Characterization of Cochlear Implant Artifact and Removal Based on Multi-Channel Wiener Filter in Unilateral Child Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Population
2.2. Protocol
2.3. Data Acquisition and Preprocessing
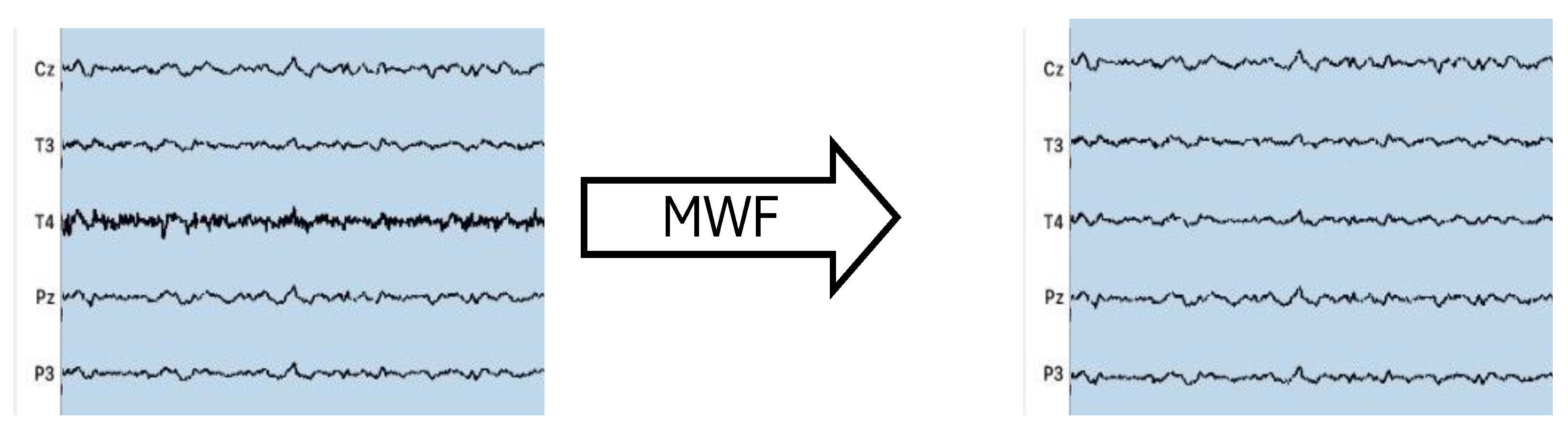
2.4. CI Artifact Removal
2.5. Similarity Evaluation
2.6. Neurophysiological Evaluation
3. Results
3.1. CI Artifact Characterization
3.2. CI Artifact Reduction
3.3. Neurophysiological Results
4. Discussion
Limitations and Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Stadio, A.; De Luca, P.; Ippolito, V.; Vedova, P.; Garofalo, S.; Turchetta, R.; Ferlito, S.; Della Volpe, A. Comparative Analysis of Intellectual Quotient in Developmental Population with Severe Hearing Loss: Hearing Aids vs. Cochlear Implant Users. Life 2023, 14, 12. [Google Scholar] [CrossRef]
- Intartaglia, B.; Zeitnouni, A.G.; Lehmann, A. Recording EEG in Cochlear Implant Users: Guidelines for Experimental Design and Data Analysis for Optimizing Signal Quality and Minimizing Artifacts. J. Neurosci. Methods 2022, 375, 109592. [Google Scholar] [CrossRef]
- Nicastri, M.; Dincer D’Alessandro, H.; Baccolini, V.; Migliara, G.; Sciurti, A.; De Vito, C.; Ranucci, L.; Giallini, I.; Greco, A.; Mancini, P. Executive Functions in Preschool and School-Age Cochlear Implant Users: Do They Differ from Their Hearing Peers? A Systematic Review and Meta-Analysis. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 579–600. [Google Scholar] [CrossRef]
- Soncini, A.; Franzini, S.; Di Marco, F.; Riccardi, P.; Bacciu, A.; Pasanisi, E.; Di Lella, F. Early Fitting in Cochlear Implant Surgery: Preliminary Results. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 61–66. [Google Scholar] [CrossRef]
- Geers, A.; Uchanski, R.; Brenner, C.; Tye-Murray, N.; Nicholas, J.; Tobey, E. Rehabilitation Factors Contributing to Implant Benefit in Children. Ann. Otol. Rhinol. Laryngol. 2002, 111, 127–130. [Google Scholar] [CrossRef]
- Sharma, A.; Nash, A.A.; Dorman, M. Cortical Development, Plasticity and Re-Organization in Children with Cochlear Implants. J. Commun. Disord. 2009, 42, 272–279. [Google Scholar] [CrossRef]
- Bauer, P.W.; Sharma, A.; Martin, K.; Dorman, M. Central Auditory Development in Children with Bilateral Cochlear Implants. Arch. Otolaryngol.-Head Neck Surg. 2006, 132, 1133–1136. [Google Scholar] [CrossRef]
- Gilley, P.M.; Sharma, A.; Dorman, M.F. Cortical Reorganization in Children with Cochlear Implants. Brain Res. 2008, 1239, 56–65. [Google Scholar] [CrossRef]
- Finke, M.; Billinger, M.; Büchner, A. Toward Automated Cochlear Implant Fitting Procedures Based on Event-Related Potentials. Ear Hear. 2017, 38, e118–e127. [Google Scholar] [CrossRef]
- Sandmann, P.; Plotz, K.; Hauthal, N.; Vos, M.; Schönfeld, R.; Debener, S. Rapid Bilateral Improvement in Auditory Cortex Activity in Postlingually Deafened Adults Following Cochlear Implantation. Clin. Neurophysiol. 2015, 126, 594–607. [Google Scholar] [CrossRef]
- Sandmann, P.; Eichele, T.; Buechler, M.; Debener, S.; Jäncke, L.; Dillier, N.; Hugdahl, K.; Meyer, M. Evaluation of Evoked Potentials to Dyadic Tones after Cochlear Implantation. Brain 2009, 132, 1967–1979. [Google Scholar] [CrossRef]
- Cartocci, G.; Maglione, A.G.; Rossi, D. Alpha and Theta EEG Variations as Indices of Listening Effort to Be Implemented in Neurofeedback Among Cochlear Implant Users. In Symbiotic Interaction. Lecture Notes in Computer Science; Ham, J., Spagnolli, A., Blankertz, B., Gamberini, L., Jacucci, G., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; Volume 2018, pp. 30–41. [Google Scholar]
- Marsella, P.; Scorpecci, A.; Cartocci, G. EEG Activity as an Objective Measure of Cognitive Load during Effortful Listening: A Study on Pediatric Subjects with Bilateral, Asymmetric Sensorineural Hearing Loss. Int. J. Pediatr. Otorhinolaryngol. 2017, 99, 1–7. [Google Scholar] [CrossRef]
- Cartocci, G.; Inguscio, B.M.S.; Giorgi, A.; Vozzi, A.; Leone, C.A.; Grassia, R.; Di Nardo, W.; Di Cesare, T.; Fetoni, A.R.; Freni, F.; et al. Music in Noise Recognition: An EEG Study of Listening Effort in Cochlear Implant Users and Normal Hearing Controls. PLoS ONE 2023, 18, e0288461. [Google Scholar] [CrossRef]
- Mason, S. Electrophysiologic and Objective Monitoring of the Cochlear Implant during Surgery: Implementation, Audit and Outcomes. Int. J. Audiol. 2004, 43, S33–S38. [Google Scholar]
- Debener, S.; Hine, J.; Bleeck, S.; Eyles, J. Source Localization of Auditory Evoked Potentials after Cochlear Implantation. Psychophysiology 2008, 45, 20–24. [Google Scholar] [CrossRef]
- Hofmann, M.; Wouters, J. Electrically Evoked Auditory Steady State Responses in Cochlear Implant Users. J. Assoc. Res. Otolaryngol. 2010, 11, 267–282. [Google Scholar] [CrossRef]
- Martin, B.A. Can the Acoustic Change Complex Be Recorded in an Individual with a Cochlear Implant? Separating Neural Responses from Cochlear Implant Artifact. J. Am. Acad. Audiol. 2007, 18, 126–140. [Google Scholar] [CrossRef]
- Presacco, A.; Innes-Brown, H.; Goupell, M.J.; Anderson, S. Effects of Stimulus Duration on Event-Related Potentials Recorded from Cochlear-Implant Users. Ear. Hear. 2017, 38, e389–e393. [Google Scholar] [CrossRef]
- Somers, B.; Verschueren, E.; Francart, T. Neural Tracking of the Speech Envelope in Cochlear Implant Users. J. Neural. Eng. 2019, 16, 016003. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Khan, M.S.; Mi, F. A Self-Adaptive Frequency Selection Common Spatial Pattern and Least Squares Twin Support Vector Machine for Motor Imagery Electroencephalography Recognition. Biomed. Signal Process. Control 2018, 41, 222–232. [Google Scholar] [CrossRef]
- Gratton, G.; Coles, M.G.; Donchin, E. A New Method for Off-Line Removal of Ocular Artifact. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 468–484. [Google Scholar] [CrossRef]
- Unser, M.; Aldroubi, A. A Review of Wavelets in Biomedical Applications. Proc. IEEE 1996, 84, 626–638. [Google Scholar] [CrossRef]
- Deprez, H.; Gransier, R.; Hofmann, M.; van Wieringen, A.; Wouters, J.; Moonen, M. Independent Component Analysis for Cochlear Implant Artifacts Attenuation from Electrically Evoked Auditory Steady-State Response Measurements. J. Neural Eng. 2017, 15, 016006. [Google Scholar] [CrossRef]
- Urigüen, J.A. EEG Artifact Removal—State-of-the-Art and Guidelines. J. Neural Eng. 2015, 12, 031001. [Google Scholar] [CrossRef]
- Viola, F.C.; Thorne, J.D.; Bleeck, S.; Eyles, J.; Debener, S. Uncovering Auditory Evoked Potentials from Cochlear Implant Users with Independent Component Analysis. Psychophysiology 2011, 48, 1470–1480. [Google Scholar] [CrossRef]
- James, C.J.; Hesse, C.W. Independent Component Analysis for Biomedical Signals. Physiol. Meas. 2004, 26, R15. [Google Scholar] [CrossRef]
- Mammone, N. Preprocessing the EEG of Alzheimer’s Patients to Automatically Remove Artifacts. Multidiscip. Approaches Neural Comput. 2018, 279–287. [Google Scholar] [CrossRef]
- Gabard-Durnam, L.J.; Mendez Leal, A.S.; Wilkinson, C.L.; Levin, A.R. The Harvard Automated Processing Pipeline for Electroencephalography (HAPPE): Standardized Processing Software for Developmental and High-Artifact Data. Front. Neurosci. 2018, 12, 316496. [Google Scholar] [CrossRef]
- Kanoga, S.; Nakanishi, M.; Mitsukura, Y. Assessing the Effects of Voluntary and Involuntary Eyeblinks in Independent Components of Electroencephalogram. Neurocomputing 2016, 193, 20–32. [Google Scholar] [CrossRef]
- Sweeney, K.T.; McLoone, S.F. Artifact Removal in Physiological Signals—Practices and Possibilities. Ieee Trans. Inf. Technol. Biomed. 2012, 16, 488–500. [Google Scholar] [CrossRef]
- Narmada, A.; Shukla, M. A Novel Adaptive Artifacts Wavelet Denoising for EEG Artifacts Removal Using Deep Learning with Meta-Heuristic Approach. Multimed. Tools Appl. 2023, 82, 40403–40441. [Google Scholar] [CrossRef]
- Stalin, S.; Roy, V.; Shukla, P.K.; Zaguia, A.; Khan, M.M.; Shukla, P.K.; Jain, A. A Machine Learning-Based Big EEG Data Artifact Detection and Wavelet-Based Removal: An Empirical Approach. Math. Probl. Eng. 2021, 2021, 2942808. [Google Scholar] [CrossRef]
- Somers, B.; Long, C.; Francart, T. EEG-Based Diagnostics of the Auditory System Using Cochlear Implant Electrodes as Sensors. Sci. Rep. 2021, 11, 5383. [Google Scholar] [CrossRef]
- Koirala, N.; Deroche, M.L.; Wolfe, J.; Neumann, S.; Bien, A.G.; Doan, D.; Goldbeck, M.; Muthuraman, M.; Gracco, V.L. Dynamic Networks Differentiate the Language Ability of Children with Cochlear Implants. Front. Neurosci. 2023, 17, 1141886. [Google Scholar] [CrossRef]
- Somers, B.; Francart, T.; Bertrand, A. A Generic EEG Artifact Removal Algorithm Based on the Multi-Channel Wiener Filter. J. Neural Eng. 2018, 15, 036007. [Google Scholar] [CrossRef]
- Germano, D.; Sciaraffa, N.; Ronca, V.; Giorgi, A.; Trulli, G.; Borghini, G.; Di Flumeri, G.; Babiloni, F.; Aricò, P. Unsupervised Detection of Covariate Shift Due to Changes in EEG Headset Position: Towards an Effective Out-of-Lab Use of Passive Brain–Computer Interface. Appl. Sci. 2023, 13, 12800. [Google Scholar] [CrossRef]
- Ranjan, R.; Sahana, B.C.; Bhandari, A.K. Ocular Artifact Elimination from Electroencephalography Signals: A Systematic Review. Biocybern. Biomed. Eng. 2021, 41, 960–996. [Google Scholar] [CrossRef]
- Sauter, D.A.; Eisner, F.; Calder, A.J.; Scott, S.K. Perceptual Cues in Nonverbal Vocal Expressions of Emotion. Q. J. Exp. Psychol. 2010, 63, 2251–2272. [Google Scholar] [CrossRef]
- Sauter, D.A.; Eisner, F.; Ekman, P.; Scott, S.K. Cross-Cultural Recognition of Basic Emotions through Nonverbal Emotional Vocalizations. Proc. Natl. Acad. Sci. USA 2010, 107, 2408–2412. [Google Scholar] [CrossRef]
- Sauter, D.A.; Panattoni, C.; Happé, F. Children’s Recognition of Emotions from Vocal Cues. Br. J. Dev. Psychol. 2013, 31, 97–113. [Google Scholar] [CrossRef]
- Mc Laughlin, M.; Valdes, A.L.; Reilly, R.B.; Zeng, F.-G. Cochlear Implant Artifact Attenuation in Late Auditory Evoked Potentials: A Single Channel Approach. Hear. Res. 2013, 302, 84–95. [Google Scholar] [CrossRef]
- Cartocci, G. The Influence of Different Cochlear Implant Features Use on the Mental Workload Index during a Word in Noise Recognition Task. Int. J. Bioelectromagn. 2016, 18, 60–66. [Google Scholar]
- Gainotti, G. Emotions and the Right Hemisphere: Can New Data Clarify Old Models? Neuroscientist 2019, 25, 258–270. [Google Scholar] [CrossRef]
- Balconi, M.; Lucchiari, C. Consciousness and Arousal Effects on Emotional Face Processing as Revealed by Brain Oscillations. A Gamma Band Analysis. Int. J. Psychophysiol. 2008, 67, 41–46. [Google Scholar] [CrossRef]
- Cartocci, G.; Maglione, A.G.; Vecchiato, G. Mental Workload Estimations in Unilateral Deafened Children. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 5 November 2015; IEEE: New York, NY, USA, 2015; pp. 1654–1657. [Google Scholar]
- Cartocci, G.; Giorgi, A.; Inguscio, B.M.S. Higher Right Hemisphere Gamma Band Lateralization and Suggestion of a Sensitive Period for Vocal Auditory Emotional Stimuli Recognition in Unilateral Cochlear Implant Children: An EEG Study. Front. Neurosci. 2021, 15, 608156. [Google Scholar] [CrossRef]
- Li, X.; Nie, K.; Karp, F.; Tremblay, K.L.; Rubinstein, J.T. Characteristics of Stimulus Artifacts in EEG Recordings Induced by Electrical Stimulation of Cochlear Implants. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics, Yantai, China, 16–18 October 2010; IEEE: New York, NY, USA, 2010; Volume 2, pp. 799–803. [Google Scholar]
- Wagner, L.; Maurits, N.; Maat, B.; Başkent, D.; Wagner, A.E. The Cochlear Implant EEG Artifact Recorded from an Artificial Brain for Complex Acoustic Stimuli. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 392–399. [Google Scholar] [CrossRef]
- Cartocci, G.; Inguscio, B.M.S.; Giliberto, G. Listening Effort in Tinnitus: A Pilot Study Employing a Light EEG Headset and Skin Conductance Assessment during the Listening to a Continuous Speech Stimulus under Different SNR Conditions. Brain Sci. 2023, 13, 1084. [Google Scholar] [CrossRef]
- Cartocci, G.; Scorpecci, A.; Borghini, G. EEG Rhythms Lateralization Patterns in Children with Unilateral Hearing Loss Are Different from the Patterns of Normal Hearing Controls during Speech-in-Noise Listening. Hear. Res. 2019, 379, 31–42. [Google Scholar] [CrossRef]
- Inguscio, B.M.S.; Mancini, P.; Greco, A.; Nicastri, M.; Giallini, I.; Leone, C.A.; Grassia, R.; Di Nardo, W.; Di Cesare, T.; Rossi, F.; et al. ‘Musical Effort’and ‘Musical Pleasantness’: A Pilot Study on the Neurophysiological Correlates of Classical Music Listening in Adults Normal Hearing and Unilateral Cochlear Implant Users. Hear. Balance Commun. 2022, 20, 79–88. [Google Scholar] [CrossRef]
- Henry, F.; Glavin, M.; Jones, E. Noise Reduction in Cochlear Implant Signal Processing: A Review and Recent Developments. IEEE Rev. Biomed. Eng. 2023, 16, 319–331. [Google Scholar] [CrossRef]
- Sciaraffa, N.; Di Flumeri, G.; Germano, D.; Giorgi, A.; Di Florio, A.; Borghini, G.; Vozzi, A.; Ronca, V.; Varga, R.; van Gasteren, M.; et al. Validation of a Light EEG-Based Measure for Real-Time Stress Monitoring during Realistic Driving. Brain Sci. 2022, 12, 304. [Google Scholar] [CrossRef]
- Borghini, G.; Di Flumeri, G.; Aricò, P.; Sciaraffa, N.; Bonelli, S.; Ragosta, M.; Tomasello, P.; Drogoul, F.; Turhan, U.; Acikel, B.; et al. A Multimodal and Signals Fusion Approach for Assessing the Impact of Stressful Events on Air Traffic Controllers. Sci. Rep. 2020, 10, 8600. [Google Scholar] [CrossRef]
- Giorgi, A.; Ronca, V.; Vozzi, A.; Sciaraffa, N.; Di Florio, A.; Tamborra, L.; Simonetti, I.; Aricò, P.; Di Flumeri, G.; Rossi, D.; et al. Wearable Technologies for Mental Workload, Stress, and Emotional State Assessment during Working-like Tasks: A Comparison with Laboratory Technologies. Sensors 2021, 21, 2332. [Google Scholar] [CrossRef]
- Inguscio, B.M.S.; Cartocci, G.; Sciaraffa, N.; Nasta, C.; Giorgi, A.; Nicastri, M.; Giallini, I.; Greco, A.; Babiloni, F.; Mancini, P. Neurophysiological Verbal Working Memory Patterns in Children: Searching for a Benchmark of Modality Differences in Audio/Video Stimuli Processing. Comput. Intell. Neurosci. 2021, 2021, 4158580. [Google Scholar] [CrossRef]
- Giorgi, A.; Ronca, V.; Vozzi, A.; Aricò, P.; Borghini, G.; Capotorto, R.; Tamborra, L.; Simonetti, I.; Sportiello, S.; Petrelli, M.; et al. Neurophysiological Mental Fatigue Assessment for Developing User-Centered Artificial Intelligence as a Solution for Autonomous Driving. Front. Neurorobot. 2023, 17, 1240933. [Google Scholar] [CrossRef]
- Di Flumeri, G.; Giorgi, A.; Germano, D.; Ronca, V.; Vozzi, A.; Borghini, G.; Tamborra, L.; Simonetti, I.; Capotorto, R.; Ferrara, S.; et al. A Neuroergonomic Approach Fostered by Wearable EEG for the Multimodal Assessment of Drivers Trainees. Sensors 2023, 23, 8389. [Google Scholar] [CrossRef]
- Ronca, V.; Brambati, F.; Napoletano, L.; Marx, C.; Trösterer, S.; Vozzi, A.; Aricò, P.; Giorgi, A.; Capotorto, R.; Borghini, G.; et al. A Novel EEG-Based Assessment of Distraction in Simulated Driving under Different Road and Traffic Conditions. Brain Sci. 2024, 14, 193. [Google Scholar] [CrossRef]
- Inguscio, B.M.S.; Cartocci, G.; Sciaraffa, N.; Nicastri, M.; Giallini, I.; Aricò, P.; Greco, A.; Babiloni, F.; Mancini, P. Two Are Better than One: Differences in Cortical EEG Patterns during Auditory and Visual Verbal Working Memory Processing between Unilateral and Bilateral Cochlear Implanted Children. Hear. Res. 2024, 446, 109007. [Google Scholar] [CrossRef]
- Inguscio, B.M.S.; Cartocci, G.; Sciaraffa, N.; Nicastri, M.; Giallini, I.; Greco, A.; Babiloni, F.; Mancini, P. Gamma-Band Modulation in Parietal Area as the Electroencephalographic Signature for Performance in Auditory–Verbal Working Memory: An Exploratory Pilot Study in Hearing and Unilateral Cochlear Implant Children. Brain Sci. 2022, 12, 1291. [Google Scholar] [CrossRef]
- Vozzi, A.; Martinez Levy, A.; Ronca, V.; Giorgi, A.; Ferrara, S.; Mancini, M.; Capotorto, R.; Cherubino, P.; Trettel, A.; Babiloni, F.; et al. Time-Dependent Analysis of Human Neurophysiological Activities during an Ecological Olfactory Experience. Brain Sci. 2023, 13, 1242. [Google Scholar] [CrossRef]
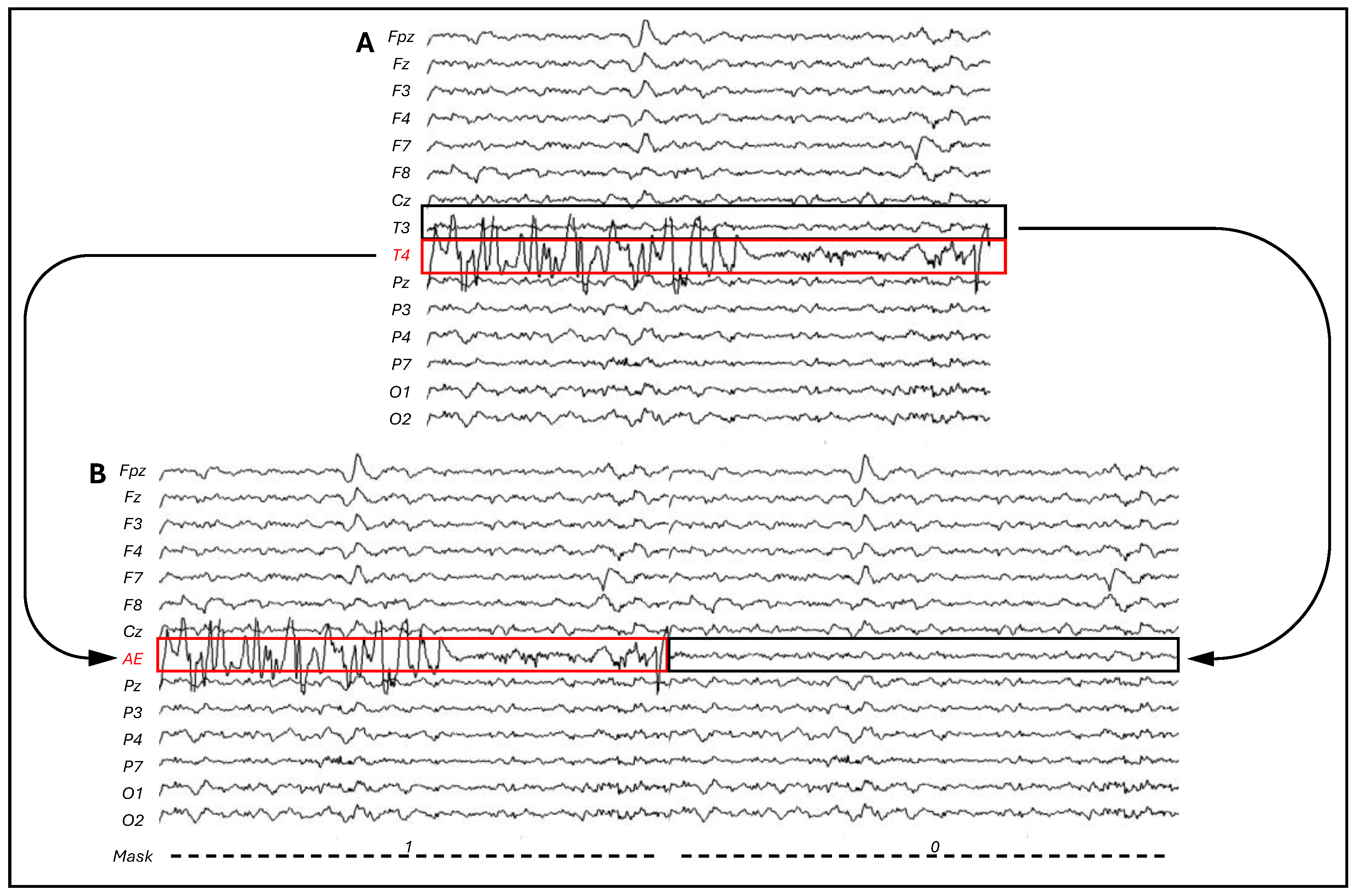
| UCI Patient | CI Side | Removed Electrode(s) | CI Contaminated Electrode | Contralateral Electrode |
|---|---|---|---|---|
| P1 | Right | P8 | T4 | T3 |
| P2 | Right | T4, p4 | P8 | P7 |
| P3 | Right | F8, P8 | T4 | T3 |
| P4 | Left | Fpz, P7 | T3 | T4 |
| P5 | Left | FZ, P7 | T3 | T4 |
| P6 | Right | P8 | T4 | T3 |
| P7 | Right | P4, P8 | T4 | T3 |
| P8 | Right | P8 | T4 | T3 |
| P9 | Right | P4, P8 | T4 | T3 |
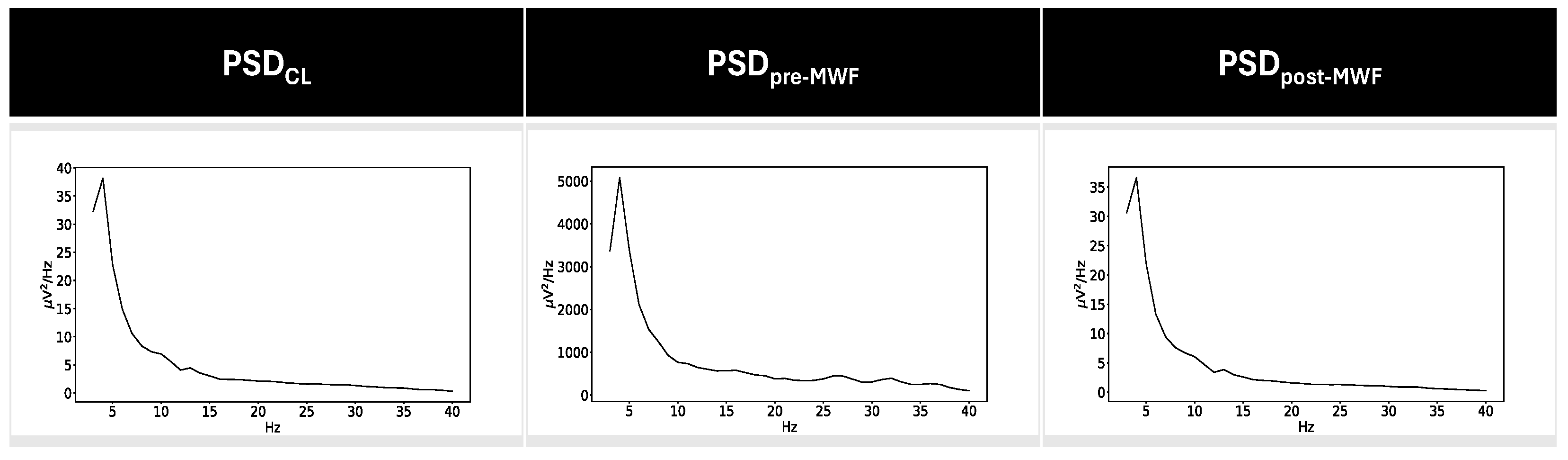
| PSDx | PSDy | RMSE Label |
|---|---|---|
| PSDpre-WMF | PSDCL | RMSEpre-CL |
| PSDpre-WMF | PSDartifact | RMSEpre-artifact |
| PSDpre-WMF | PSDpost-MWF | RMSEpre-post |
| PSDpost-MWF | PSDCL | RMSEpost-CL |
| PSDpost-MWF | PSDartifact | RMSEpost-artifact |
| PSDCL | PSDartifact | RMSECL-artifact |
| Band | Conditions | Groups | Mean (SE) |
|---|---|---|---|
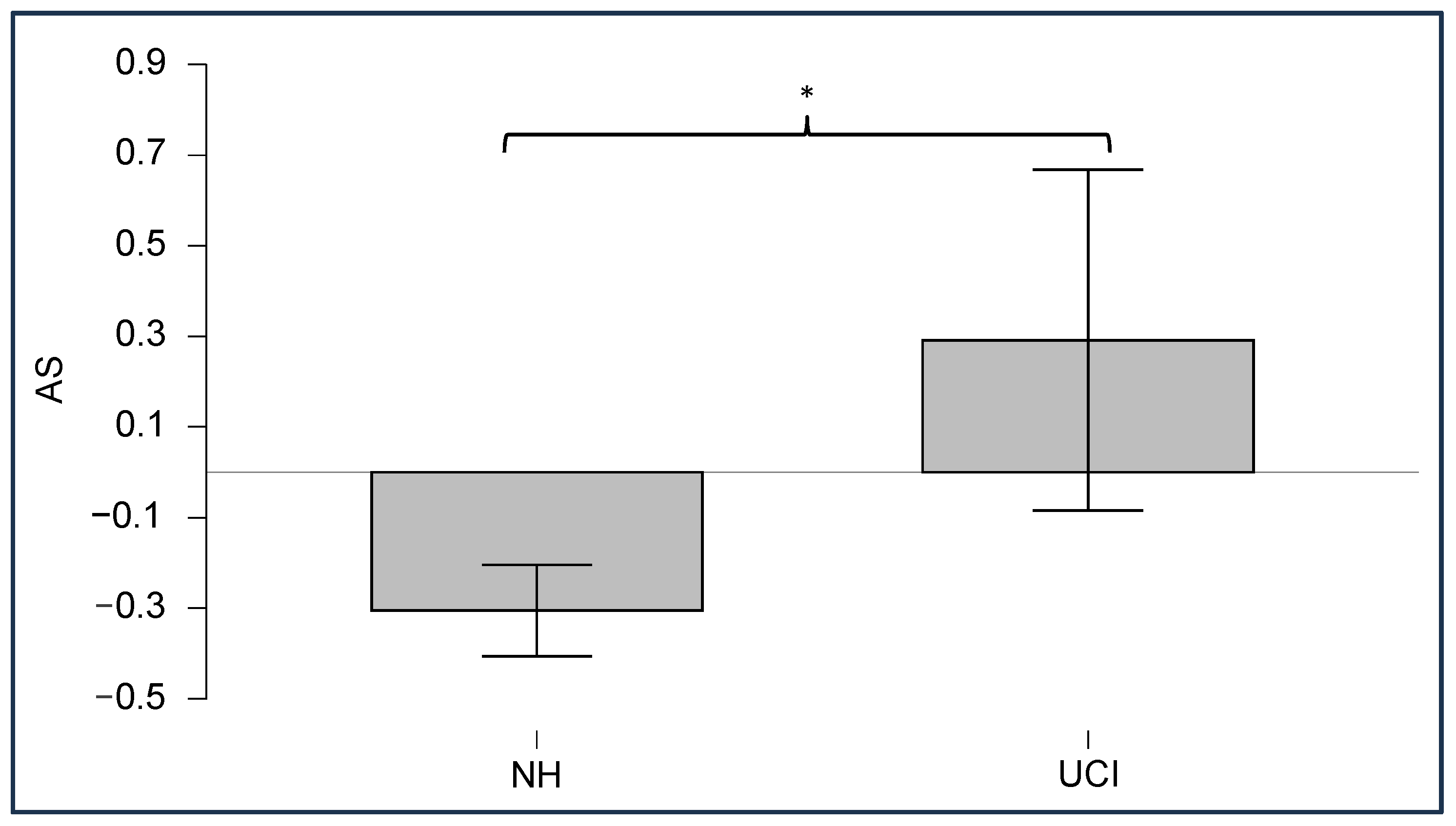
| Theta | Rest * | NH UCI | 3.66 (0.75) 2041.97 (1415.53) |
| Sound * | NH UCI | 9.84 (3.39) 3188.16 (1947.68) | |
| Alpha | Rest * | NH UCI | 2.09 (0.31) 765.37 (556.76) |
| Sound * | NH UCI | 3.33 (1.15) 1351.36 (739.09) | |
| Beta | Rest * | NH UCI | 1.16 (0.28) 442.27 (345.20) |
| Sound * | NH UCI | 1.98 (0.76) 618.42 (415.89 | |
| Low Gamma | Rest * | NH UCI | 0.41 (0.17) 247.42 (201.24) |
| Sound * | NH UCI | 0.85 (0.36) 342.82 (219.45) |
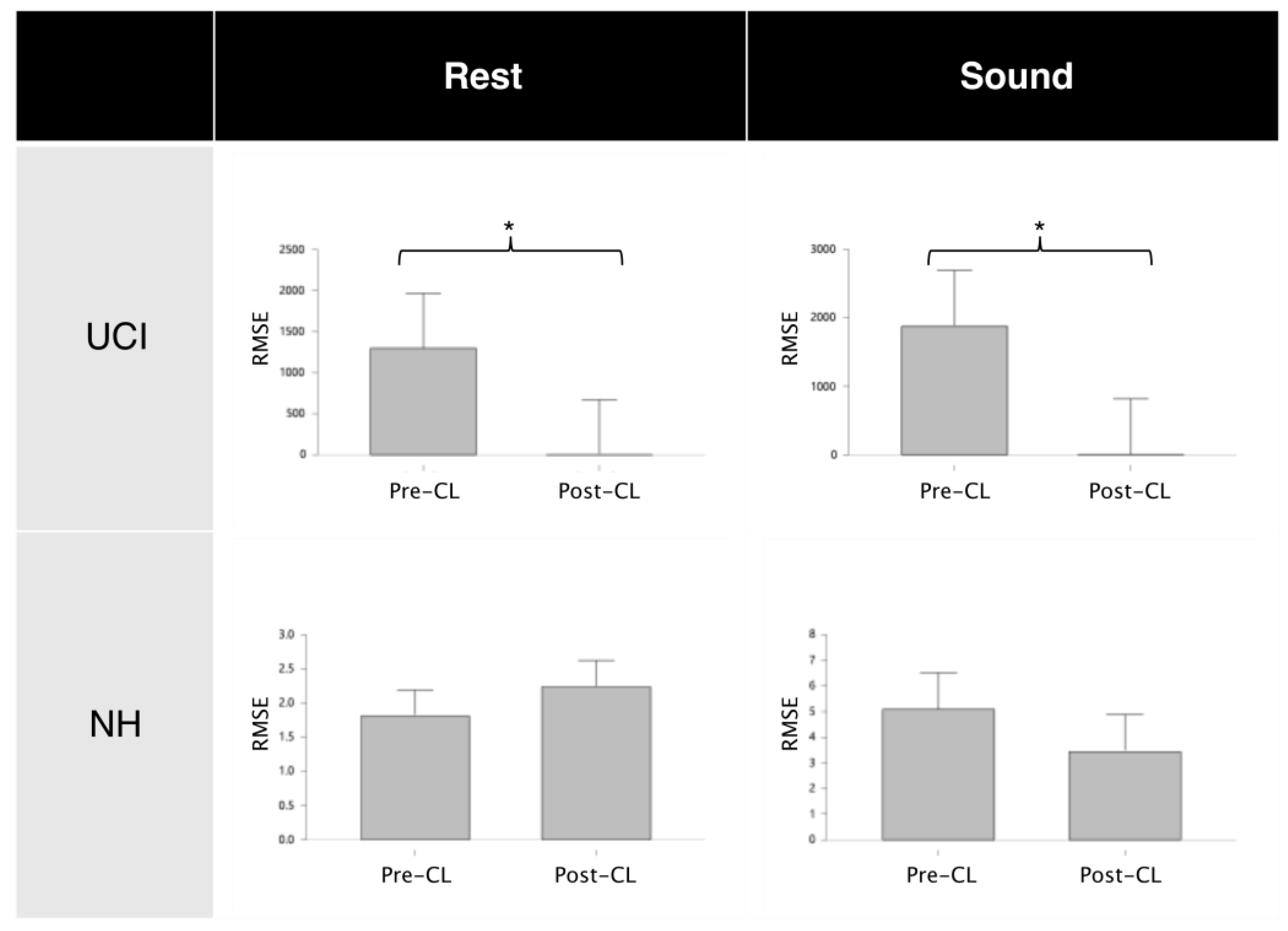
| Mean (SE) | z | p | |
|---|---|---|---|
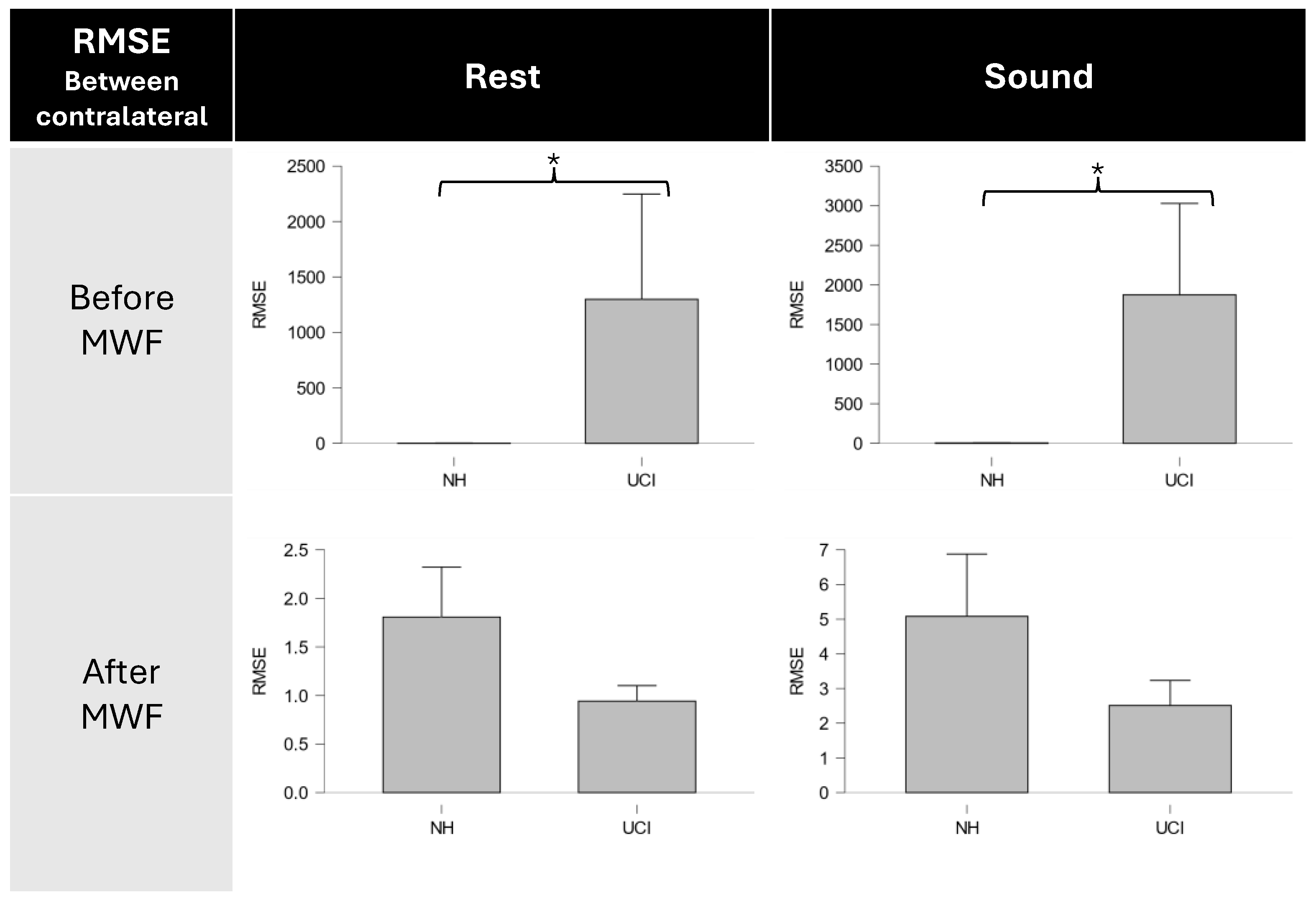
| RMSEpre-artifact RMSECL-artifact | 40.12 (8.38) 1848.31 (1153.22) | −2.67 | 0.03 * |
| RMSEpre-artifact RMSEpost-artifact | 40.12 (8.38) 1849.11 (1153.22) | −2.67 | 0.03 * |
| RMSEpre-CL RMSEpost-CL | 1875.24 (1152.84) 2.51 (0.72) | 2.67 | 0.03 * |
| RMSEpost-CL RMSEpost-artifact | 2.51 (0.72) 1849.11 (1153.22) | −2.67 | 0.03 * |
| Mean (SE) | z | p | |
|---|---|---|---|
| RMSEpre-artifact RMSECL-artifact | 15.42 (5.47) 1286.66 (948.61) | −2.67 | 0.03 * |
| RMSEpre-artifact RMSEpost-artifact | 15.42 (5.47) 1287.20 (948.63) | −2.67 | 0.03 * |
| RMSEpre-CL RMSEpost-CL | 1299.13 (948.83) 0.94 (0.16) | 2.67 | 0.03 * |
| RMSEpost-CL RMSEpost-artifact | 0.94 (0.16) 1287.20 (948.63) | −2.67 | 0.03 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, D.; Cartocci, G.; Inguscio, B.M.S.; Capitolino, G.; Borghini, G.; Di Flumeri, G.; Ronca, V.; Giorgi, A.; Vozzi, A.; Capotorto, R.; et al. Characterization of Cochlear Implant Artifact and Removal Based on Multi-Channel Wiener Filter in Unilateral Child Patients. Bioengineering 2024, 11, 753. https://doi.org/10.3390/bioengineering11080753
Rossi D, Cartocci G, Inguscio BMS, Capitolino G, Borghini G, Di Flumeri G, Ronca V, Giorgi A, Vozzi A, Capotorto R, et al. Characterization of Cochlear Implant Artifact and Removal Based on Multi-Channel Wiener Filter in Unilateral Child Patients. Bioengineering. 2024; 11(8):753. https://doi.org/10.3390/bioengineering11080753
Chicago/Turabian StyleRossi, Dario, Giulia Cartocci, Bianca M. S. Inguscio, Giulia Capitolino, Gianluca Borghini, Gianluca Di Flumeri, Vincenzo Ronca, Andrea Giorgi, Alessia Vozzi, Rossella Capotorto, and et al. 2024. "Characterization of Cochlear Implant Artifact and Removal Based on Multi-Channel Wiener Filter in Unilateral Child Patients" Bioengineering 11, no. 8: 753. https://doi.org/10.3390/bioengineering11080753
APA StyleRossi, D., Cartocci, G., Inguscio, B. M. S., Capitolino, G., Borghini, G., Di Flumeri, G., Ronca, V., Giorgi, A., Vozzi, A., Capotorto, R., Babiloni, F., Scorpecci, A., Giannantonio, S., Marsella, P., Leone, C. A., Grassia, R., Galletti, F., Ciodaro, F., Galletti, C., & Aricò, P. (2024). Characterization of Cochlear Implant Artifact and Removal Based on Multi-Channel Wiener Filter in Unilateral Child Patients. Bioengineering, 11(8), 753. https://doi.org/10.3390/bioengineering11080753