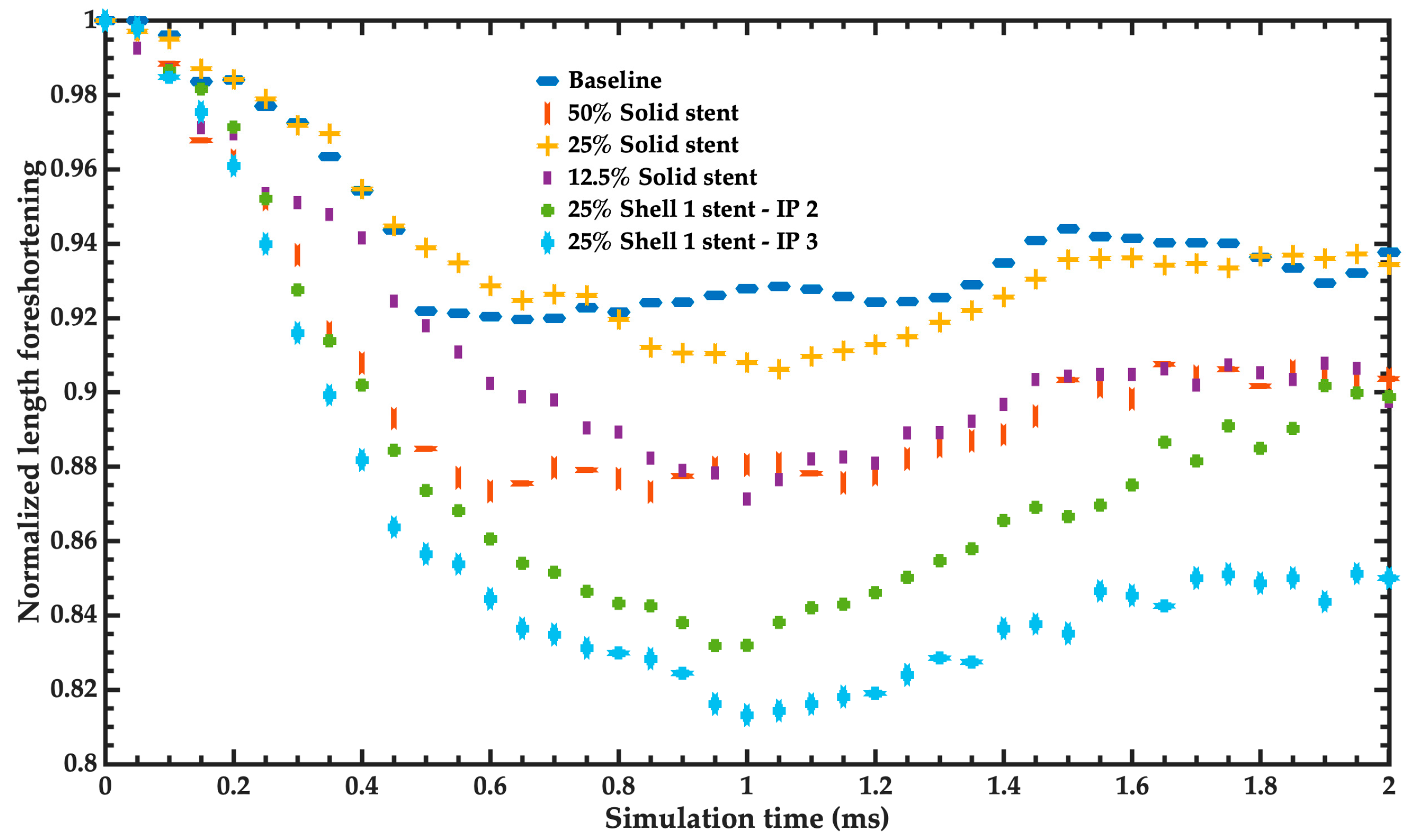
- Foreshortening: Dimensional changes may occur when deploying a stent, influencing the final length. Knowledge of the foreshortening characteristics aids in proper stent length selection and proper placement in the body. Percent foreshortening is determined as 100·(change in length/loaded length).
- Recoil for balloon expandable stents: The recoil behavior of balloon expandable stents influences proper device selection, sizing, acute post-implant results, and long-term clinical outcomes. Recoil is a function of stent design and material selection. Therefore, knowledge of stent recoil helps to characterize the behavior of a particular stent design. Recoil is calculated as the diameter of the expanded balloon/diameter of the deflated balloon.
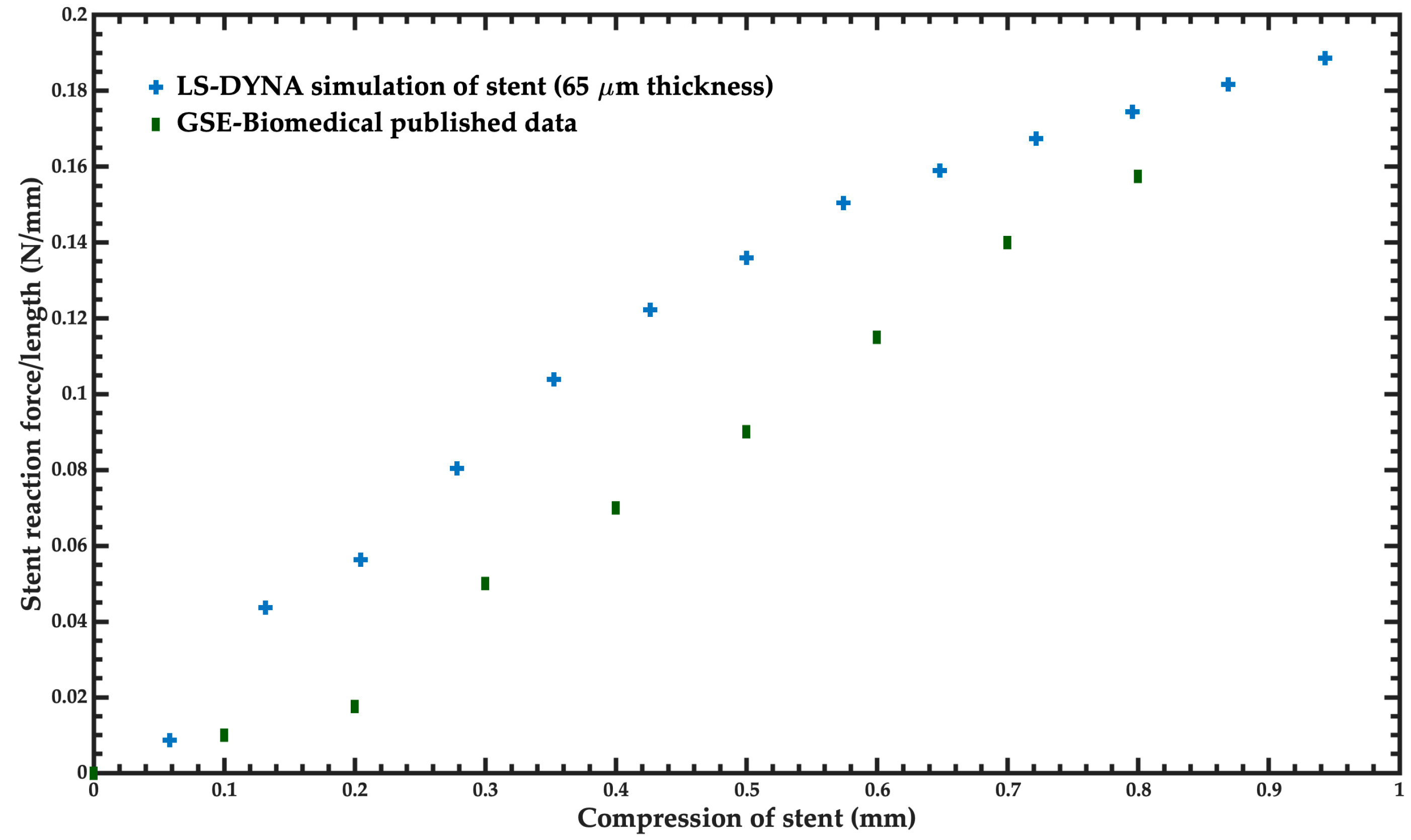
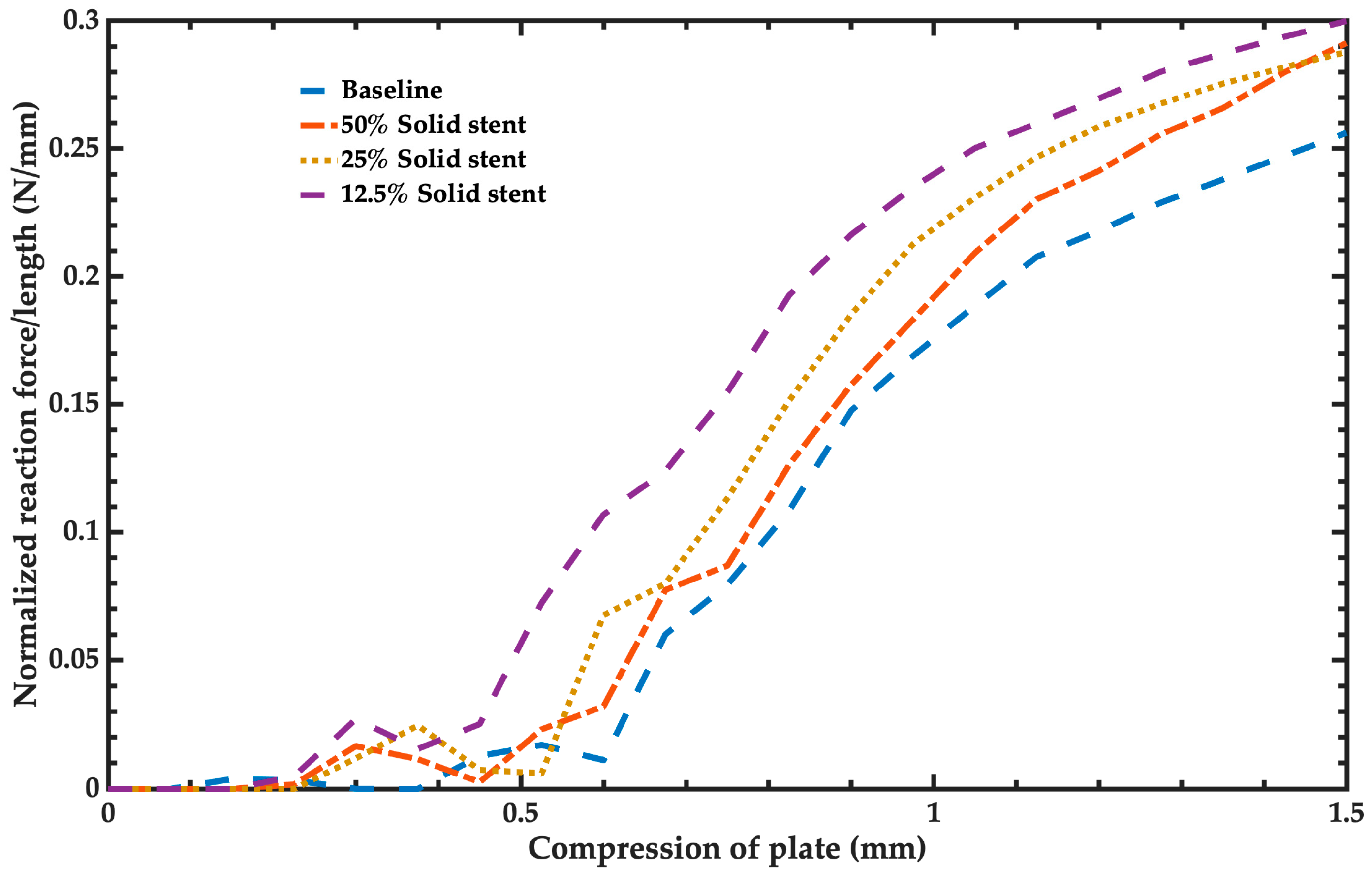
- Radial stiffness and radial strength: Radial stiffness and stent recoil determine the diameter of balloon expandable stents deployed in compliant vessels. Radial stiffness and radial strength characterize the stent’s ability to resist collapse under short-term or long-term external loads. The radial stiffness of the stent is calculated as the force required to compress a length normalized stent/amount of compression. The units of the radial stiffness are N/mm. This radial strength is defined as the force that generates a plastic deformation on the expanded stent. It is computed when the radial stiffness slope decreases. The plastic deformation of the stent began when its radial strength was achieved.
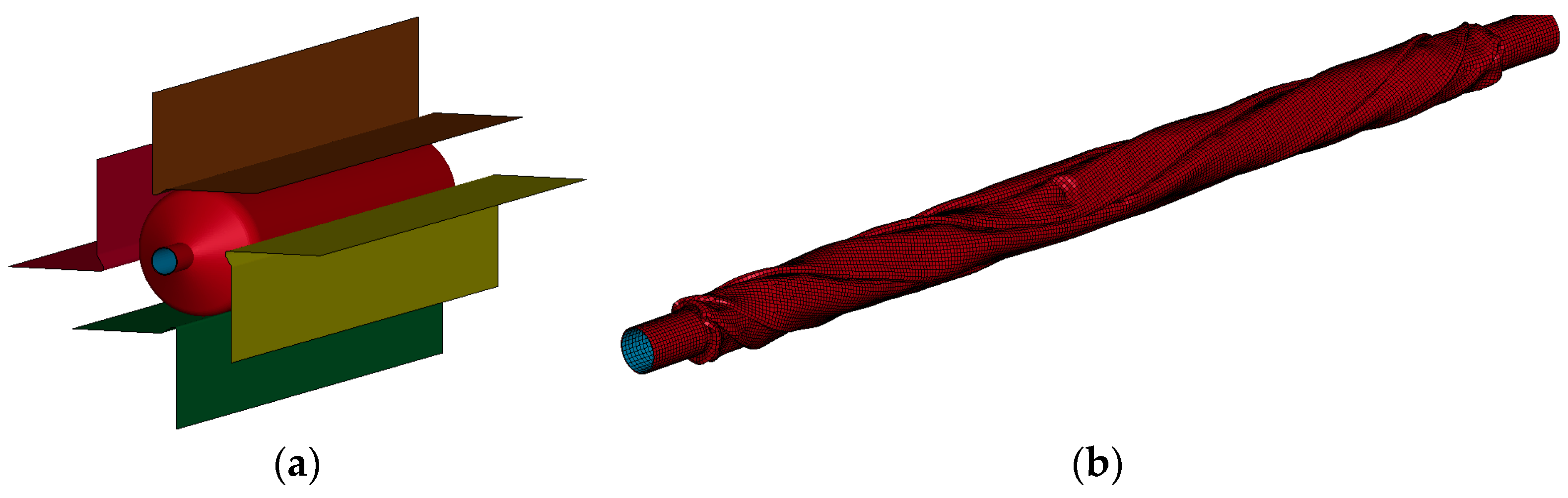
2.2. Geometry and FEM for Baseline Stent and Balloon Model
2.3. Materials for Balloon and Stent
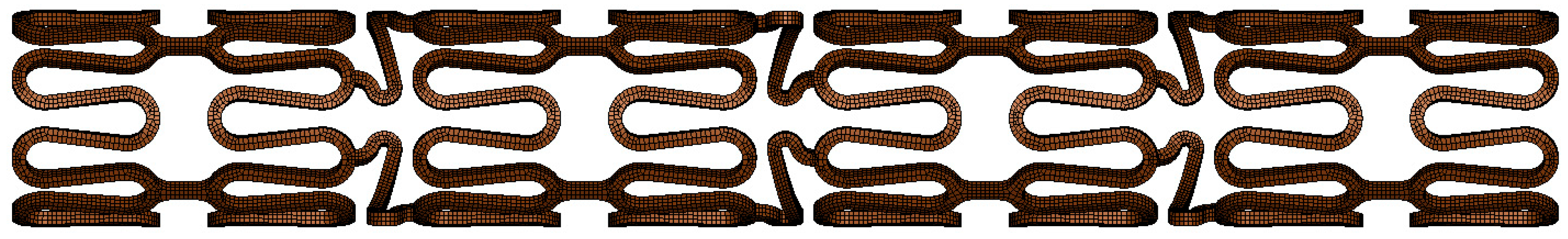
2.4. Geometry and FEM Model of Simplified Stent
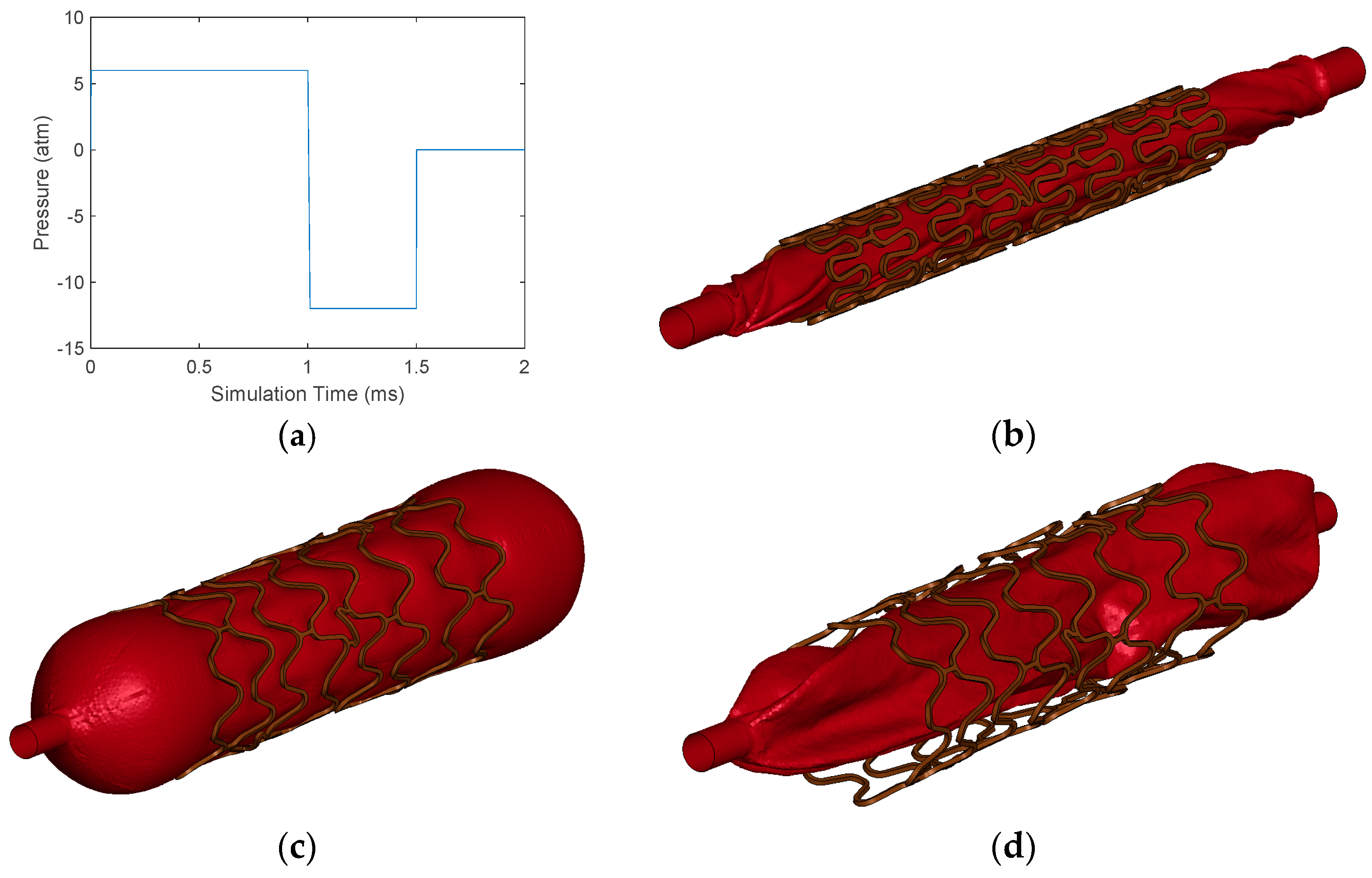
2.5. Simulations for Mechanical Performance Parameters of Stent
3. Results and Discussion
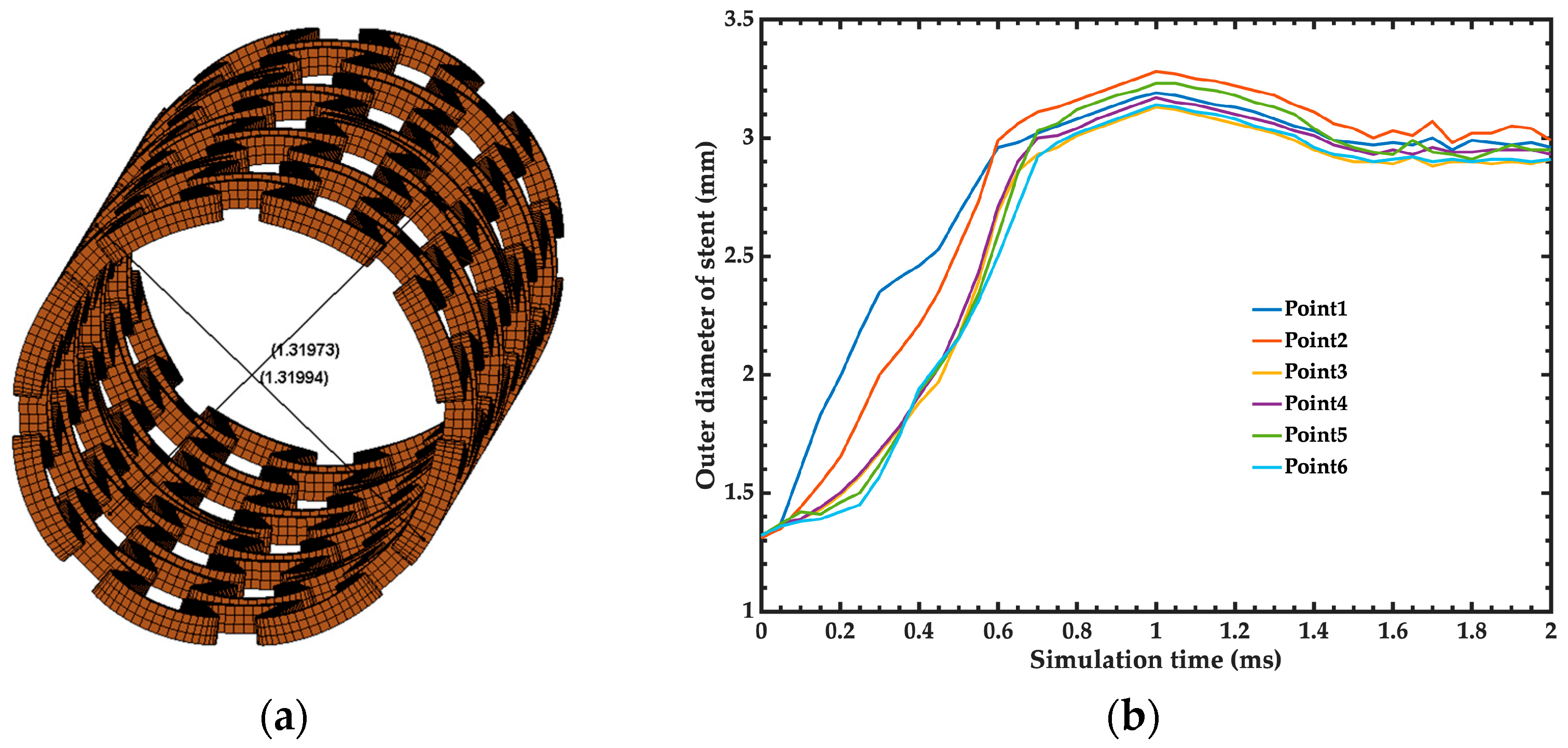
3.1. Baseline Simulation
3.2. Mechanical Performance Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization, “The Top 10 Causes of Death”. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 3 April 2024).
- Ahmad, F.B.; Cisewski, J.A.; Xu, J.; Anderson, R.N. Provisional mortality data—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Petzold, M.; Cao, J.; Zhang, Y.; Wang, W. Direct medical costs of hospitalizations for cardiovascular diseases in Shanghai, China: Trends and projections. Medicine 2015, 94, e837. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global epidemiology of ischemic heart disease: Results from the global burden of disease study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Hwang, K.-C.; Lee, K.-Y.; Kim, Y.-H. Ischemic heart diseases: Current treatments and future. J. Control Release 2009, 140, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Etave, F.; Finet, G.; Boivin, M.; Boyer, J.C.; Rioufol, G.; Thollet, G. Mechanical properties of coronary stents determined by using finite element analysis. J. Biomech. 2001, 34, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.K.; Yang, D.Z.; Qi, M.; Wang, W.Q. Finite element analysis of the implantation of a balloon-expandable stent in a stenosed artery. Int. J. Cardiol. 2005, 104, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Lally, C.; Dolan, F.; Prendergast, P.J. Cardiovascular stent design and vessel stresses: A finite element analysis. J. Biomech. 2005, 38, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Q.; Liang, D.-K.; Yang, D.-Z.; Qi, M. Analysis of the transient expansion behavior and design optimization of coronary stents by finite element method. J. Biomech. 2006, 39, 21–32. [Google Scholar] [CrossRef]
- De Beule, M.; Mortier, P.; Carlier, S.G.; Verhegghe, B.; Van Impe, R.; Verdonck, P. Realistic finite element-based stent design: The impact of balloon folding. J. Biomech. 2008, 41, 383–389. [Google Scholar] [CrossRef]
- Zahedmanesh, H.; John Kelly, D.; Lally, C. Simulation of a balloon expandable stent in a realistic coronary artery-determination of the optimum modelling strategy. J. Biomech. 2010, 43, 2126–2132. [Google Scholar] [CrossRef]
- Pant, S.; Limbert, G.; Curzen, N.P.; Bressloff, N.W. Multiobjective design optimisation of coronary stents. Biomaterials 2011, 32, 7755–7773. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems; Guidance for Industry and FDA Staff; Food and Drug Admnistration: Silver Spring, MD, USA, 2010. [Google Scholar]
- Gundert, T.J.; Marsden, A.L.; Yang, W.; LaDisa, J.F. Optimization of cardiovascular stent design using computational fluid dynamics. J. Biomech. Eng. 2012, 134, 011002. [Google Scholar] [CrossRef] [PubMed]
- Bressloff, N.W.; Ragkousis, G.; Curzen, N. Design optimisation of coronary artery stent systems. Ann. Biomed. Eng. 2016, 44, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Ragkousis, G.E.; Curzen, N.; Bressloff, N.W. Multi-objective optimisation of stent dilation strategy in a patient-specific coronary artery via computational and surrogate modelling. J. Biomech. 2016, 49, 205–215. [Google Scholar] [CrossRef]
- Tammareddi, S.; Sun, G.; Li, Q. Multiobjective robust optimization of coronary stents. Mater. Des. 2016, 90, 682–692. [Google Scholar] [CrossRef]
- Li, H.; Liu, T.; Wang, M.; Zhao, D.; Qiao, A.; Wang, X.; Gu, J.; Li, Z.; Zhu, B. Design optimization of stent and its dilatation balloon using Kriging surrogate model. Biomed. Eng. Online 2017, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Geith, M.A.; Swidergal, K.; Hochholdinger, B.; Schratzenstaller, T.G.; Wagner, M.; Holzapfel, G.A. On the importance of modeling balloon folding, pleating, and stent crimping: An FE study comparing experimental inflation tests. Int. J. Numer. Method Biomed. Eng. 2019, 35, e3249. [Google Scholar] [CrossRef]
- Gharleghi, R.; Wright, H.; Luvio, V.; Jepson, N.; Luo, Z.; Senthurnathan, A.; Babaei, B.; Prusty, B.G.; Ray, T.; Beier, S. A multi-objective optimization of stent geometries. J. Biomech. 2021, 125, 110575. [Google Scholar] [CrossRef]
- GSE Biomedical. Available online: https://www.gse-biomedical.com (accessed on 4 April 2024).
- Oberhofer, G.; Gese, H.; Groß, M.; Kühling, M. Numerical analysis of the balloon dilatation process using the explicit finite element method for the optimization of a stent. In Proceedings of the LS-DYNA Anwenderforum, Ulm, Germany, 7–9 October 2006; Volume 35, pp. 35–46. [Google Scholar]
- Terumo.com. Accuforce—Brochure ENG Latam. Available online: https://www.terumo-radialedge.com/es/product/accuforce/pdf01.pdf (accessed on 15 May 2024).
- Gervaso, F.; Capelli, C.; Petrini, L.; Lattanzio, S.; Di Virgilio, L.; Migliavacca, F. On the effects of different strategies in modelling balloon-expandable stenting by means of finite element method. J. Biomech. 2008, 41, 1206–1212. [Google Scholar] [CrossRef]
- Pant, S.; Bressloff, N.W.; Limbert, G. Geometry parameterization and multidisciplinary constrained optimization of coronary stents. Biomech. Model Mechanobiol. 2012, 11, 61–82. [Google Scholar] [CrossRef]
- Ragkousis, G.E.; Curzen, N.; Bressloff, N.W. Simulation of longitudinal stent deformation in a patient-specific coronary artery. Med. Eng. Phys. 2014, 36, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Abundes-Velasco, A.; Jiménez-Rodríguez, G.M.; Romero-Ibarra, J.L.; Sandoval-Jones, J.P.; Sánchez-Pérez, E.; Galaz-Méndez, R.; Ulacia-Flores, P.; Farjat-Pasos, J.I.; Padilla-Ibarra, J.; González, S.A.-D.; et al. Design and manufacture of a coronary stent INC-1 and initial tests in experimental animal model. Arch. Cardiol. Mex. 2020, 90, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Galaz-Méndez, R. Cardiovascular Stent Device Optimization | Complx. Available online: https://www.complx.com.mx/Reporte_tecnico_en_extenso_GALAZ_Stent.pdf (accessed on 22 April 2024).
- Martin, D.; Boyle, F. Sequential structural and fluid dynamics analysis of balloon-expandable coronary stents: A multivariable statistical analysis. Cardiovasc. Eng. Technol. 2015, 6, 314–328. [Google Scholar] [CrossRef] [PubMed]






| Point | Strain | Stress (MPa) |
|---|---|---|
| 1 | 6.6730 × 10−3 | 1117 |
| 2 | 7.6940 × 10−3 | 1178 |
| 3 | 8.7150 × 10−3 | 1235 |
| 4 | 1.0256 × 10−2 | 1293 |
| 5 | 1.2835 × 10−2 | 1345 |
| 6 | 1.6444 × 10−2 | 1389 |
| 7 | 2.0562 × 10−2 | 1416 |
| 8 | 2.5945 × 10−2 | 1442 |
| 9 | 3.0791 × 10−2 | 1461 |
| 10 | 3.5871 × 10−2 | 1472 |
| 11 | 4.2940 × 10−2 | 1486 |
| 12 | 4.9211 × 10−2 | 1496 |
| 13 | 1.6077 × 10−1 | 1677 |
| Simulation Models | Recoil Index | Recoil |
|---|---|---|
| Baseline | 2.00 | 1.09 |
| 12.5% Solid stent | 2.00 | 1.11 |
| 25% Sell 1 stent-IP3 | 2.02 | 1.17 |
| 25% Shell 1 stent-IP5 | 2.03 | 1.16 |
| 25% Shell 2 stent-IP3 | 2.05 | 1.14 |
| 25% Shell 2 stent-IP5 | 2.06 | 1.12 |
| 25% Solid stent | 1.93 | 1.11 |
| 25% Shell 1 stent-IP2 | 2.09 | 1.10 |
| 25% Shell 2 stent-IP2 | 2.10 | 1.10 |
| 50% solid stent | 1.89 | 1.23 |
| 25% Shell 16 stent-IP5 | 2.22 | 1.10 |
| 25% Shell 16 stent-IP7 | 2.23 | 1.06 |
| 25% Shell 16 stent-IP3 | 2.23 | 1.07 |
| 25% Shell 16 stent-IP6 | 2.24 | 1.06 |
| Model | Simulation Time (min) | Time Reduction (%) | Radial Stiffness Error @ 0.75 mm Compression | Recoil Index Error | Recoil Error | Foreshortening Error Difference |
|---|---|---|---|---|---|---|
| Baseline | 835.25 | - | - | - | - | - |
| 50% Solid | 280.03 | 66.5% | 11.70% | 5.5% | 12.84% | 3.40% |
| 25% Solid | 234.13 | 72.0% | 16.46% | 3.5% | 1.83% | 3.06% |
| 12.5% Solid | 216.70 | 74.1% | 21.71% | 0.0% | 1.83% | 3.67% |
| 25% Shell 2-IP 5 | 187.67 | 77.4% | 100.00% | 3.0% | 2.75% | 2.80% |
| 25% Shell 1-IP 5 | 192.05 | 77.0% | 100.00% | 1.5% | 6.42% | 1.87% |
| 25% Shell 1-IP 2 | 189.20 | 77.6% | 100.00% | 4.5% | 0.92% | 6.78% |
| 25% Shell 2-IP 2 | 185.17 | 77.5% | 100.00% | 5.0% | 0.92% | 6.71% |
| 25% Shell 1-IP 3 | 188.92 | 77.3% | 100.00% | 1.0% | 7.34% | 4.76% |
| 25% Shell 2-IP 3 | 187.00 | 77.8% | 100.00% | 2.5% | 4.59% | 6.56% |
| 25% Shell 16-IP 7 | 151.60 | 82.4% | 100.00% | 11.5% | 2.75% | 1.47% |
| 25% Shell 16-IP 3 | 144.68 | 81.8% | 100.00% | 11.5% | 1.83% | 5.10% |
| 25% Shell 16-IP 5 | 146.93 | 82.7% | 100.00% | 11.0% | 0.92% | 10.40% |
| 25% Shell 16-IP 6 | 149.38 | 82.1% | 100.00% | 12.0% | 2.75% | 8.24% |
| Reference | Number of Computer Cores | Computer Time per Point Evaluation (h) | Software |
|---|---|---|---|
| Our work | 14 | 3.6 | LS-DYNA Explicit (R11.1.0) |
| [19] | 32 | 33.5 | LS-DYNA Implicit (R10.1.0) |
| [16] | 32 | 160 | Abaqus Explicit (V 6.12) |
| [17] | 8 | 26 | Abaqus Explicit (V 6.9.2) |
| [29] | 6 | 24 | Abaqus Explicit (V 6.9.1) |
| [12] | 8 | >24 | Abaqus Explicit (V 6.9.1) |
| [24] | 4 | 120 | Abaqus Explicit (V 6.9.1) |
| [10] | * | 48 | Abaqus Explicit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo, J.P.; Martínez-Castillo, J.; Cardenas, D.; Delgado-Alvarado, E.; Vigueras-Zuñiga, M.O.; Herrera-May, A.L. Simplified Models to Assess the Mechanical Performance Parameters of Stents. Bioengineering 2024, 11, 583. https://doi.org/10.3390/bioengineering11060583
Toledo JP, Martínez-Castillo J, Cardenas D, Delgado-Alvarado E, Vigueras-Zuñiga MO, Herrera-May AL. Simplified Models to Assess the Mechanical Performance Parameters of Stents. Bioengineering. 2024; 11(6):583. https://doi.org/10.3390/bioengineering11060583
Chicago/Turabian StyleToledo, Juan P., Jaime Martínez-Castillo, Diego Cardenas, Enrique Delgado-Alvarado, Marco Osvaldo Vigueras-Zuñiga, and Agustín L. Herrera-May. 2024. "Simplified Models to Assess the Mechanical Performance Parameters of Stents" Bioengineering 11, no. 6: 583. https://doi.org/10.3390/bioengineering11060583








