The Potential of a New Natural Vessel Source: Decellularized Intercostal Arteries as Sufficiently Long Small-Diameter Vascular Grafts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Harvest and Decellularization of Intercostal Artery
2.3. DNA Content and Characterization of Tissue
2.4. Scanning Electron Microscopy (SEM)
2.5. Mechanical Characterizations
2.6. Cytocompatibility
2.7. Hemocompatibility
2.8. Statistical Analysis
3. Results
3.1. The Diameters and Lengths of PIAs and BIAs Meet the Requirements for CABG
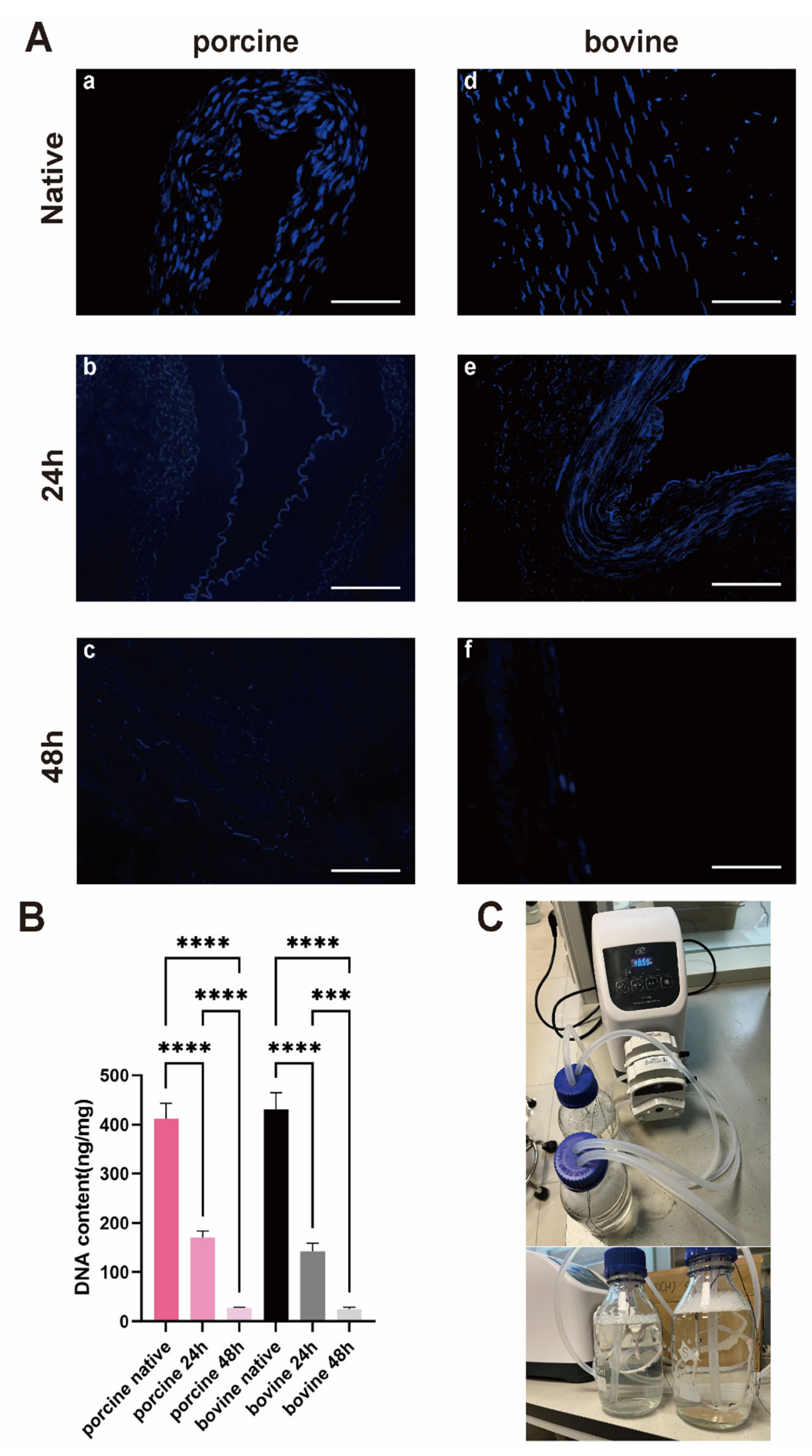
3.2. 48*2 h Protocol Can Yield DIAs with Low Immunogenicity
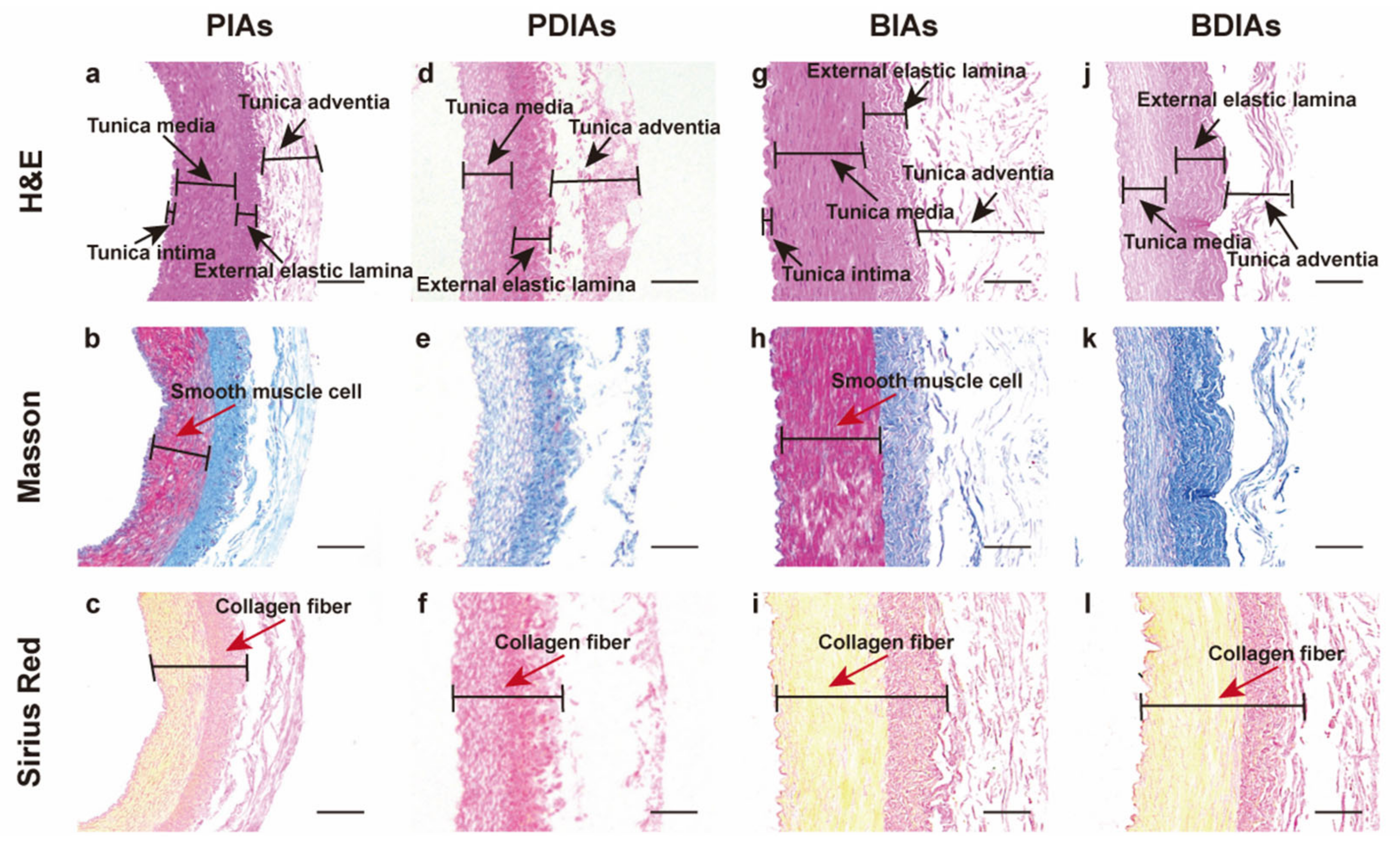
3.3. Tissue Staining Indicated That the Collagen Fibers Were Largely Preserved
3.4. Surface Morphology
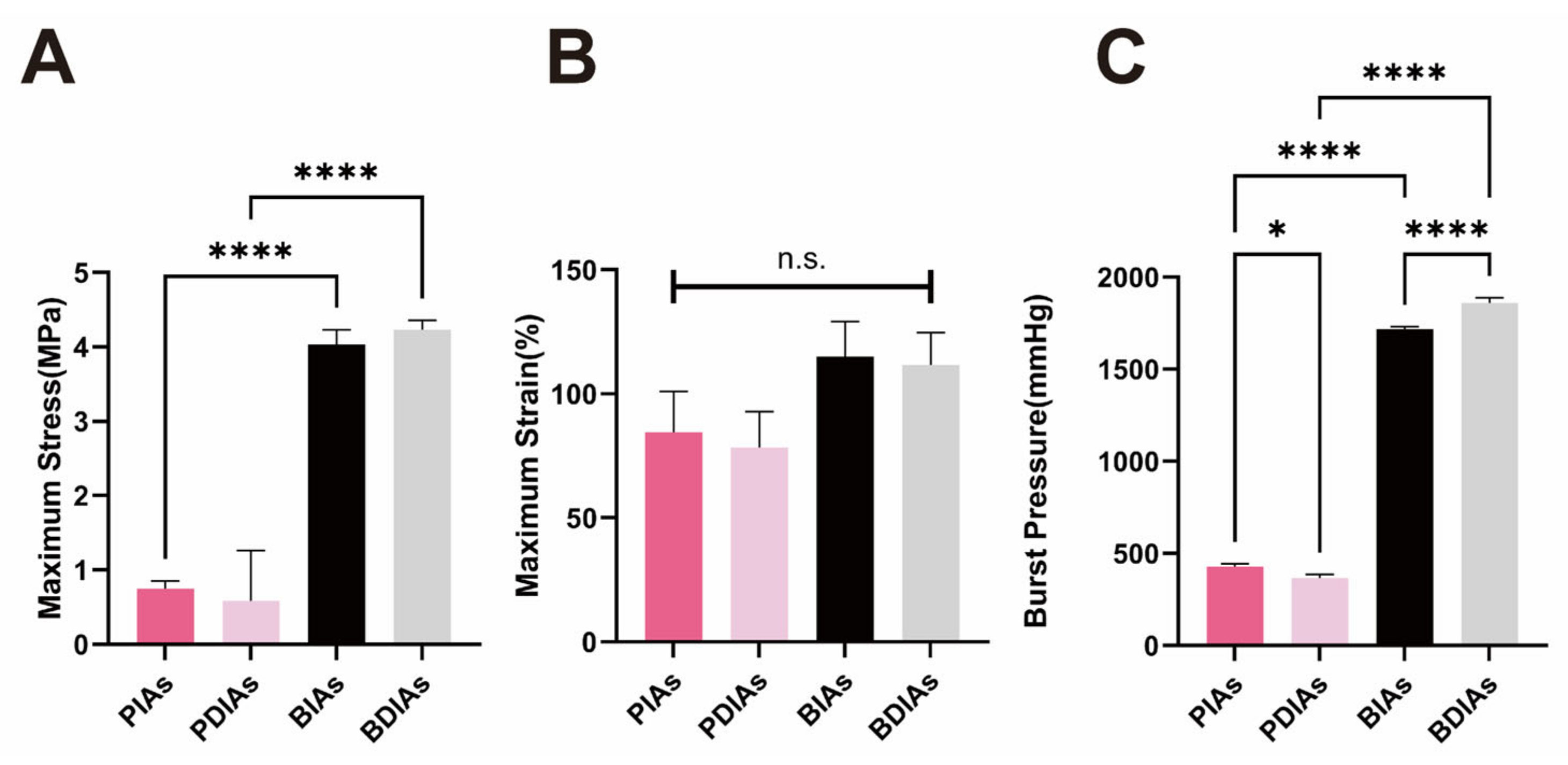
3.5. PDIAs and BDIAs Maintain Favorable Mechanical Properties
3.6. Adequate Cellular Compatibility Yet Suboptimal Hemocompatibility for BDIAs and PDIAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mo, Z.W.; Peng, Y.M.; Zhang, Y.X.; Li, Y.; Kang, B.A.; Chen, Y.T.; Li, L.; Sorci-Thomas, M.G.; Lin, Y.J.; Cao, Y.; et al. High-density lipoprotein regulates angiogenesis by long non-coding RNA HDRACA. Signal Transduct. Target. Ther. 2023, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.A.; Li, H.M.; Chen, Y.T.; Deng, M.J.; Li, Y.; Peng, Y.M.; Gao, J.J.; Mo, Z.W.; Zhou, J.G.; Ou, Z.J.; et al. High-density lipoprotein regulates angiogenesis by affecting autophagy via miRNA-181a-5p. Sci. China Life Sci. 2024, 67, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Z.Q.; Turner, N.J.; Teng, S.F.; Cheng, W.Y.; Zhou, H.Y.; Zhang, L.; Hu, H.W.; Wang, Q.; Badylak, S.F. Perfusion-decellularized skeletal muscle as a three-dimensional scaffold with a vascular network template. Biomaterials 2016, 89, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.E.M.; Lamm, P.; Wellmann, P.; Milz, S.; Hagl, C.; Juchem, G. Autologous endothelialized vein allografts in coronary artery bypass surgery—Long term results. Biomaterials 2019, 212, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Balayla, J.; Edwards, M.; Lefkowitz, A. Uterine artery as an arterial conduit for coronary artery bypass graft (CABG) surgery in women: A role for estrogen-receptor alpha (ER-α) in the prevention of post-CABG accelerated atherosclerosis and graft disease. Med. Hypotheses 2013, 80, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Hiob, M.A.; She, S.; Muiznieks, L.D.; Weiss, A.S. Biomaterials and Modifications in the Development of Small-Diameter Vascular Grafts. ACS Biomater. Sci. Eng. 2017, 3, 712–723. [Google Scholar] [CrossRef]
- Obiweluozor, F.O.; Emechebe, G.A.; Kim, D.; Cho, H.; Park, C.; Kim, C.-S.; Jeong, I. Considerations in the Development of Small-Diameter Vascular Graft as an Alternative for Bypass and Reconstructive Surgeries: A Review. Cardiovasc. Eng. Technol. 2020, 11, 495–521. [Google Scholar] [CrossRef]
- Soldani, G.; Losi, P.; Bernabei, M.; Burchielli, S.; Chiappino, D.; Kull, S.; Briganti, E.; Spiller, D. Long term performance of small-diameter vascular grafts made of a poly(ether)urethane-polydimethylsiloxane semi-interpenetrating polymeric network. Biomaterials 2010, 31, 2592–2605. [Google Scholar] [CrossRef]
- Cai, Z.; Gu, Y.; Xiao, Y.; Wang, C.; Wang, Z. Porcine carotid arteries decellularized with a suitable concentration combination of Triton X-100 and sodium dodecyl sulfate for tissue engineering vascular grafts. Cell Tissue Bank. 2021, 22, 277–286. [Google Scholar] [CrossRef]
- Eufrásio-da-Silva, T.; Ruiz-Hernandez, E.; O’Dwyer, J.; Picazo-Frutos, D.; Duffy, G.P.; Murphy, B.P. Enhancing medial layer recellularization of tissue-engineered blood vessels using radial microchannels. Regen. Med. 2019, 14, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Cahill, P.A.; Lally, C. Investigation of a small-diameter decellularised artery as a potential scaffold for vascular tissue engineering; biomechanical evaluation and preliminary cell seeding. J. Mech. Behav. Biomed. Mater. 2012, 14, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Xie, X.; Yuan, H.; Tang, Z.; Qian, T.; Liu, Y.; Song, M.; Liu, S.; Lu, T.; et al. Photooxidation and Pentagalloyl Glucose Cross-Linking Improves the Performance of Decellularized Small-Diameter Vascular Xenograft In Vivo. Front. Bioeng. Biotechnol. 2022, 10, 816513. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Fukuda, H.; Toda, K.; Yoshitatsu, M.; Funatsu, T.; Masai, T.; Miyamoto, Y. Remodeling of the radial artery anastomosed to the internal thoracic artery as a composite straight graft. J. Thorac. Cardiovasc. Surg. 2007, 134, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Kiyokawa, K.; Asada, H.; Kawakami, T.; Haga, M.; Akasaka, N. Evaluation of an internal thoracic artery as a coronary artery bypass graft by intercostal duplex scanning ultrasonography. Jpn. J. Thorac. Cardiovasc. Surg. 2001, 49, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Qiao, W.; Shi, J.; Qiu, X.; Dong, N. Application of decellularized vascular matrix in small-diameter vascular grafts. Front. Bioeng. Biotechnol. 2022, 10, 1081233. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.L.; Koh, J.; Prabhakar, V.; Niklason, L.E. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transpl. 2003, 12, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Lei, D.; Li, S.; Huang, P.; Qi, Q.; Sun, Y.; Zhang, Y.; Wang, Z.; You, Z.; Ye, X.; et al. Hybrid small-diameter vascular grafts: Anti-expansion effect of electrospun poly ε-caprolactone on heparin-coated decellularized matrices. Biomaterials 2016, 76, 359–370. [Google Scholar] [CrossRef]
- Gui, L.; Muto, A.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng. Part A 2009, 15, 2665–2676. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, V.E.; Martínez-González, B.; Quiroga-Garza, A.; Reyes-Hernández, C.G.; de la Fuente-Villarreal, D.; de la Garza-Castro, O.; Guzmán-López, S.; Elizondo-Omaña, R.E. Human Umbilical Vessels: Choosing the Optimal Decellularization Method. ASAIO J. 2018, 64, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.H.; Aigner, P.; Holnthoner, W.; Monforte, X.; Nürnberger, S.; Rünzler, D.; Redl, H.; Teuschl, A.H. Decellularized human placenta chorion matrix as a favorable source of small-diameter vascular grafts. Acta Biomater. 2016, 29, 125–134. [Google Scholar] [CrossRef]
- Dahan, N.; Zarbiv, G.; Sarig, U.; Karram, T.; Hoffman, A.; Machluf, M. Porcine small diameter arterial extracellular matrix supports endothelium formation and media remodeling forming a promising vascular engineered biograft. Tissue Eng. Part A 2012, 18, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Kimicata, M.; Allbritton-King, J.D.; Navarro, J.; Santoro, M.; Inoue, T.; Hibino, N.; Fisher, J.P. Assessment of decellularized pericardial extracellular matrix and poly(propylene fumarate) biohybrid for small-diameter vascular graft applications. Acta Biomater. 2020, 110, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Zouhair, S.; Sasso, E.D.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; Giorgio, R.; et al. A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef]
- Negishi, J.; Funamoto, S.; Kimura, T.; Nam, K.; Higami, T.; Kishida, A. Porcine radial artery decellularization by high hydrostatic pressure. J. Tissue Eng. Regen. Med. 2015, 9, E144–E151. [Google Scholar] [CrossRef]
- Seiffert, N.; Tang, P.; Keshi, E.; Reutzel-Selke, A.; Moosburner, S.; Everwien, H.; Wulsten, D.; Napierala, H.; Pratschke, J.; Sauer, I.M.; et al. In vitro recellularization of decellularized bovine carotid arteries using human endothelial colony forming cells. J. Biol. Eng. 2021, 15, 15. [Google Scholar] [CrossRef]
- Lee, P.F.; Chau, E.; Cabello, R.; Yeh, A.T.; Sampaio, L.C.; Gobin, A.S.; Taylor, D.A. Inverted orientation improves decellularization of whole porcine hearts. Acta Biomater. 2017, 49, 181–191. [Google Scholar] [CrossRef]
- Boccafoschi, F.; Botta, M.; Fusaro, L.; Copes, F.; Ramella, M.; Cannas, M. Decellularized biological matrices: An interesting approach for cardiovascular tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 1648–1657. [Google Scholar] [CrossRef]
- Schneider, K.H.; Rohringer, S.; Kapeller, B.; Grasl, C.; Kiss, H.; Heber, S.; Walter, I.; Teuschl, A.H.; Podesser, B.K.; Bergmeister, H. Riboflavin-mediated photooxidation to improve the characteristics of decellularized human arterial small diameter vascular grafts. Acta Biomater. 2020, 116, 246–258. [Google Scholar] [CrossRef]
- Guyette, J.P.; Gilpin, S.E.; Charest, J.M.; Tapias, L.F.; Ren, X.; Ott, H.C. Perfusion decellularization of whole organs. Nat. Protoc. 2014, 9, 1451–1468. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Gu, Y.; Cheng, J.; Li, J.; Xu, Z.; Xing, Y.; Wang, C.; Wang, Z. Decellularization, cross-linking and heparin immobilization of porcine carotid arteries for tissue engineering vascular grafts. Cell Tissue Bank. 2019, 20, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Ye, Z.; Fu, M.; Wang, Q.; Wu, H.; Lin, S.; Yin, T.; Hu, T.; Wang, G. Design, Preparation, and Performance of a Novel Bilayer Tissue-Engineered Small-Diameter Vascular Graft. Macromol. Biosci. 2019, 19, e1800189. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.S.; Kochová, P.; Pálek, R.; Rosendorf, J.; Červenková, L.; Dahmen, U.; Liška, V.; Moulisová, V. Decellularization of Porcine Carotid Arteries: From the Vessel to the High-Quality Scaffold in Five Hours. Front. Bioeng. Biotechnol. 2022, 10, 833244. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, J.; Wang, R.; Gu, Y.; Li, J.; Wang, C. Triton X-100 combines with chymotrypsin: A more promising protocol to prepare decellularized porcine carotid arteries. Biomed. Mater. Eng. 2017, 28, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Simsa, R.; Vila, X.M.; Salzer, E.; Teuschl, A.; Jenndahl, L.; Bergh, N.; Fogelstrand, P. Effect of fluid dynamics on decellularization efficacy and mechanical properties of blood vessels. PLoS ONE 2019, 14, e0220743. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, Y.; Ke, Z.; Yang, H.; Lu, C.; Li, Y.; Guo, Y.; Wang, W. History, progress and future challenges of artificial blood vessels: A narrative review. Biomater. Transl. 2022, 3, 81–98. [Google Scholar] [PubMed]
- Matsuda, T.; Ito, S. Surface coating of hydrophilic-hydrophobic block co-polymers on a poly(acrylonitrile) haemodialyser reduces platelet adhesion and its transmembrane stimulation. Biomaterials 1994, 15, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Nie, N.; Tu, Q.; Wang, J.-C.; Chao, F.; Liu, R.; Zhang, Y.; Liu, W. Synthesis of copolymers using dendronized polyethylene glycol and assay of their blood compatibility and antibacterial adhesion activity. Colloids Surf. B Biointerfaces 2012, 97, 226–235. [Google Scholar] [CrossRef]
- Knetsch, M.; Koole, L. VEGF-E enhances endothelialization and inhibits thrombus formation on polymeric surfaces. J. Biomed. Mater. Res. Part A 2009, 93, 77–85. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Zhou, H.; Ou, J.-S.; Liu, Y. The Potential of a New Natural Vessel Source: Decellularized Intercostal Arteries as Sufficiently Long Small-Diameter Vascular Grafts. Bioengineering 2024, 11, 700. https://doi.org/10.3390/bioengineering11070700
Xia Y, Zhou H, Ou J-S, Liu Y. The Potential of a New Natural Vessel Source: Decellularized Intercostal Arteries as Sufficiently Long Small-Diameter Vascular Grafts. Bioengineering. 2024; 11(7):700. https://doi.org/10.3390/bioengineering11070700
Chicago/Turabian StyleXia, Yuan, Haiyun Zhou, Jing-Song Ou, and Yunqi Liu. 2024. "The Potential of a New Natural Vessel Source: Decellularized Intercostal Arteries as Sufficiently Long Small-Diameter Vascular Grafts" Bioengineering 11, no. 7: 700. https://doi.org/10.3390/bioengineering11070700
APA StyleXia, Y., Zhou, H., Ou, J.-S., & Liu, Y. (2024). The Potential of a New Natural Vessel Source: Decellularized Intercostal Arteries as Sufficiently Long Small-Diameter Vascular Grafts. Bioengineering, 11(7), 700. https://doi.org/10.3390/bioengineering11070700







