The Effects of Khat Chewing among Djiboutians: Dental Chemical Studies, Gingival Histopathological Analyses and Bioinformatics Approaches
Abstract
:1. Introduction
2. Material and Methods
2.1. Physicochemical Analysis of the Teeth
2.1.1. Sampling of Human Teeth
2.1.2. Analysis Techniques
2.2. Biological Test of Affected Tissues in the Oral Gums
2.3. Bioinformatic Approach
2.4. Statistical Studies
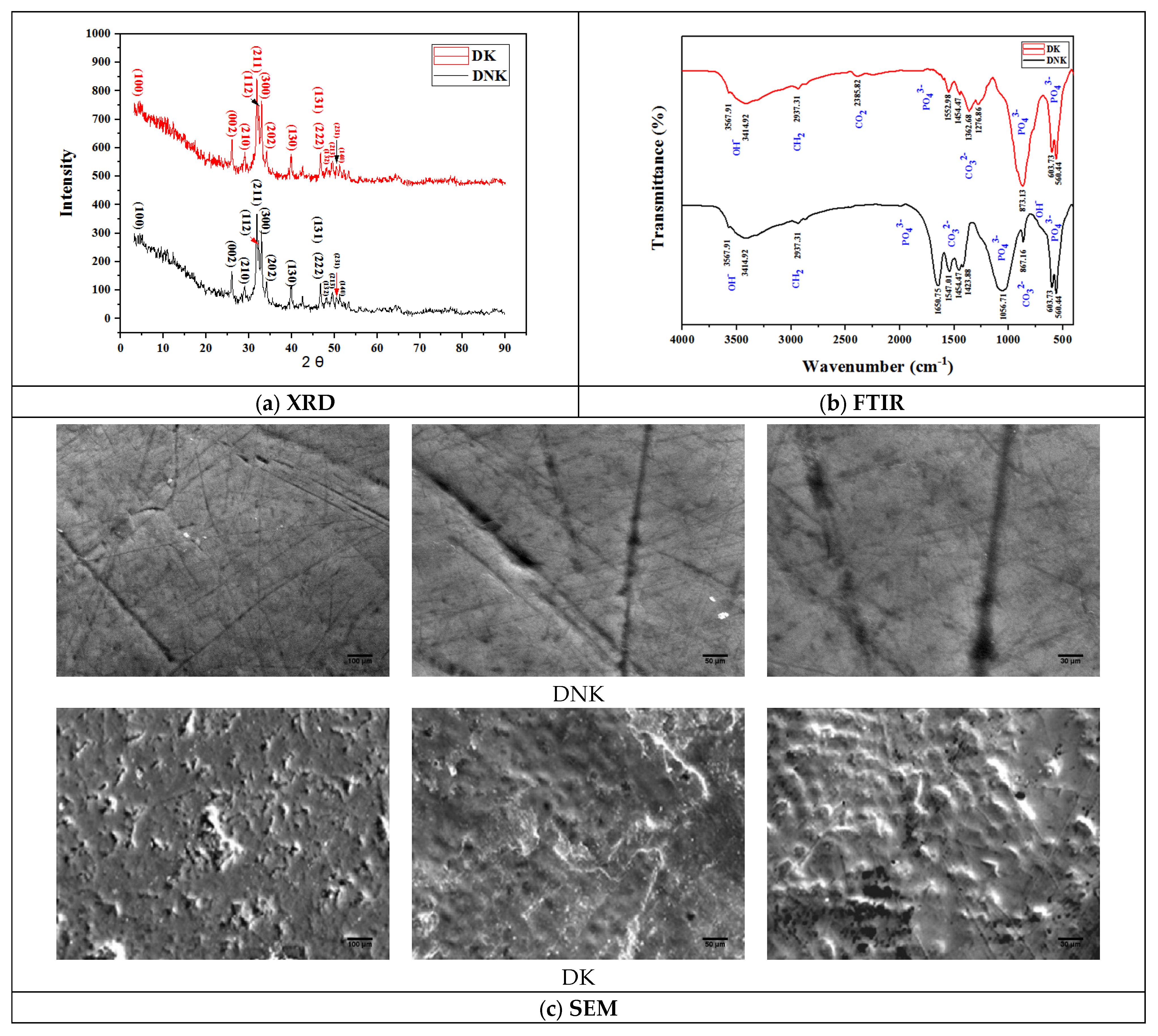
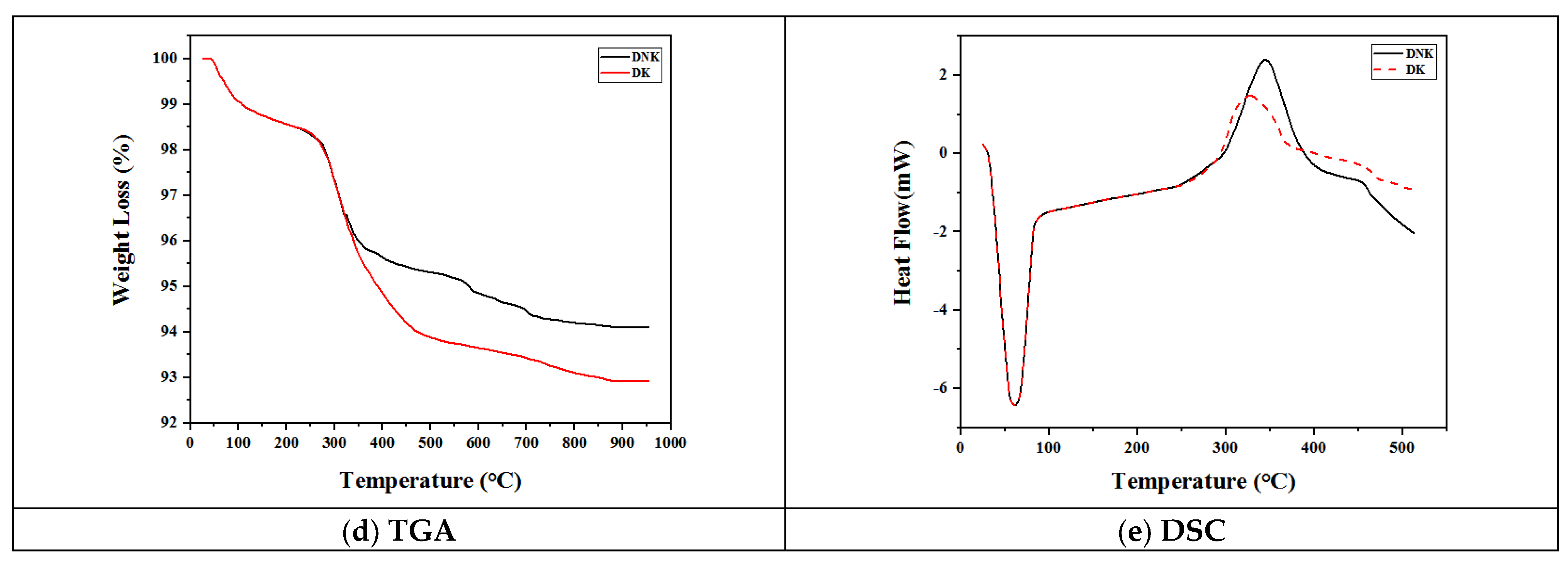
3. Results and Discussion
3.1. Teeth Characterization
3.2. Histological Test of Affected Tissues in the Oral Gums
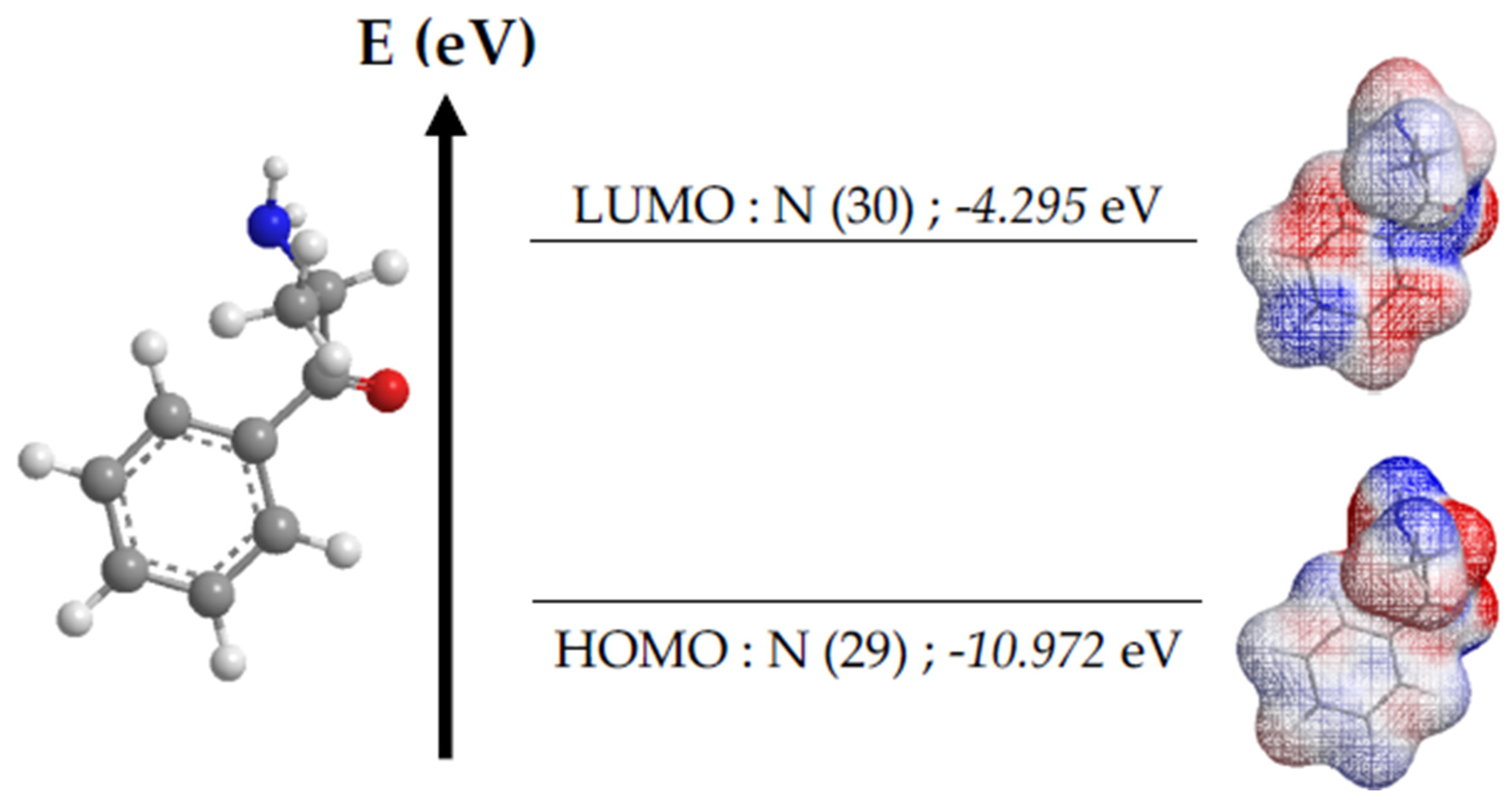
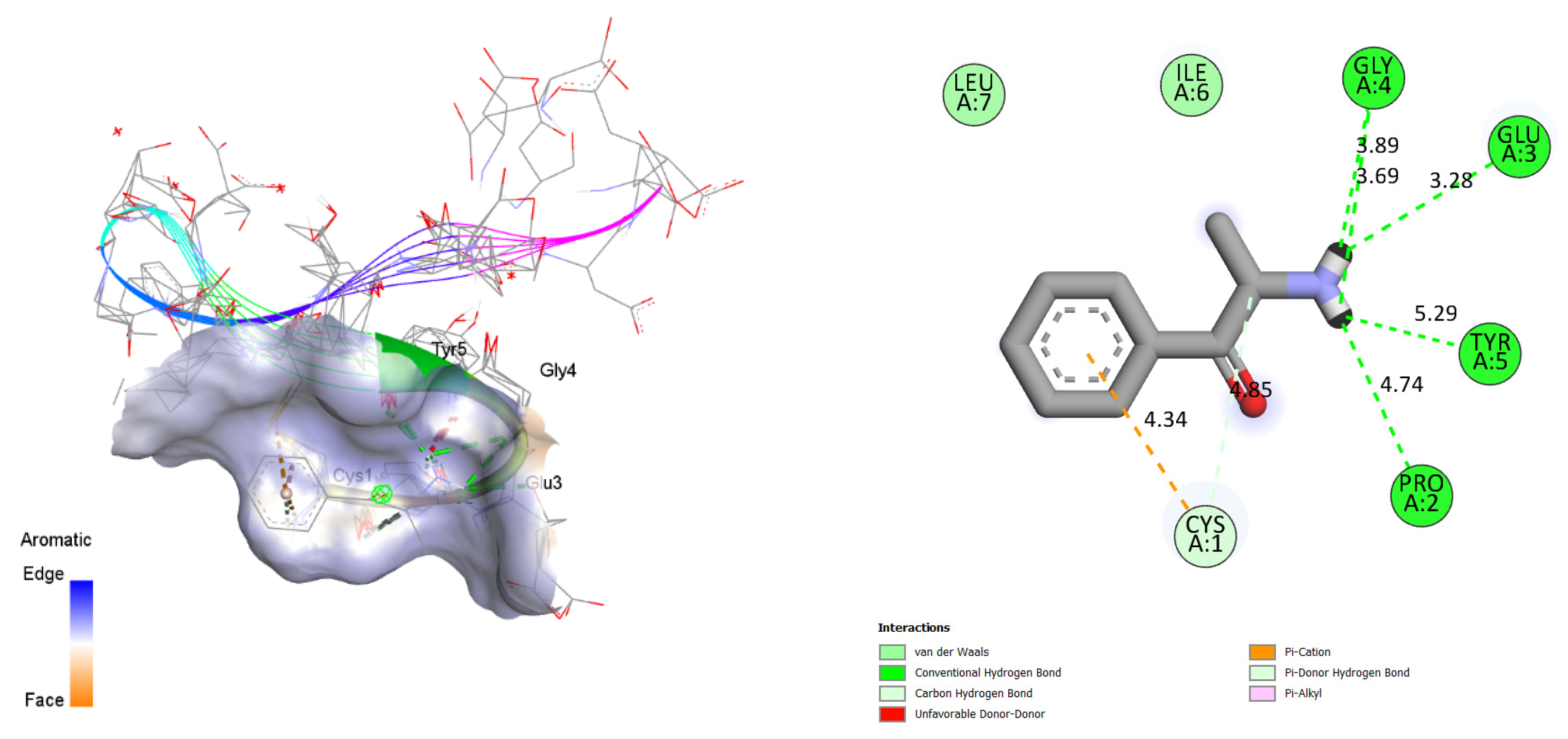
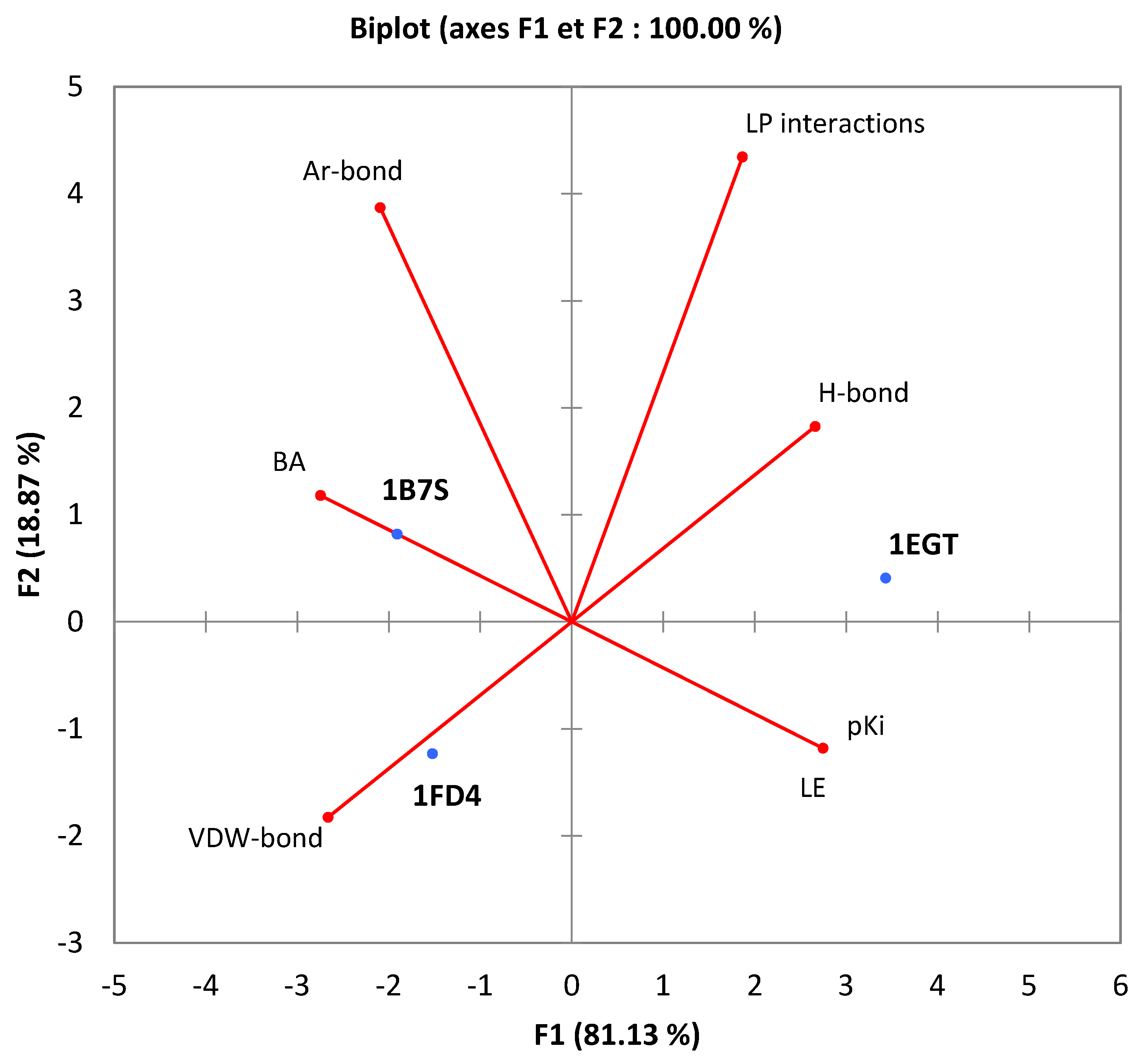
3.3. Bioinformatic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, B.; Soares, J.; Rocha-Pereira, C.; Mladěnka, P.; Remião, F.; OEMONOM Researchers. Khat, a cultural chewing drug: A toxicokinetic and toxicodynamic summary. Toxins 2022, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Latif, F.M.; Ainane, A.; Houmed Aboubaker, I.; Mohamed, J.; Ainane, T. An Overview of Cancer in Djibouti: Current Status, Therapeutic Approaches, and Promising Endeavors in Local Essential Oil Treatment. Pharmaceuticals 2023, 16, 1617. [Google Scholar] [CrossRef] [PubMed]
- Bennani, A.; Mohamed, S. Effect of Khat consumption on oral health: Study carried out in Djibouti City. Oral Health Dent. Sci. 2021, 5, 1–8. [Google Scholar] [CrossRef]
- Ayano, G.; Ayalew, M.; Bedaso, A.; Duko, B. Epidemiology of Khat (Catha edulis) Chewing in Ethiopia: A Systematic Review and meta-analysis. J. Psychoact. Drugs 2022, 40–49. [Google Scholar] [CrossRef]
- Costa, V.M.; Grando, L.G.R.; Milandri, E.; Nardi, J.; Teixeira, P.; Mladěnka, P.; OEMONOM. Natural Sympathomimetic Drugs: From Pharmacology to Toxicology. Biomolecules 2022, 12, 1793. [Google Scholar] [CrossRef] [PubMed]
- Dhabbah, A.M. Determination of chiral cathinone in fresh samples of Catha edulis. Forensic Sci. Int. 2020, 307, 110105. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Merito Ali, A.; El Montassir, Z.; Kciuk, M.; Ainane, T. Chemical Composition of the Essential Oil of Catha edulis Forsk from Djibouti and Its Toxicological Investigations In Vivo and In Vitro. Processes 2023, 11, 1324. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Mohamed, M.M.E.T. A comprehensive bibliometric analysis of Catha edulis (Vahl) Endli (Khat) research (1961–2021). Bull. Natl. Res. Cent. 2022, 46, 279. [Google Scholar] [CrossRef]
- Kithinji, D.; Maina, S.; Ndwigah, S.; Mugo, H.; Oyugi, J. Antimicrobial properties of Catha edulis (Miraa) against selected bacterial and fungal pathogens, an in-vitro experimental study. J. Pharmacogn. Phytochem. 2023, 12, 53–62. [Google Scholar] [CrossRef]
- Edwards, B.; Atkins, N. Exploring the association between khat use and psychiatric symptoms: A systematic review. BMJ Open 2022, 12, 7. [Google Scholar] [CrossRef]
- Middleton, N.; Kashani, S.S.; Attarchi, S.; Rahnama, M.; Mosalman, S.T. Synoptic causes and socio-economic consequences of a severe dust storm in the Middle East. Atmosphere 2021, 12, 1435. [Google Scholar] [CrossRef]
- Ye, S.; Hu, J.; Liu, Z.; Liang, M. Progress and research trends on Catha edulis (Vahl) Endl. (Catha edulis): A review and bibliometric analysis. Front. Pharmacol. 2021, 12, 705376. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.A.; Alsahli, M.A.; Alarifi, F.F.; Hakami, B.O.; Alkeraithe, F.W.; Alhuqbani, M.; Almannie, R. A Narrative Review of the Toxic Effects on Male Reproductive and Sexual Health of Chewing the Psychostimulant, Catha edulis (Khat). Med. Sci. Monit. 2023, 29, e939455-1. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.X.; Ho, W.Y.; Yan, P.; Alshagga, M.A. Evaluation of khat (Catha edulis) use as a risk factor of cancer: A systematic review. Asian Pac. J. Cancer Prev. 2020, 21, 881. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.; Hobani, Y.H.; Mosbah, N.; Abdalla, S.E.; Zaino, M.; Mohan, S.; El-Setouhy, M. Chronic khat (Catha edulis) chewing and genotoxicity: The role of antioxidant defense system and oxidative damage of DNA. Pharmacogn. Mag. 2020, 16, 68. [Google Scholar]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.K.; Mandhan, K.; Dash, D.; Bhardwaj, S.; Kumari, M.; Sharma, N. Density functional theory studies on molecular geometry, spectroscopy, HOMO–LUMO and reactivity descriptors of titanium (IV) and oxidozirconium (IV) complexes of phenylacetohydroxamic acid. J. Comput. Chem. 2022, 43, 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Khemis, I.B.; Sagaama, A.; Issaoui, N.; Lamine, A.B. Steric and energetic characterizations of mouse and human musk receptors activated by nitro musk smelling compounds at molecular level: Statistical physics treatment and molecular docking analysis. Int. J. Biol. Macromol. 2021, 188, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Shaimardanov, A.R.; Shulga, D.A.; Palyulin, V.A. Is an Inductive Effect Explicit Account Required for Atomic Charges Aimed at Use within the Force Fields? J. Phys. Chem. A 2022, 126, 6278–6294. [Google Scholar] [CrossRef]
- Azad, I.; Akhter, Y.; Khan, T.; Azad, M.I.; Chandra, S.; Singh, P.; Nasibullah, M. Synthesis, quantum chemical study, AIM simulation, in silico ADMET profile analysis, molecular docking and antioxidant activity assessment of aminofuran derivatives. J. Mol. Struct. 2020, 1203, 127285. [Google Scholar] [CrossRef]
- Ahmad, S.; Kumar, M.; Garima, K.; Ali, A.; Arora, H.; Muthu, S.; Javed, S. DFT, Molecular Docking, Molecular Dynamics Simulation, and Hirshfeld Surface Analysis of 2-Phenylthioaniline. Polycycl. Aromat. Compd. 2023, 1–23. [Google Scholar] [CrossRef]
- Ramalingam, A.; Guerroudj, A.R.; Sambandam, S.; Kumar, A.; Krishnamoorthy, R.; Boukabcha, N.; Elayaperumal, M. Synthesis, Vibrational Spectra, Hirshfeld Surface Analysis, DFT Calculations, and In Silico ADMET Study of 3-(2-Chloroethyl)-2,6-Bis(4-fluorophenyl)piperidin-4-one: A Potent Anti-Alzheimer Agent. J. Mol. Struct. 2022, 1269, 133845. [Google Scholar] [CrossRef]
- Abdoul-Latif, F.M.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Ainane, T. Essential Oil of Ruta chalepensis L. from Djibouti: Chemical Analysis and Modeling of In Vitro Anticancer Profiling. Separations 2022, 9, 387. [Google Scholar] [CrossRef]
- Ainane, A.; Abdoul-Latif, F.M.; Mohamed, J.; Attahar, W.; Ouassil, M.; Shybat, Z.L.; Ainane, T. Behaviour Desorption Study of the Essential Oil of Cedrus atlantica in a Porous Clay versus Insecticidal Activity against Sitophilus granarius: Explanation of the Phenomenon by Statistical Studies. Int. J. Metrol. Qual. Eng. 2021, 12, 12. [Google Scholar] [CrossRef]
- Hiraishi, N.; Gondo, T.; Shimada, Y.; Hill, R.; Hayashi, F. Crystallographic and Physicochemical Analysis of Bovine and Human Teeth Using X-ray Diffraction and Solid-State Nuclear Magnetic Resonance. J. Funct. Biomater. 2022, 13, 254. [Google Scholar] [CrossRef]
- Mirjalili, F.; Navabazam, A.; Samanizadeh, N. Preparation of Hydroxyapatite Nanoparticles from Natural Teeth. Russ. J. Nondestruct. Test. 2021, 57, 152–162. [Google Scholar] [CrossRef]
- Rahmat, R.A.; Humphries, M.A.; Austin, J.J.; Linacre, A.M.; Raven, M.; Self, P. Integrating Spectrophotometric and XRD Analyses in the Investigation of Burned Dental Remains. Forensic Sci. Int. 2020, 310, 110236. [Google Scholar] [CrossRef] [PubMed]
- Todica, M.; Muresan-Pop, M.; Niculaescu, C.; Constantiniuc, M. XRD and FTIR Investigation of the Structural Changes of the Human Tooth Induced by Citric Acid. Rom. J. Phys. 2020, 65, 706. [Google Scholar]
- Babot-Marquillas, C.; Sánchez-Martín, M.J.; Amigo, J.M.; Yousef, I.; Valido, I.H.; Boada, R.; Valiente, M. Tooth Whitening, Oxidation or Reduction? Study of Physicochemical Alterations in Bovine Enamel Using Synchrotron Based Micro-FTIR. Dent. Mater. 2022, 38, 670–679. [Google Scholar] [CrossRef]
- Loganathan, S.; Santhanakrishnan, S.; Bathe, R.; Arunachalam, M. FTIR and Raman as a Noninvasive Probe for Predicting the Femtosecond Laser Ablation Profile on Heterogeneous Human Teeth. J. Mech. Behav. Biomed. Mater. 2021, 115, 104256. [Google Scholar] [CrossRef]
- Vitiello, F.; Tosco, V.; Monterubbianesi, R.; Orilisi, G.; Gatto, M.L.; Sparabombe, S.; Orsini, G. Remineralization Efficacy of Four Remineralizing Agents on Artificial Enamel Lesions: SEM-EDS Investigation. Materials 2022, 15, 4398. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.M.C.; Delbem, A.C.B.; Gomes, L.F.; Emerenciano, N.G.; dos Passos Silva, M.; Cannon, M.L.; Danelon, M. Combined Effect of Casein Phosphopeptide-Amorphous Calcium Phosphate and Sodium Trimetaphosphate on the Prevention of Enamel Demineralization and Dental Caries: An In Vitro Study. Clin. Oral Investig. 2021, 25, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Ruengrungsom, C.; Burrow, M.F.; Parashos, P.; Palamara, J.E. Evaluation of F, Ca, and P Release and Micro-Hardness of Eleven Ion-Leaching Restorative Materials and the Recharge Efficacy Using a New Ca/P Containing Fluoride Varnish. J. Dent. 2020, 102, 103474. [Google Scholar] [CrossRef]
- Muhammad, N.; Sarfraz, Z.; Zafar, M.S.; Liaqat, S.; Rahim, A.; Ahmad, P.; Khandaker, M.U. Characterization of Various Acrylate Based Artificial Teeth for Denture Fabrication. J. Mater. Sci. Mater. Med. 2022, 33, 17. [Google Scholar] [CrossRef]
- Lozano-Peral, D.; Arango-Díaz, A.; Martín-de-las-Heras, S.; Rubio, L. Thermogravimetric Analysis of Teeth for Forensic Purposes. J. Therm. Anal. Calorim. 2020, 139, 1121–1129. [Google Scholar] [CrossRef]
- Hsu, K.; Yuh, D.Y.; Lin, S.C.; Lyu, P.S.; Pan, G.X.; Zhuang, Y.C.; Juan, C.J. Improving Performance of Deep Learning Models Using 3.5 D U-Net via Majority Voting for Tooth Segmentation on Cone Beam Computed Tomography. Sci. Rep. 2022, 12, 19809. [Google Scholar] [CrossRef]
- Barbuzan-Caragyov, A.; Hajaj, T.; Cojocariu, A.C.; Christa, S.; Sinescu, C. Efficiency of Natural Ingredients in Teeth Whitening. Dent. Mater. 2022, 38, e6–e7. [Google Scholar] [CrossRef]
- Meng, F.H.; Schricker, S.R.; Brantley, W.A.; Mendel, D.A.; Rashid, R.G.; Fields, H.W., Jr.; Alapati, S.B. Differential Scanning Calorimetry (DSC) and Temperature-Modulated DSC Study of Three Mouthguard Materials. Dent. Mater. 2007, 23, 1492–1499. [Google Scholar] [CrossRef]
- Kunin, A.A.; Evdokimova, A.Y.; Moiseeva, N.S. Age-Related Differences of Tooth Enamel Morphochemistry in Health and Dental Caries. EPMA J. 2015, 6, 3. [Google Scholar] [CrossRef]
- Bhadila, G.; Filemban, H.; Wang, X.; Melo, M.A.S.; Arola, D.D.; Tay, F.R.; Xu, H.H. Bioactive Low-Shrinkage-Stress Nanocomposite Suppresses S. mutans Biofilm and Preserves Tooth Dentin Hardness. Acta Biomater. 2020, 114, 146–157. [Google Scholar] [CrossRef]
- Louisy, A.; Rochefort, J.; Plantier, F.; Kervarrec, T.; Quilhot, P.; Agbo Godeau, S.; Samimi, M. “Plasma Cell Gingivitis” Encompasses Multiple Entities: A Retrospective Series of 37 Cases. Eur. J. Dermatol. 2023, 33, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, G.; Panico, R.; Garola, F.; Jara, R.; Villarroel-Dorrego, M.; Martinez, B. Unusual Clinical Presentations of Plasma Cell Mucositis Involving Oral Mucosa: Presentation of 2 Cases and Review of the Literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 136, e92–e108. [Google Scholar] [CrossRef] [PubMed]
- Leuci, S.; Coppola, N.; Adamo, N.; Bizzoca, M.E.; Russo, D.; Spagnuolo, G.; Mignogna, M.D. Clinico-Pathological Profile and Outcomes of 45 Cases of Plasma Cell Gingivitis. J. Clin. Med. 2021, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Capodiferro, S.; Limongelli, L.; Favia, G. Oral and Maxillo-Facial Manifestations of Systemic Diseases: An Overview. Medicina 2021, 57, 271. [Google Scholar] [CrossRef] [PubMed]
- Sciuca, A.M.; Toader, M.P.; Stelea, C.G.; Maftei, G.A.; Ciurcanu, O.E.; Stefanescu, O.M.; Popa, C. Desquamative Gingivitis in the Context of Autoimmune Bullous Dermatoses and Lichen Planus—Challenges in the Diagnosis and Treatment. Diagnostics 2022, 12, 1754. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Singh, D.K.; Pathi, J. Host Response in Periodontology: The Defensive Shield of the Supporting Structures of Teeth. J. Prim. Care Dent. Oral Health 2021, 2, 25–39. [Google Scholar] [CrossRef]
- Gomez-Casado, C.; Sanchez-Solares, J.; Izquierdo, E.; Díaz-Perales, A.; Barber, D.; Escribese, M.M. Oral Mucosa as a Potential Site for Diagnosis and Treatment of Allergic and Autoimmune Diseases. Foods 2021, 10, 970. [Google Scholar] [CrossRef]
- Figueredo, C.M.; Lira-Junior, R.; Love, R.M. T and B Cells in Periodontal Disease: New Functions in a Complex Scenario. Int. J. Mol. Sci. 2019, 20, 3949. [Google Scholar] [CrossRef]
- Challacombe, S.J.; Shirlaw, P.J.; Thornhill, M.H. Immunology of Diseases of the Oral Cavity. In Mucosal Immunology, 1943–1983; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Sherina, N.; de Vries, C.; Kharlamova, N.; Sippl, N.; Jiang, X.; Brynedal, B.; Lundberg, K. Antibodies to a Citrullinated Porphyromonas gingivalis Epitope Are Increased in Early Rheumatoid Arthritis, and Can Be Produced by Gingival Tissue B Cells: Implications for a Bacterial Origin in RA Etiology. Front. Immunol. 2022, 13, 804822. [Google Scholar] [CrossRef]
- Thorat, B.B.; Baburaj, M.D.; Kadam, P.D.; Pmiple, S.K. B Cells in Periodontitis—A Friend or Foe? Acta Sci. Dent. Sci. 2022, 6, 102–107. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, N.; Arce, R.M. Periodontal Inflammation: Integrating Genes and Dysbiosis. Periodontol. 2000 2020, 82, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Kubaib, A.; Afroze, N.N.; Imran, P.M.; Natarajan, P.; Balu, D.; Ansari, A. Insights into the Binding Mechanism of 2,5-Substituted 4-Pyrone Derivatives as Therapeutic Agents for Fused Dimeric Interactions: A Computational Study Using QTAIM, Dynamics and Docking Simulations of Protein–Ligand Complexes. Int. J. Quantum Chem. 2024, 124, e27330. [Google Scholar] [CrossRef]
- Adindu, E.A.; Godfrey, O.C.; Agwupuye, E.I.; Ekpong, B.O.; Agurokpon, D.C.; Ogbodo, S.E.; Louis, H. Structural Analysis, Reactivity Descriptors (HOMO-LUMO, ELF, NBO), Effect of Polar (DMSO, EtOH, H2O) Solvation, and Libido-Enhancing Potential of Resveratrol by Molecular Docking. Chem. Phys. Impact 2023, 7, 100296. [Google Scholar] [CrossRef]
- Ejaz, S.A.; Farid, A.; Zargar, S.; Channar, P.A.; Aziz, M.; Wani, T.A.; Erben, M.F. Computational and Theoretical Chemistry of Newly Synthesized and Characterized 2,2′-(5,5′-(1,4-Phenylene) bis(1 H-Tetrazole-5,1-Diyl)) Bis-N-Acetamides. BMC Chem. 2023, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, U.K.; Gürsoy, M.; Liukkonen, A.; Suominen, A.L.; Könönen, E. Salivary Human β-Defensin 1-3 and Human α-Defensin-1 Levels in Relation to the Extent of Periodontal Disease and Tooth Loss in the Elderly. J. Clin. Med. 2023, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Hertel, S.; Hannig, C.; Sterzenbach, T. The Abundance of Lysozyme, Lactoferrin and Cystatin S in the Enamel Pellicle of Children—Potential Biomarkers for Caries? Arch. Oral Biol. 2023, 146, 105598. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, J.D.; Roumenina, L.T.; Perrella, G.; Rayes, J. Basic Mechanisms of Hemolysis-Associated Thrombo-Inflammation and Immune Dysregulation. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1349–1361. [Google Scholar] [CrossRef]
- Vieira, R.; de Sousa, K.A.; da Silva, G.S.; Silva, D.H.S.; Castro-Gamboa, I. CHEIC: Chemical Image Classificator. An Intelligent System for Identification of Volatiles Compounds with Potential for Respiratory Diseases Using Deep Learning. Expert Syst. Appl. 2023, 234, 121178. [Google Scholar] [CrossRef]
- Laus, A.; Kumar, A.; Caboni, P.; De Luca, M.A.; Baumann, M.H.; Pieroni, E.; Tocco, G. In Silico Characterization of Ligand–Receptor Interactions for U-47700, N, N-Didesmethyl-U-47700, U-50488 at Mu-and Kappa-Opioid Receptors. Arch. Pharm. 2023, 356, 2300256. [Google Scholar] [CrossRef]
- da Rocha, J.M.; Campos, D.M.D.O.; Esmaile, S.C.; Menezes, G.D.L.; Bezerra, K.S.; da Silva, R.A.; Oliveira, J.I. Quantum Biochemical Analysis of the Binding Interactions Between a Potential Inhibitory Drug and the Ebola Viral Glycoprotein. J. Biomol. Struct. Dyn. 2024, 1–17. [Google Scholar] [CrossRef]
- Odey, M.O.; Antai, E.E.; Adindu, E.A.; Godfrey, O.C.; Bassey, I.U.; Nwaokolrie, F.O.; Louis, H. Unraveling the Impact of Polar Solvation on the Molecular Geometry, Spectroscopy (FT-IR, UV, NMR), Reactivity (ELF, NBO, HOMO-LUMO) and Antiviral Inhibitory Potential of Cissampeline by Molecular Docking Approach. Chem. Phys. Impact 2023, 7, 100346. [Google Scholar] [CrossRef]
- Sharma, N.; Vuppu, S. Computational Modelling and Molecular Docking of Industrial Leather Enzymes. Mol. Biotechnol. 2023, 1–19. [Google Scholar] [CrossRef]





| Descriptors | Symbol | Equation |
|---|---|---|
| Energy gap | EGAP | |
| Chemical hardness | η | |
| Electronegativity | χ | |
| Electrophilicity index | ω | |
| Molecular flexibility | S |
| Protein | 1FD4 | 1B7S | 1EGT |
|---|---|---|---|
| Size (Å) | x = 40; y = 40; z = 40 | x = 40; y = 40; z = 40 | x = 40; y = 40; z = 40 |
| Center (Å) | x = 7.675 | x = 13.157 | x = 0.199 |
| y = 21.015 | y = 14.900 | y = 0.148 | |
| z = 18.444 | z = 28.924 | z = −0.114 |
| Teeth | Calcium (%) | Phosphorus (%) | Ca/P Ratio | Microhardness (HV) |
|---|---|---|---|---|
| DK | 17.45 ± 2.54 | 6.32 ± 1.01 | 2.76 ± 0.11 | 52.16 ± 5.64 |
| DNK | 32.87 ± 1.74 | 15.09 ± 1.36 | 2.17 ± 0.16 | 244.68 ± 5.79 |
| EGAP | η | χ | ω | S |
|---|---|---|---|---|
| 6.677 | 3.338 | 7.634 | 8.729 | 0.149 |
| Proteins | Binding Affinity (kcal.mol−1) | pKi | Ligand Efficiency (kcal.mol−1) | Ligand–Protein Interactions | Number of Conventional Hydrogen Bonds | Number of Aromatic Bonds | Number of Van Der Waals Bonds |
|---|---|---|---|---|---|---|---|
| 1FD4 | −5.7 | 4.18 | 0.5182 | 4 (Leu9—Pro17—Val18—Lys10) | 1 | 1 | 5 |
| 1B7S | −5.6 | 4.11 | 0.5091 | 5 (GLN58—TRP64—Val99—ALA108—TRP109) | 1 | 4 | 5 |
| 1EGT | −9.7 | 7.11 | 0.8818 | 6 (GLY4—GLY4—GLU3—TYR5—PRO2—CYS1) | 5 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Abdoul-Latif, F.; Ainane, A.; Merito, A.; Houmed Aboubaker, I.; Mohamed, H.; Cherroud, S.; Ainane, T. The Effects of Khat Chewing among Djiboutians: Dental Chemical Studies, Gingival Histopathological Analyses and Bioinformatics Approaches. Bioengineering 2024, 11, 716. https://doi.org/10.3390/bioengineering11070716
Mohamed Abdoul-Latif F, Ainane A, Merito A, Houmed Aboubaker I, Mohamed H, Cherroud S, Ainane T. The Effects of Khat Chewing among Djiboutians: Dental Chemical Studies, Gingival Histopathological Analyses and Bioinformatics Approaches. Bioengineering. 2024; 11(7):716. https://doi.org/10.3390/bioengineering11070716
Chicago/Turabian StyleMohamed Abdoul-Latif, Fatouma, Ayoub Ainane, Ali Merito, Ibrahim Houmed Aboubaker, Houda Mohamed, Sanaa Cherroud, and Tarik Ainane. 2024. "The Effects of Khat Chewing among Djiboutians: Dental Chemical Studies, Gingival Histopathological Analyses and Bioinformatics Approaches" Bioengineering 11, no. 7: 716. https://doi.org/10.3390/bioengineering11070716






