Bladder Reconstruction in Cats Using In-Body Tissue Architecture (iBTA)-Induced Biosheet
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
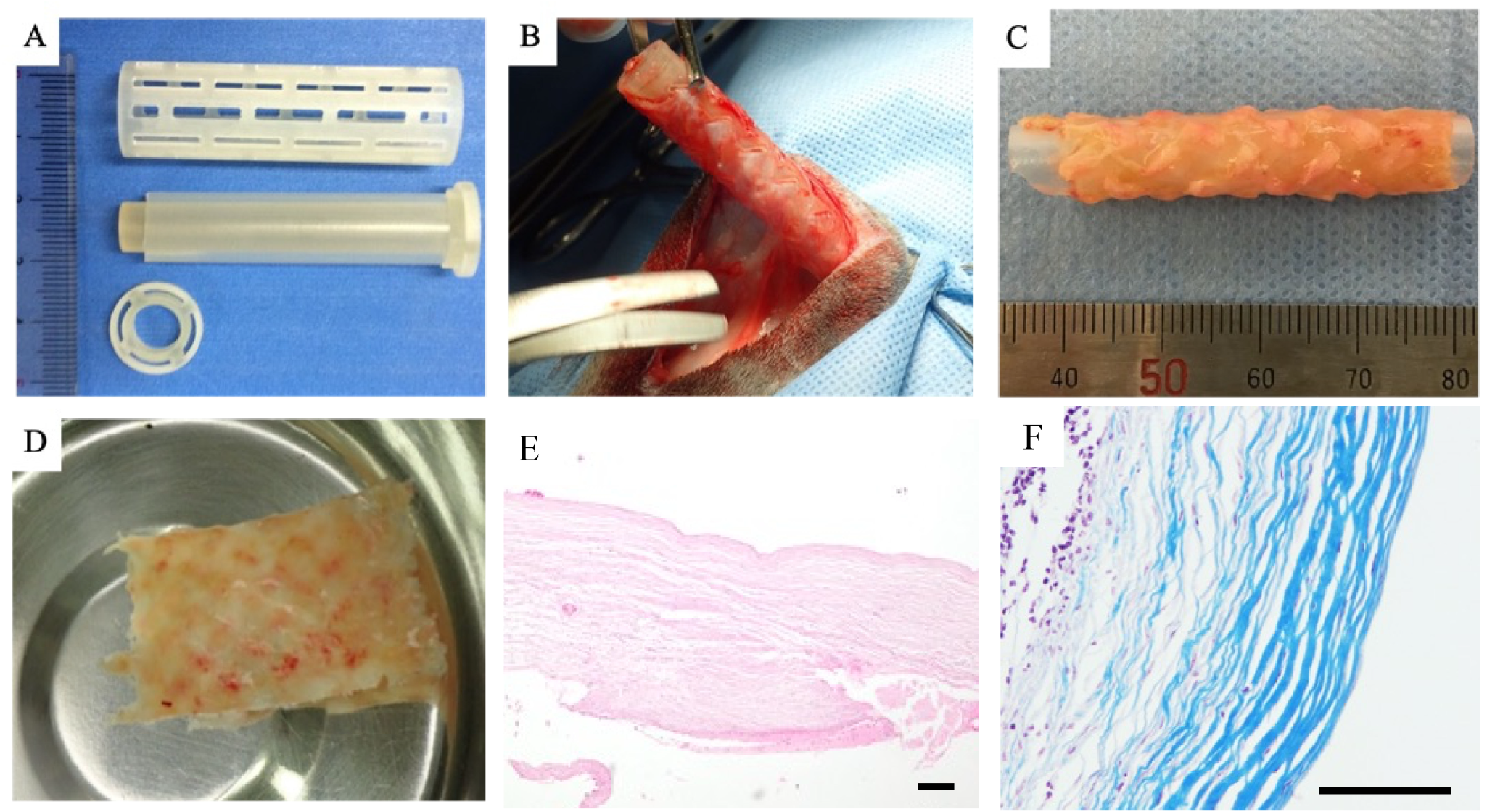
2.2. Mold Preparation for Graft Fabrication
2.3. Embedding of Mold and Graft (Biosheet) Fabrication
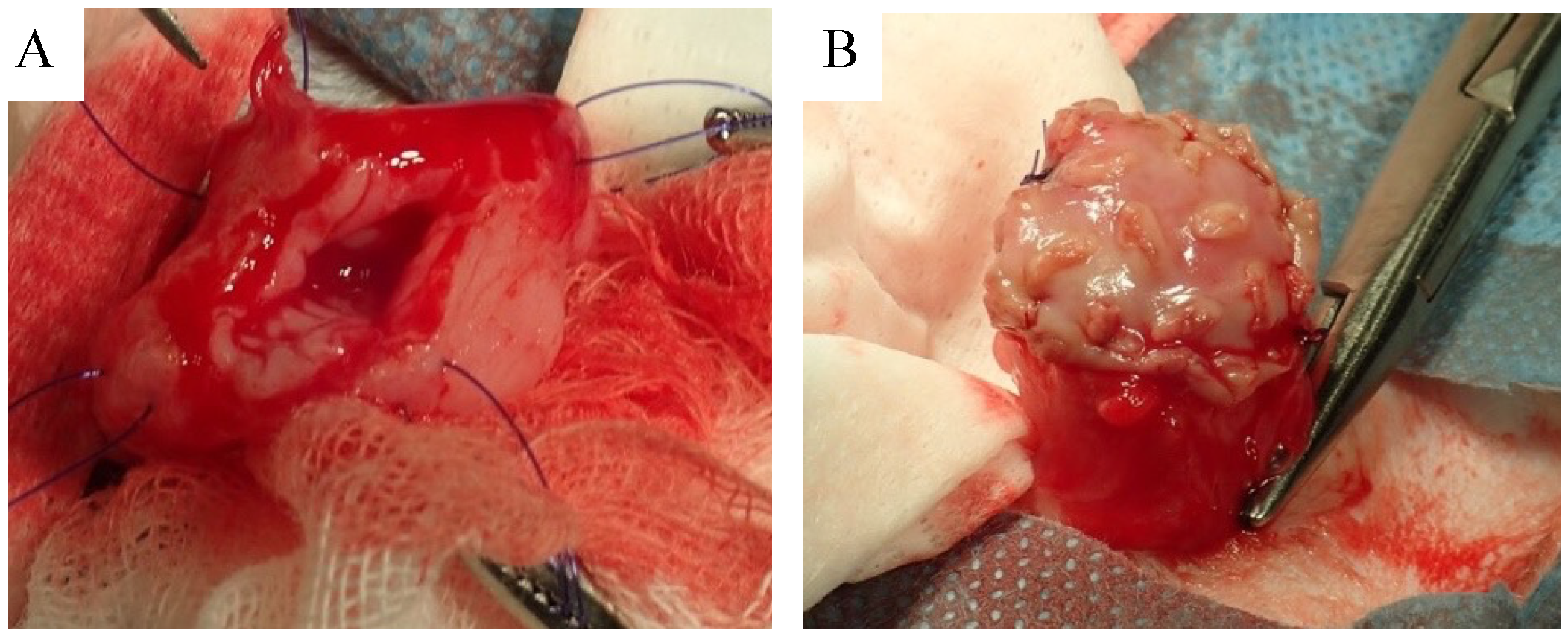
2.4. Biosheet Implantation
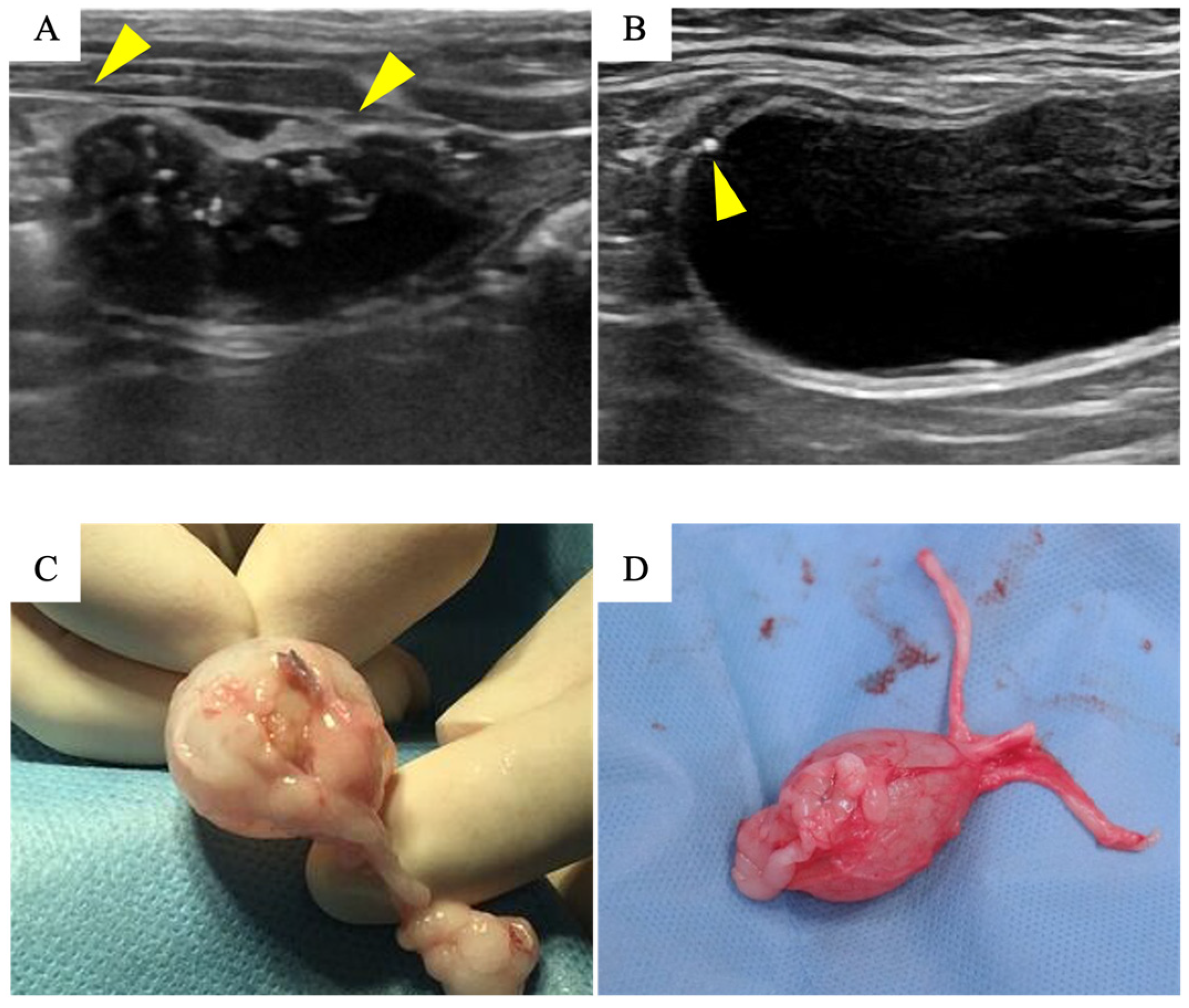
2.5. Clinical Evaluation after Implantation
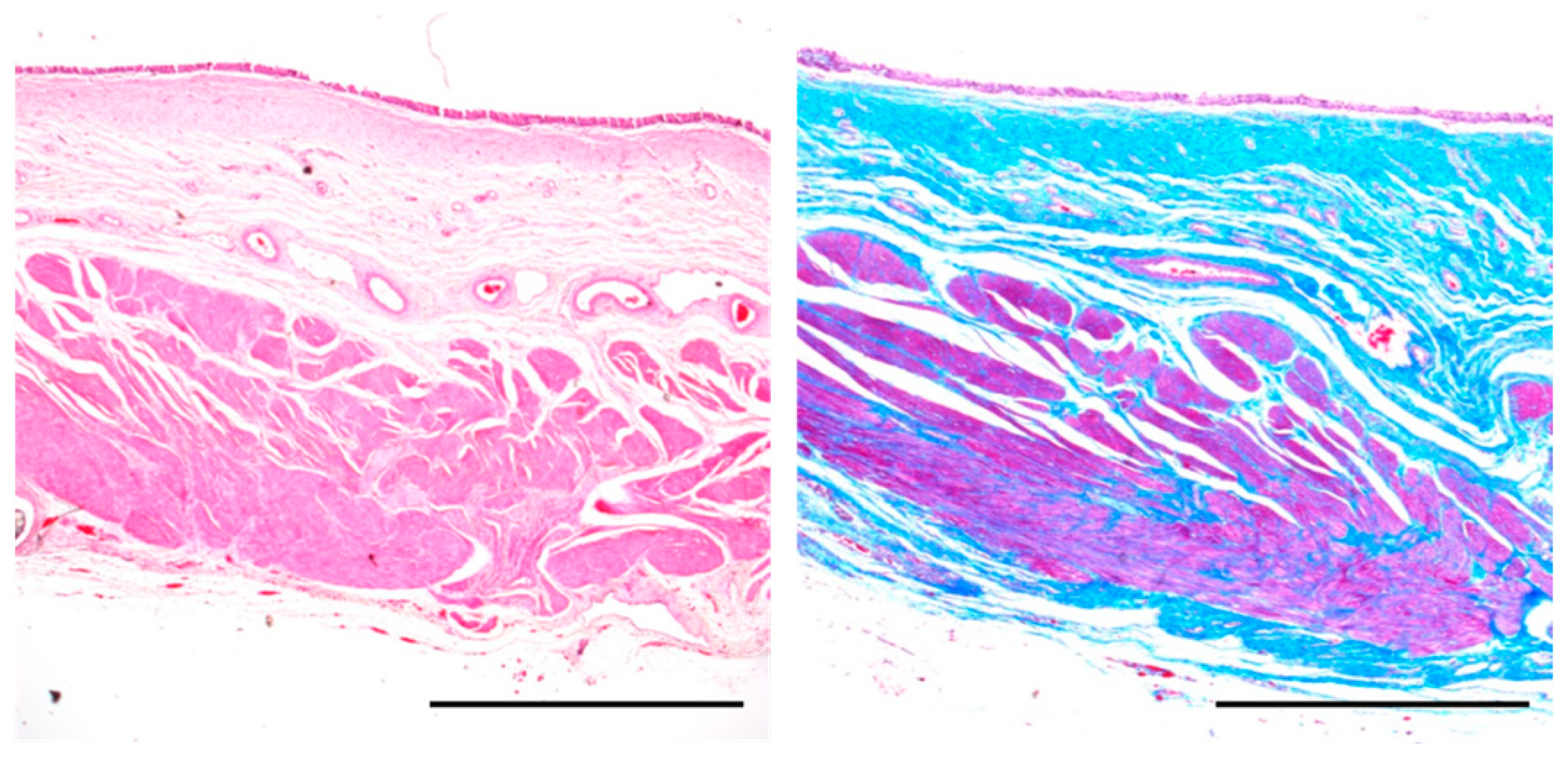
2.6. Histological Evaluation
3. Results
3.1. Biosheet Characterization
3.2. Biosheet Implantation and Postoperative Evaluation
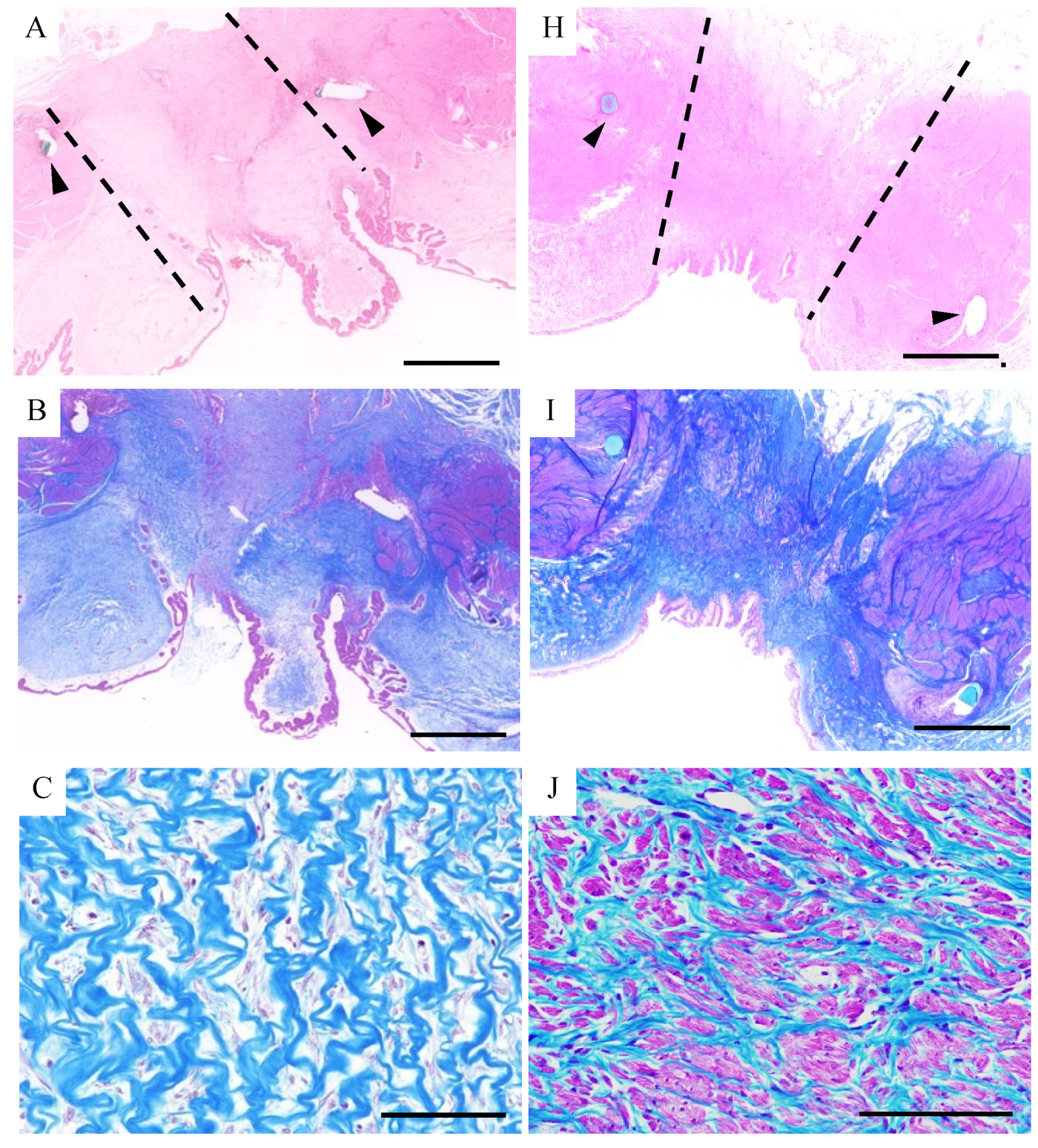
3.3. Histological Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Palm, C.; Westropp, J. Cats and Calcium Oxalate. Strategies for Managing Lower and Upper Tract Stone Disease. J. Feline Med. Surg. 2011, 13, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Kyles, A.E.; Hardie, E.M.; Wooden, B.G.; Adin, C.A.; Stone, E.A.; Gregory, C.R.; Mathews, K.G.; Cowgill, L.D.; Vaden, S.; Nyland, T.G. Management and Outcome of Cats with Ureteral Calculi. J. Am. Vet. Med. Assoc. 2005, 226, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.F.; Aronson, L.R.; Brown, D.C. Postoperative Mortality in Cats After Ureterolithotomy. Vet. Surg. 2011, 40, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Wormser, C.; Clarke, D.L.; Aronson, L.R. Outcomes of Ureteral Surgery and Ureteral Stenting in Cats: 117 Cases (2006–2014). J. Am. Vet. Med. Assoc. 2016, 248, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Vera-Donoso, C.D.; Moreno-Manzano, V. Bioengineering Approaches for Bladder Regeneration. Int. J. Mol. Sci. 2018, 19, 1796. [Google Scholar] [CrossRef] [PubMed]
- Kurzrock, E.A. Pediatric Enterocystoplasty: Long-Term Complications and Controversies. World J. Urol. 2009, 27, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Mingin, G.C.; Stock, J.A.; Hanna, M.K. Gastrocystoplasty: Long-Term Complications in 22 Patients. J. Urol. 1999, 162, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, S.; Özer, G.; Özer, E.; Soygür, T.; Beşalti, Ö.; Anafarta, K. Failure of Ureteral Replacement with Gore-Tex Tube Grafts. Urology 1998, 51, 400–403. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, G.L.; Liu, G.H.; Jiang, J.T.; Xia, S.J.; Sun, J.; Zhu, Y.J.; Zhu, J. Ureteral Reconstruction Using Autologous Tubular Grafts for the Management of Ureteral Strictures and Defects: An Experimental Study. Urol. Int. 2012, 88, 60–65. [Google Scholar] [CrossRef]
- Del Gaudio, C.; Vianello, A.; Bellezza, G.; Maulà, V.; Sidoni, A.; Zucchi, A.; Bianco, A.; Porena, M. Evaluation of Electrospun Bioresorbable Scaffolds for Tissue-Engineered Urinary Bladder Augmentation. Biomed. Mater. 2013, 8, 045013. [Google Scholar] [CrossRef]
- Noe, H.N.; Williams, R.S.; Causey, J.; Smith, D.P. Long-Term Effects of Polytetrafluoroethylene Injected into the Rat Bladder Submucosa. Urology 1994, 43, 852–855. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, P.K.J.D.; Sloff, M.; Janke, H.P.; Versteegden, L.R.M.; Kortmann, B.B.M.; De Gier, R.P.E.; Geutjes, P.J.; Oosterwijk, E.; Feitz, W.F.J. Ureteral Reconstruction in Goats Using Tissue-Engineered Templates and Subcutaneous Preimplantation. Tissue Eng. Part A 2018, 24, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, W.; Zhang, W.; Zhang, Z.; Zhou, G.; Cao, Y.; Liu, W. Hyaluronic Acid Coating Enhances Biocompatibility of Nonwoven PGA Scaffold and Cartilage Formation. Tissue Eng. Part C Methods 2017, 23, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, X.; Petersen, S.; Jin, X.; Qiu, Z.; Zhu, J. Mineralogy and Geochemistry of Hydrothermal Precipitates from Kairei Hydrothermal Field, Central Indian Ridge. Mar. Geol. 2014, 354, 69–80. [Google Scholar] [CrossRef]
- Kropp, B.P.; Rippy, M.K.; Badylak, S.F.; Adams, M.C.; Keating, M.A.; Rink, R.C.; Thor, K.B. Regenerative Urinary Bladder Augmentation Using Small Intestinal Submucosa: Urodynamic and Histopathologic Assessment in Long-Term Canine Bladder Augmentations. J. Urol. 1996, 155, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F. Xenogeneic Extracellular Matrix as a Scaffold for Tissue Reconstruction. Transpl. Immunol. 2004, 12, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.F.; McGuire, B.B.; Callanan, A.; Flood, H.D.; McGloughlin, T.M. Xenogenic Extracellular Matrices as Potential Biomaterials for Interposition Grafting in Urological Surgery. J. Urol. 2010, 184, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Madihally, S.V.; Palmer, B.; Frimberger, D.; Fung, K.M.; Kropp, B.P. Biomatrices for Bladder Reconstruction. Adv. Drug Deliv. Rev. 2015, 82, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Farhat, W.; Merguerian, P.A.; Wilson, G.J.; Khoury, A.E.; Woodhouse, K.A. 22 Week Assessment of Bladder Acellular Matrix as a Bladder Augmentation Material in a Porcine Model. Biomaterials 2002, 23, 2179–2190. [Google Scholar] [CrossRef]
- Roth, C.C.; Mondalek, F.G.; Kibar, Y.; Ashley, R.A.; Bell, C.H.; Califano, J.A.; Madihally, S.V.; Frimberger, D.; Lin, H.K.; Kropp, B.P. Bladder Regeneration in a Canine Model Using Hyaluronic Acid-Poly(Lactic-Co-Glycolic-Acid) Nanoparticle Modified Porcine Small Intestinal Submucosa. BJU Int. 2011, 108, 148–155. [Google Scholar] [CrossRef]
- Hayashida, K.; Kanda, K.; Yaku, H.; Ando, J.; Nakayama, Y. Development of an in Vivo Tissue-Engineered, Autologous Heart Valve (the Biovalve): Preparation of a Prototype Model. J. Thorac. Cardiovasc. Surg. 2007, 134, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.H.; Efendy, J.L.; Campbell, G.R. Novel Vascular Graft Grown within Recipient’s Own Peritoneal Cavity. Circ. Res. 1999, 85, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Satake, R.; Komura, M.; Komura, H.; Kodaka, T.; Terawaki, K.; Ikebukuro, K.; Komuro, H.; Yonekawa, H.; Hoshi, K.; Takato, T.; et al. Patch Tracheoplasty in Body Tissue Engineering Using Collagenous Connective Tissue Membranes (Biosheets). J. Pediatr. Surg. 2016, 51, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kanda, K.; Ishibashi-Ueda, H.; Yaku, H.; Nakayama, Y. Autologous Small-Caliber “Biotube” Vascular Grafts with Argatroban Loading: A Histomorphological Examination after Implantation to Rabbits. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Ishii, D.; Enmi, J.I.; Moriwaki, T.; Ishibashi-Ueda, H.; Kobayashi, M.; Iwana, S.; Iida, H.; Satow, T.; Takahashi, J.C.; Kurisu, K.; et al. Development of In Vivo Tissue-Engineered Microvascular Grafts with an Ultra Small Diameter of 0.6 mm (MicroBiotubes): Acute Phase Evaluation by Optical Coherence Tomography and Magnetic Resonance Angiography. J. Artif. Organs 2016, 19, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Kaneko, Y.; Takewa, Y.; Okumura, N. Mechanical Properties of Human Autologous Tubular Connective Tissues (Human Biotubes) Obtained from Patients Undergoing Peritoneal Dialysis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Kaneko, Y.; Okumura, N.; Terazawa, T. Initial 3-Year Results of First Human Use of an in-Body Tissue-Engineered Autologous “Biotube” Vascular Graft for Hemodialysis. J. Vasc. Access 2020, 21, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Iimori, Y.; Iwai, R.; Nagatani, K.; Inoue, Y.; Funayama-Iwai, M.; Okamoto, M.; Nakata, M.; Mie, K.; Nishida, H.; Nakayama, Y.; et al. Urinary Bladder Reconstruction Using Autologous Collagenous Connective Tissue Membrane “Biosheet®” Induced by in-Body Tissue Architecture: A Pilot Study. Regen. Ther. 2020, 15, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Takewa, Y.; Yamanami, M.; Kishimoto, Y.; Arakawa, M.; Kanda, K.; Matsui, Y.; Oie, T.; Ishibashi-Ueda, H.; Tajikawa, T.; Ohba, K.; et al. In Vivo Evaluation of an In-Body, Tissue-Engineered, Completely Autologous Valved Conduit (Biovalve Type VI) as an Aortic Valve in a Goat Model. J. Artif. Organs 2013, 16, 176–184. [Google Scholar] [CrossRef]
- Higashita, R.; Miyazaki, M.; Oi, M.; Ishikawa, N. First-in-Human Results of an in-Body Tissue Architecture-Induced Tissue-Engineered Vascular Graft “Biotube” for Application in Distal Bypass for Chronic Limb-Threatening Ischemia. J. Vasc. Surg. Cases, Innov. Tech. 2022, 8, 488–493. [Google Scholar] [CrossRef]
- Funayama, M.; Matsui, Y.; Tajikawa, T.; Sasagawa, T.; Saito, Y.; Sagishima, S.; Mizuno, T.; Mizuno, M.; Harada, K.; Uchida, S.; et al. Successful Implantation of Autologous Valved Conduits with Self-Expanding Stent (Stent-Biovalve) within the Pulmonary Artery in Beagle Dogs. J. Vet. Cardiol. 2015, 17, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, M.M.; de Mel, A. Biofabrication and Biomaterials for Urinary Tract Reconstruction. Res. Rep. Urol. 2017, 9, 79–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pokrywczynska, M.; Gubanska, I.; Drewa, G.; Drewa, T. Application of Bladder Acellular Matrix in Urinary Bladder Regeneration: The State of the Art and Future Directions. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.C., IV; Davis, M.M.; Smith, E.R.; Walsh, M.J.; Ellison, P.K.; Rink, R.C.; Kropp, B.P. The Ontogeny of Canine Small Intestinal Submucosa Regenerated Bladder. J. Urol. 1997, 158, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Chen, W.; Chen, B.; Shan, T.; Jia, W.; Hou, X.; Li, L.; Ye, G.; Dai, J. Bladder Regeneration in a Canine Model Using a Bladder Acellular Matrix Loaded with a Collagen-Binding BFGF. Biomater. Sci. 2017, 5, 2427–2436. [Google Scholar] [CrossRef]
- Agrawal, C.M.; Athanasiou, K.A. Technique to Control PH in Vicinity of Biodegrading PLA-PGA Implants. J. Biomed. Mater. Res. 1997, 38, 105–114. [Google Scholar] [CrossRef]

| No. | Sex | Year | Body Weight (kg) | Observation Period | Success Rate ※1 | Implanted Biosheet |
|---|---|---|---|---|---|---|
| 1 | Male | 0.5 | 2.7 | 1 month | 2/2 | autologous |
| 2 | Male | 0.5 | 2.4 | 1 month | 2/2 | autologous |
| 3 | Male | 0.5 | 2.5 | 1 month | 1/2 | autologous |
| 4 | Female | 0.5 | 2.4 | 3 months | 2/2 | autologous |
| 5 | Female | 0.5 | 2.4 | 3 months | 1/2 | autologous |
| 6 | Female | 0.5 | 2.5 | 3 months | 0/2 | biosheet from cat#4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, N.; Sugiyama, F.; Tsuboi, M.; Nakamura, H.K.; Nishimura, R.; Nakayama, Y.; Fujita, A. Bladder Reconstruction in Cats Using In-Body Tissue Architecture (iBTA)-Induced Biosheet. Bioengineering 2024, 11, 615. https://doi.org/10.3390/bioengineering11060615
Fujita N, Sugiyama F, Tsuboi M, Nakamura HK, Nishimura R, Nakayama Y, Fujita A. Bladder Reconstruction in Cats Using In-Body Tissue Architecture (iBTA)-Induced Biosheet. Bioengineering. 2024; 11(6):615. https://doi.org/10.3390/bioengineering11060615
Chicago/Turabian StyleFujita, Naoki, Fumi Sugiyama, Masaya Tsuboi, Hazel Kay Nakamura, Ryohei Nishimura, Yasuhide Nakayama, and Atsushi Fujita. 2024. "Bladder Reconstruction in Cats Using In-Body Tissue Architecture (iBTA)-Induced Biosheet" Bioengineering 11, no. 6: 615. https://doi.org/10.3390/bioengineering11060615
APA StyleFujita, N., Sugiyama, F., Tsuboi, M., Nakamura, H. K., Nishimura, R., Nakayama, Y., & Fujita, A. (2024). Bladder Reconstruction in Cats Using In-Body Tissue Architecture (iBTA)-Induced Biosheet. Bioengineering, 11(6), 615. https://doi.org/10.3390/bioengineering11060615







