Investigating the Feasibility and Performance of Hybrid Overmolded UHMWPE 3D-Printed PEEK Structural Composites for Orthopedic Implant Applications: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Designing and Printing of Porous Test Specimens
2.2. Overmolding and Mechanical Testing
2.3. Cross-Sectional Analysis
2.4. Micro-CT Analysis
3. Results
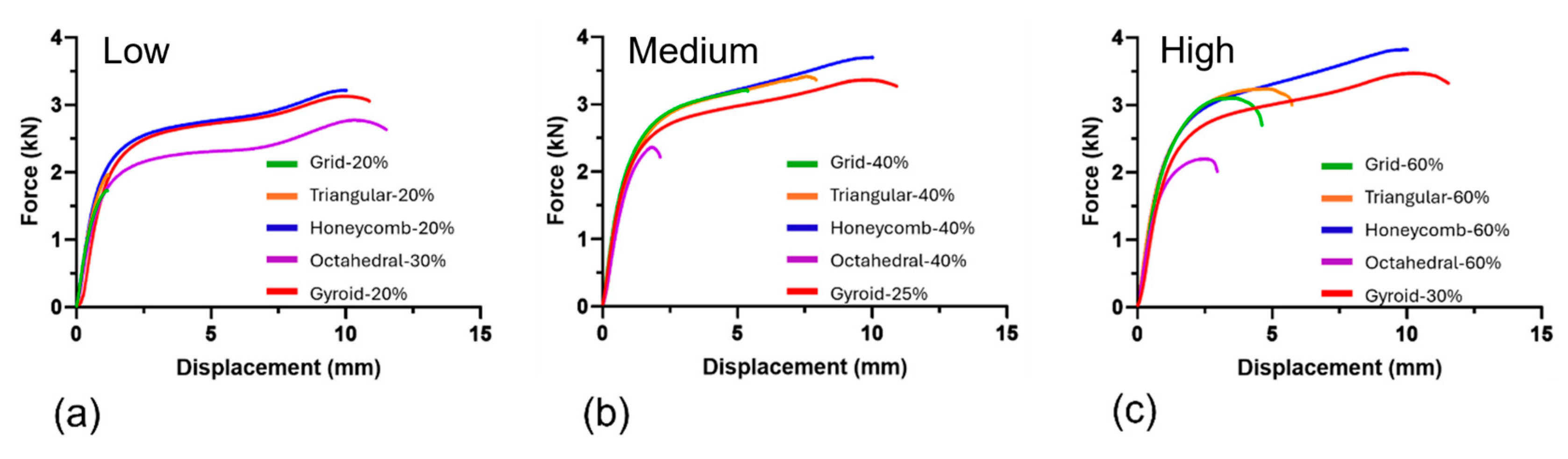
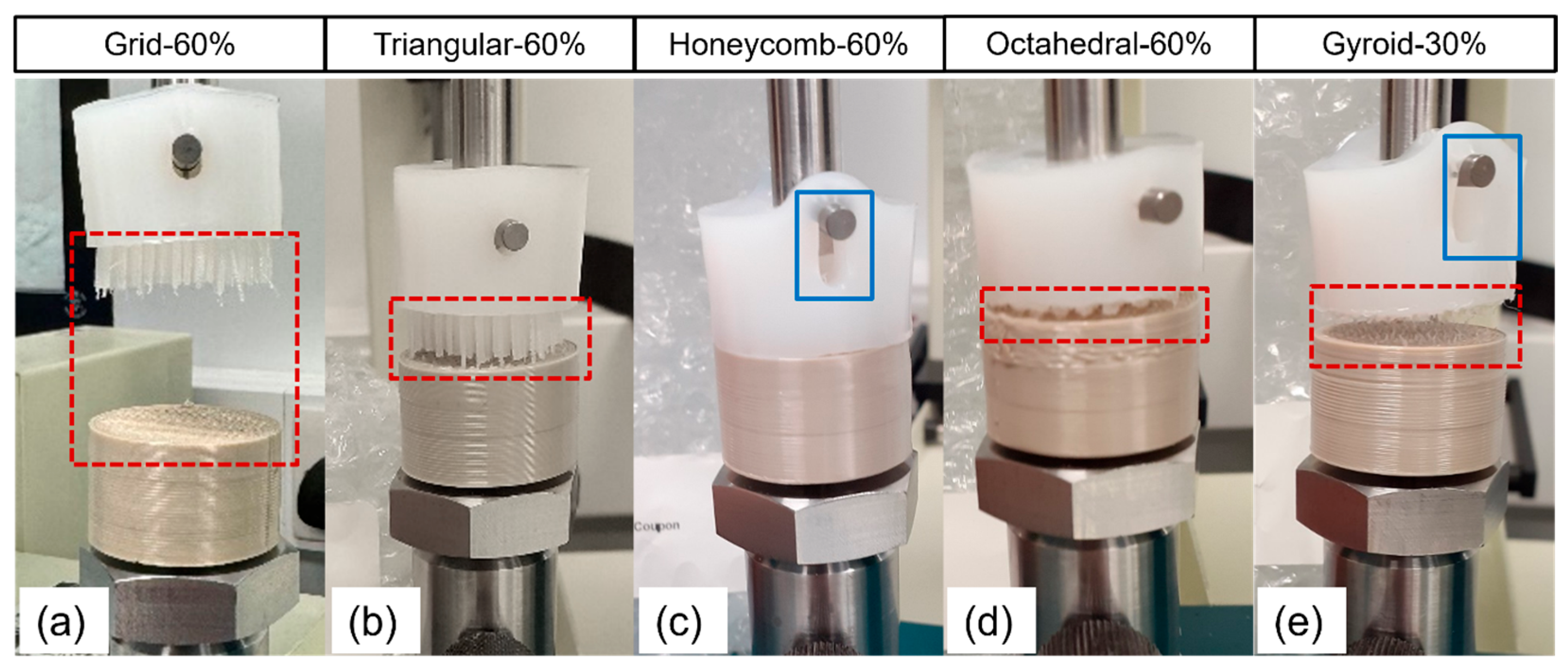
3.1. Mechanical Testing
3.2. Cross-Sectional Analysis
3.3. Micro-CT Interfacial Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aliyeva, N.; Sas, B.; Okan, S.B. Recent developments on the overmolding process for the fabrication of thermoset and thermoplastic composites by the integration of nano/micron-scale reinforcements. Compos. Part. A Appl. Sci. Manuf. 2021, 149, 106525. [Google Scholar] [CrossRef]
- Grujicic, M.; Sellappan, V.; Omar, M.A.; Seyr, A.; Obieglo, A.; Erdmann, M.; Holzleitner, J. An overview of the polymer-to-metal direct-adhesion hybrid technologies for load-bearing automotive components. J. Mater. Process Techhnol. 2008, 197, 363–373. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Sumner, D.R. Long-term implant fixation and stress-shielding in total hip replacement. J. Biomech. 2015, 48, 797–800. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kim, K.H. Ion implantation of titanium based biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric biomaterials for medical implants and devices. ACS Biomater. Sci. Eng. 2016, 2, 454–474. [Google Scholar] [CrossRef]
- Banoriya, D.; Purohit, R.; Dwivedi, R.K. Advanced applications of polymer based biomaterials. Mater. Today Proc. 2017, 4, 3534–3541. [Google Scholar] [CrossRef]
- Boros, R.; Tatyana, A.; Golcs, Á.; Krafcsik, O.H.; Kovács, J.G. Plasma treatment to improve the adhesion between ABS and PA6 in hybrid structures produced by injection overmolding. Polym. Test. 2022, 106, 107446. [Google Scholar] [CrossRef]
- Zhao, S.; Kimura, F.; Kadoya, S.; Kajihara, Y. Experimental analysis on mechanical interlocking of metal-polymer direct joining. Precis. Eng. 2020, 61, 120–125. [Google Scholar] [CrossRef]
- Kim, W.S.; Yun, I.H.; Lee, J.J.; Jung, H.T. Evaluation of mechanical interlock effect on adhesion strength of polymermetal interfaces using micro-patterned surface topography. Int. J. Adhes. Adhes. 2010, 30, 408–417. [Google Scholar] [CrossRef]
- Yin, S.; Xie, Y.; Li, R.; Zhang, J.; Zhou, T. Polymer-metal hybrid material with an ultra-high interface strength based on mechanical interlocking via Nanopores produced by electrochemistry. Ind. Eng. Chem. Res. 2020, 59, 12409–12420. [Google Scholar] [CrossRef]
- Harris, A.F.; Beevers, A. The effects of grit-blasting on surface properties for adhesion. Int. J. Adhes. Adhes. 1999, 19, 445–452. [Google Scholar] [CrossRef]
- Costantini, M.; Colosi, C.; Mozetic, P.; Jaroszewicz, J.; Tosato, A.; Rainer, A.; Trombetta, M.; Więszkowski, W.; Dentini, M.; Barbetta, A. Correlation between porous texture and cell seeding efficiency of gas foaming and microfluidic foaming scaffolds. Mater. Sci. Eng. C 2016, 62, 668–677. [Google Scholar] [CrossRef]
- Węglowski, M.S.; Blacha, S.; Phillips, A. Electron beam welding—Techniques and trends—Review. Vacuum 2016, 130, 72–92. [Google Scholar] [CrossRef]
- Sun, Z.; Karppi, R. The application of electron beam welding for the joining of dissimilar metals: An overview. J. Mater. Process. Technol. 1996, 3, 257–267. [Google Scholar] [CrossRef]
- Barienti, K.; Kahra, C.; Herbst, S.; Nürnberger, F.; Maier, H.J. Ion beam processing for sample preparation of hybrid materials with strongly differing mechanical properties. Metallogr. Microstruct. Anal. 2020, 9, 54–60. [Google Scholar] [CrossRef]
- Wagner, A.; Wendler, M.; Petschelt, A.; Belli, R.; Lohbauer, U. Bonding performance of universal adhesives in different etching modes. J. Dent. 2014, 42, 800–807. [Google Scholar] [CrossRef]
- Bötel, F.; Zimmermann, T.; Sütel, M.; Müller, W.D.; Schwitalla, A.D. Influence of different low-pressure plasma process parameters on shear bond strength between veneering composites and PEEK materials. Dent. Mater. 2018, 34, e246–e254. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, A.; Broeckhoven, C.; Yadroitsava, I.; Yadroitsava, I.; Hands, C.H.; Kunju, R.; Bhate, D. Beautiful and functional: A review of biomimetic design in additive manufacturing. Addit. Manuf. 2019, 27, 408–427. [Google Scholar] [CrossRef]
- Prakash, K.S.; Nancharaih, T.; Rao, V.V.S. Additive manufacturing techniques in manufacturing—An overview. Mater. Today Proc. 2018, 5, 3073–3882. [Google Scholar] [CrossRef]
- Nazir, A.; Gokcekaya, O.; Md Masum Billah, K.; Ertugrul, O.; Jiang, J.; Sun, J.; Hussain, S. Multi-material additive manufacturing: A systematic review of design, properties, applications, challenges and 3D printing of materials and cellular metamaterials. Mater. Des. 2023, 226, 111661. [Google Scholar] [CrossRef]
- Al-ketan, O.; Lee, D.W.; Rowshan, R.; Abu Al-Rub, R.K. Functionally graded and multi-morphology sheet TPMS lattices: Design, manufacturing, and mechanical properties. J. Mech. Behav. Biomed. Mater. 2020, 102, 103520. [Google Scholar] [CrossRef]
- Verma, S.; Yang, C.K.; Lin, C.H.; Jeng, J.Y. Additive manufacturing of lattice structures for high strength mechanical interlocking of metal and resin during injection molding. Addit. Manuf. 2022, 49, 102463. [Google Scholar] [CrossRef]
- Sengsri, P.; Fu, H.; Kaewunruen, S. Mechanical properties and energy absorption capability of a 3D-printed TPMS sanwhich lattice model for meta-functional composite bridge bearing applications. J. Compos. Sci. 2022, 6, 71. [Google Scholar] [CrossRef]
- Saleh, M.; Anwar, S.; Al-Ahmari, A.M.; Alfaify, A. Compression performance and failure analysis of 3D-printed carbon fiber/PLA composite TPMS lattice structures. Polymers 2022, 14, 4595. [Google Scholar] [CrossRef]
- Grygier, D.; Kujawa, M.; Kowalewski, P. Deposition of biocompatible polymers by 3D printing (FDM) on titanium alloy. Polymers 2022, 14, 4595. [Google Scholar] [CrossRef]
- Song, W.; Mu, G.; Feng, Z.; Huang, Z.; Liu, Y.; Huang, X.; Xiao, L. Mechanical properties of 3D printed interpenetrating phase composites with TPMS architectures. Thin-Walled Struct. 2023, 193, 111210. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, H.; Dunn, M.L.; Yu, K. 3D interfacial material-locking structures to promote bonding strength of dissimilar materials. J. Manuf. Process. 2024, 112, 92–102. [Google Scholar] [CrossRef]
- Zou, X.; Huang, L.; Chen, K.; Jiang, M.; Zhang, S.; Wang, M.; Hua, X.; Shan, A. Surface structuring via additive manufacturing to improve the performance of metal and polymer joints. Metals 2021, 11, 567. [Google Scholar] [CrossRef]
- Alhmoudi, A.; Sheikh-Ahmad, J.; Almaskari, F.; Bojanampati, S. Joining of polymer to metal using material extrusion additive manufacturing. Int. J. Adv. Manuf. Technol. 2023, 129, 3303–3319. [Google Scholar] [CrossRef]
- Smith, J.A.; Petersmann, S.; Arbeiter, F.; Schäfer, U. Optimization and manufacture of polyetheretherketone patient specific cranial implants by material extrusion—A clinical perspective. J. Mech. Behav. Biomed. Mater. 2023, 144, 105965. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B.; Turner, A.S. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 1–11. [Google Scholar] [CrossRef]
- Basgul, C.; Spece, H.; Sharma, N.; Thieringer, F.M.; Kurtz, S.M. Structure, properties, and bioactivity of 3D printed PAEKs for implant applications: A systematic review. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1924–1941. [Google Scholar] [CrossRef]
- Petersmann, S.; Smith, J.A.; Schäfer, U.; Arbeiter, F. Material extrusion-based additive manufacturing of polyetheretherketone cranial implants: Mechanical performance and print quality. J. Mater. Res. Technol. 2023, 22, 642–657. [Google Scholar] [CrossRef]
- Wang, P.; Zou, B.; Xiao, H.; Ding, S.; Huang, C. Effects of printing parameters of fused deposition modeling on mechanical properties, surface quality, and microstructure of PEEK. J. Mater. Process Technol. 2019, 271, 62–74. [Google Scholar] [CrossRef]
- Wu, W.; Geng, P.; Li, G.; Zhao, D.; Zhang, H.; Zhao, J. Influence of layer thickness and raster angle on the mechanical properties of 3D-printed PEEK and a comparative mechanical study between PEEK and ABS. Materials 2015, 8, 5834–5846. [Google Scholar] [CrossRef]
- Basgul, C.; MacDonald, D.W.; Siskey, R.; Kurtz, S.M.K. Thermal localization improves the interlayer adhesion and structural integrity of 3D printed PEEK lumbar spinal cages. Materialia. 2020, 10, 100650. [Google Scholar] [CrossRef]
- Yang, C.; Tian, X.; Li, D.; Cao, Y.; Zhao, F.; Shi, C. Influence of thermal processing conditions in 3D printing on the crystallinity and mechanical properties of PEEK material. J. Mater. Process Technol. 2017, 248, 1–7. [Google Scholar] [CrossRef]
- Basgul, C.; Yu, T.; MacDonald, D.W.; Siskey, R.; Marcolongo, M.; Kurtz, S.M.K. Does annealing improve the interlayer adhesion and structural integrity of FFF 3D printed PEEK lumbar spinal cages? J. Mech. Behav. Biomed. Mater. 2020, 102, 103455. [Google Scholar] [CrossRef]
- Sharma, N.; Aghlmandi, S.; Dalcanale, F.; Seiler, D.; Zeilhofer, H.F.; Honigmann, P.; Thieringer, F.M. Quantitative assessment of point-of-care 3D-printed patient-specific polyetheretherketone (PEEK) cranial implants. Int. J. Mol. Sci. 2021, 22, 8521. [Google Scholar] [CrossRef]
- Sharma, N.; Aghlmandi, S.; Cao, S.; Kunz, C.; Honigmann, P.; Thieringer, F.M. Quality characteristics and clinical relevance of in-house 3D-printed customized polyetheretherketone (PEEK) implants for craniofacial reconstruction. J. Clin. Med. 2020, 9, 818. [Google Scholar] [CrossRef]
- Sharma, N.; Honigmann, P.; Cao, S.; Thieringer, F.M. Dimensional characteristics of FDM 3D printed PEEK implant for craniofacial reconstruction. Trans. Addit. Manuf. Meets Med. 2020, 2. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Villarraga, M.; Herr, M.; Bergström, J.; Rimnac, C.; Edidin, A. Thermomechanical behavior of virgin and highly crosslinked ultra-high molecular weight polyethylene used in total joint replacements. Biomaterials 2002, 23, 3681–3697. [Google Scholar] [CrossRef]
- Englert, L.; Heuer, A.; Engelskirchen, M.K.; Frölich, F.; Dietrich, S.; Liebig, W.V.; Kärger, L.; Schulz, V. Hybrid material additive manufacturing: Interlocking interfaces for fused filament fabrication on laser powder bed fusion substrates. Virtual Phys. Prototyp. 2022, 17, 508–527. [Google Scholar] [CrossRef]
- Chueh, Y.H.; Wei, C.; Zhang, X.; Li, L. Integrated laser-based powder bed fusion and fused filament fabrication for three-dimensional printing of hybrid metal/polymer objects. Addit. Manuf. 2020, 31, 100928. [Google Scholar] [CrossRef]
- Ding, S.; Zou, B.; Wang, P.; Ding, H. Effects of nozzle temperature and building orientation on mechanical properties and microstructure of PEEK and PEI printed by 3D-FDM. Polym. Test. 2019, 78, 105948. [Google Scholar] [CrossRef]
- Arif, M.F.; Kumar, S.; Varadarajan, K.M.; Cantwell, W.J. Performance of biocompatible PEEK processed by fused deposition additive manufacturing. Mater. Des. 2018, 146, 249–259. [Google Scholar] [CrossRef]
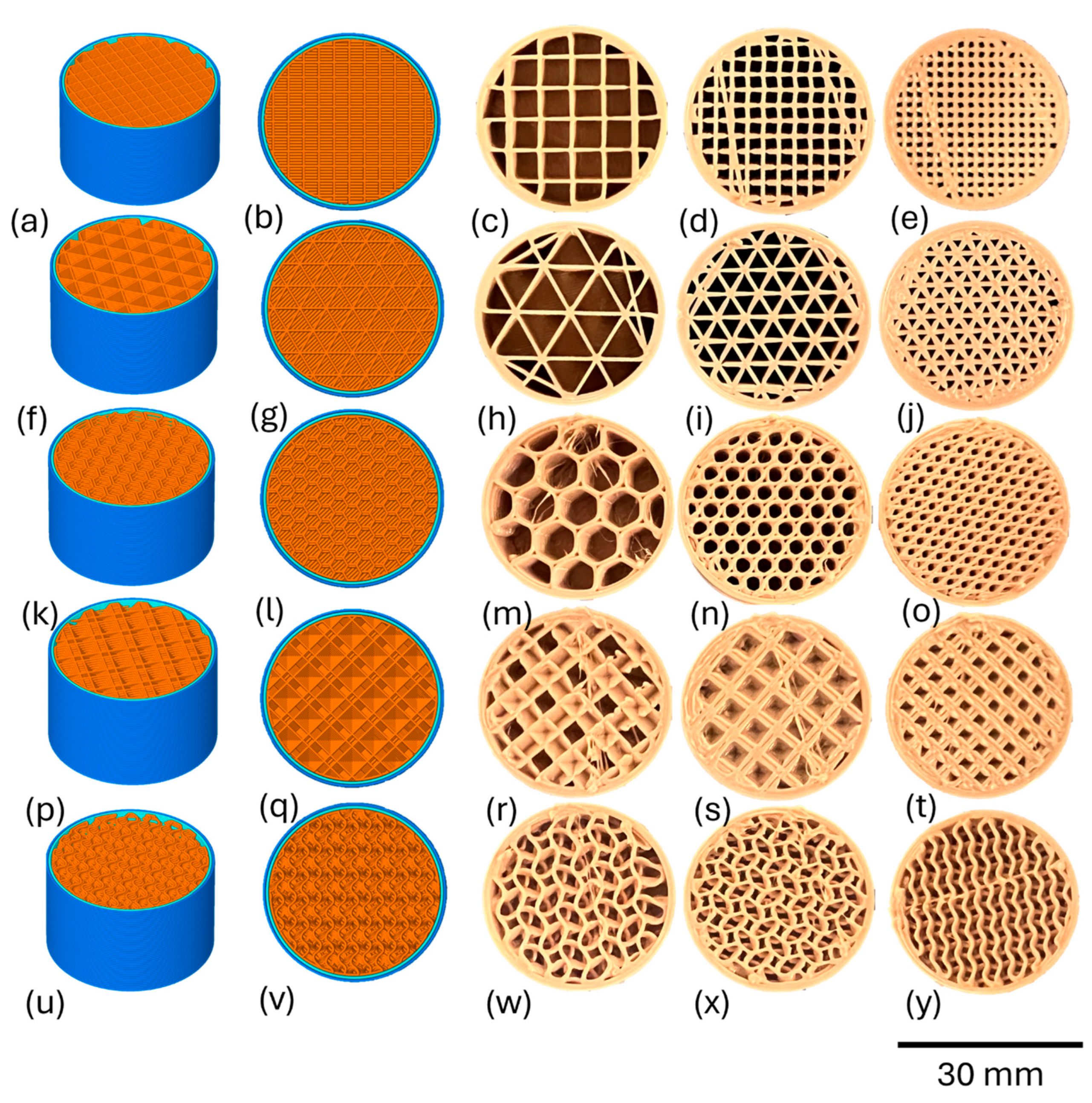



| Printing Parameter | Porous PEEK Insert |
|---|---|
| Nozzle temperature (°C) | 430 |
| Printing speed (mm/s) | 25 |
| Printing speed of the first layer (% of the printing speed of subsequent layers) | 100 |
| Nozzle material | Brass |
| Nozzle diameter (mm) | 0.4 |
| Build platform material | Glass |
| Build platform temperature (°C) | 250 |
| Build chamber temperature (°C) | 200 |
| Layer thickness (mm) | 0.3 |
| First layer thickness (mm) | 0.3 |
| Build orientation (°) | 90 |
| Number of concentric perimeters | 2 |
| Sample | Separation Force (kN) | Separation Strength (MPa) | Failure Mechanism |
|---|---|---|---|
| Grid-20% | 1.97 ± 0.02 | 2.78 ± 0.02 | Type 1 |
| Grid-40% | 3.33 ± 0.17 | 4.72 ± 0.24 | Type 1 |
| Grid-60% | 3.23 ± 0.03 | 4.56 ± 0.05 | Type 1 |
| Triangular-20% | 1.78 ± 0.05 | 2.52 ± 0.07 | Type 1 |
| Triangular-40% | 3.24 ± 0.13 * | 4.58 ± 0.18 * | Type 1 and 2 |
| Triangular-60% | 3.11 ± 0.03 | 4.4 ± 0.04 | Type 1 |
| Honeycomb-20% | 3.23 ± 0.04 * | 4.57 ± 0.05 * | Type 2 |
| Honeycomb-40% | 3.72 ± 0.1 * | 5.27 ± 0.14 * | Type 2 |
| Honeycomb-60% | 3.86 ± 0.16 * | 5.47 ± 0.23 * | Type 1 and 2 |
| Octahedral-30% | 2.22 ± 0.04 * | 3.92 ± 0.06 * | Type 1 and 2 |
| Octahedral-40% | 2.37 ± 0.13 | 3.35 ± 0.18 | Type 1 |
| Octahedral-60% | 2.22 ± 0.06 | 3.14 ± 0.08 | Type 1 |
| Gyroid-20% | 2.99 ± 0.25 * | 4.23 ± 0.36 * | Type 2 |
| Gyroid-25% | 3.34 ± 0.05 * | 4.73 ± 0.07 * | Type 2 |
| Gyroid-30% | 3.46 ± 0.03 * | 4.9 ± 0.04 * | Type 2 |
| Sample | PEEK Volume (%) | UHMWPE Volume (%) | Air Void Volume (%) |
|---|---|---|---|
| Honeycomb-20% | 23.1 | 72 | 2.4 |
| Honeycomb-40% | 40.9 | 56.8 | 2.2 |
| Honeycomb-60% | 59 | 39.8 | 0.6 |
| Gyroid-20% | 24.6 | 71.5 | 3.2 |
| Gyroid-25% | 32.4 | 63.9 | 2.9 |
| Gyroid-30% | 33.3 | 63.4 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, J.A.; Basgul, C.; Mohammadlou, B.S.; Allen, M.; Kurtz, S.M. Investigating the Feasibility and Performance of Hybrid Overmolded UHMWPE 3D-Printed PEEK Structural Composites for Orthopedic Implant Applications: A Pilot Study. Bioengineering 2024, 11, 616. https://doi.org/10.3390/bioengineering11060616
Smith JA, Basgul C, Mohammadlou BS, Allen M, Kurtz SM. Investigating the Feasibility and Performance of Hybrid Overmolded UHMWPE 3D-Printed PEEK Structural Composites for Orthopedic Implant Applications: A Pilot Study. Bioengineering. 2024; 11(6):616. https://doi.org/10.3390/bioengineering11060616
Chicago/Turabian StyleSmith, James A., Cemile Basgul, Bita Soltan Mohammadlou, Mark Allen, and Steven M. Kurtz. 2024. "Investigating the Feasibility and Performance of Hybrid Overmolded UHMWPE 3D-Printed PEEK Structural Composites for Orthopedic Implant Applications: A Pilot Study" Bioengineering 11, no. 6: 616. https://doi.org/10.3390/bioengineering11060616
APA StyleSmith, J. A., Basgul, C., Mohammadlou, B. S., Allen, M., & Kurtz, S. M. (2024). Investigating the Feasibility and Performance of Hybrid Overmolded UHMWPE 3D-Printed PEEK Structural Composites for Orthopedic Implant Applications: A Pilot Study. Bioengineering, 11(6), 616. https://doi.org/10.3390/bioengineering11060616






