On the Fused Deposition Modelling of Personalised Bio-Scaffolds: Materials, Design, and Manufacturing Aspects
Abstract
:1. Introduction
- Biocompatibility: This ensures that the implant material does not cause adverse reactions inside the body; the materials used must be non-toxic or have low-toxicity properties, and must not cause an immune reaction, and must prevent post-surgical infections; they should support cell attachment, proliferation, and differentiation;
- Bioactivity, promoting osteointegration: The scaffold should interact positively with the biological environment, promoting new bone tissue. This is achieved through the integration of osteoinductive and osteoconductive properties that promote bone growth;
- Mechanical properties: The bio-scaffold is engineered to possess the necessary mechanical strength and elasticity that mirrors the mechanical properties of the native bone at the implant site; it should provide structural support until the new tissue is formed and can bear loads;
- Permeability: Porosity (density and pore size) is relevant for the adequate interconnectivity of pores that allows for cell migration, vascularisation, and nutrient/waste exchange; the pore size is crucial for determining the type of tissue that will infiltrate the scaffold;
- Degradation rate: The degradation rate of the scaffold material must be carefully aligned with the pace of new tissue growth. This alignment allows for a systematic transition of mechanical loads to the regenerating tissue, thereby supporting seamless tissue integration and structural stability.
- Manufacturability: The ability to fabricate the scaffold into complex shapes customised to the patient’s defect, using techniques like 3D printing, ensuring a perfect customised fit, and a precision placement, is critical for the implant’s longevity, effectiveness, and integration with the bone;
- Sterilizability: The scaffold must be sterilizable without losing its structural integrity or biological properties;
- Durability: This ensures long-term functionality without the need for replacement;
- Affordability and availability: The materials and technology used should be cost-effective and readily available to ensure that the treatment can be accessed by a wide range of patients;
- Regulatory compliance: The scaffold must meet the regulatory requirements of medical devices for the specific application it is intended for.
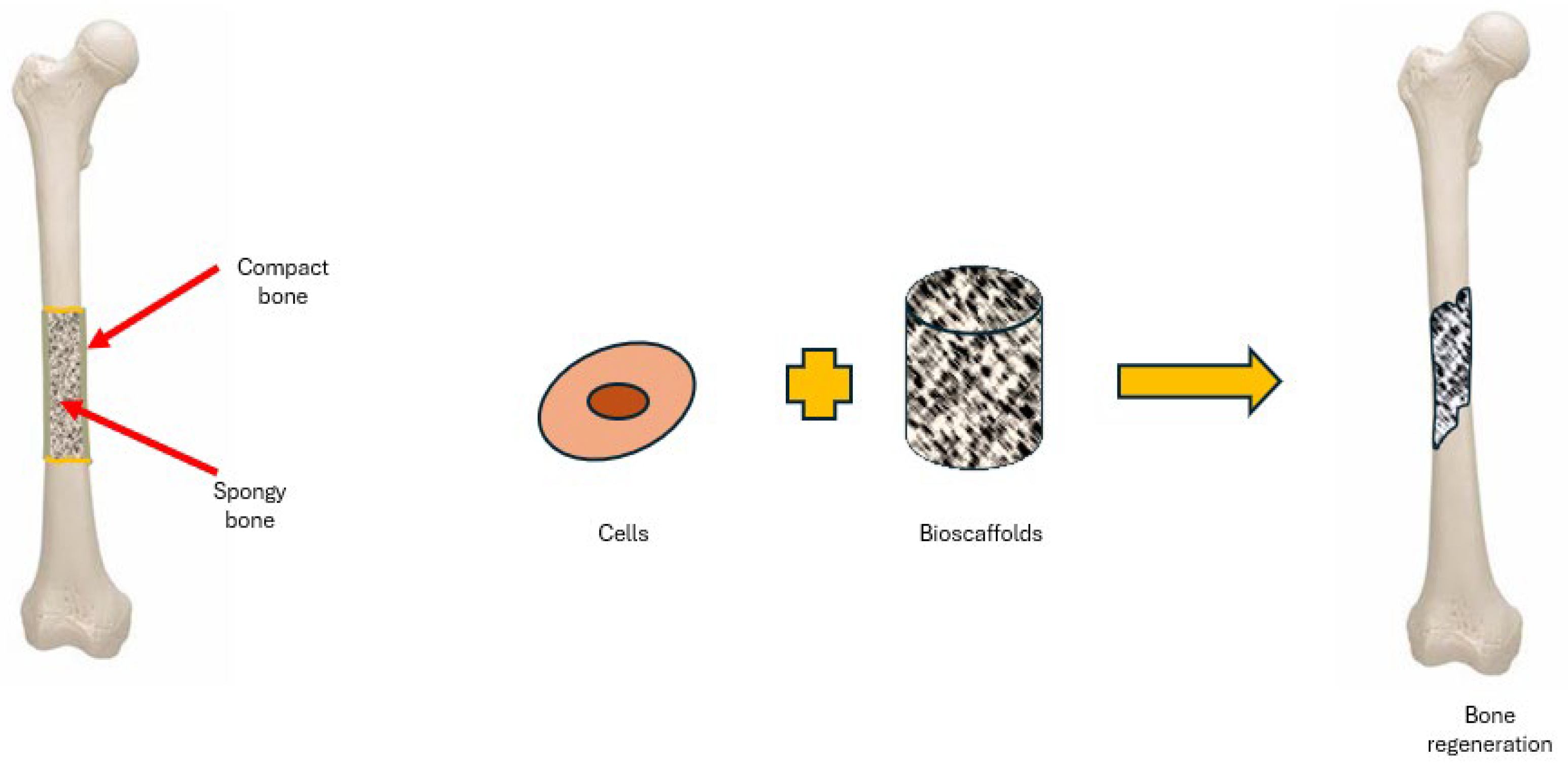
2. Bio-Scaffolds for BTE
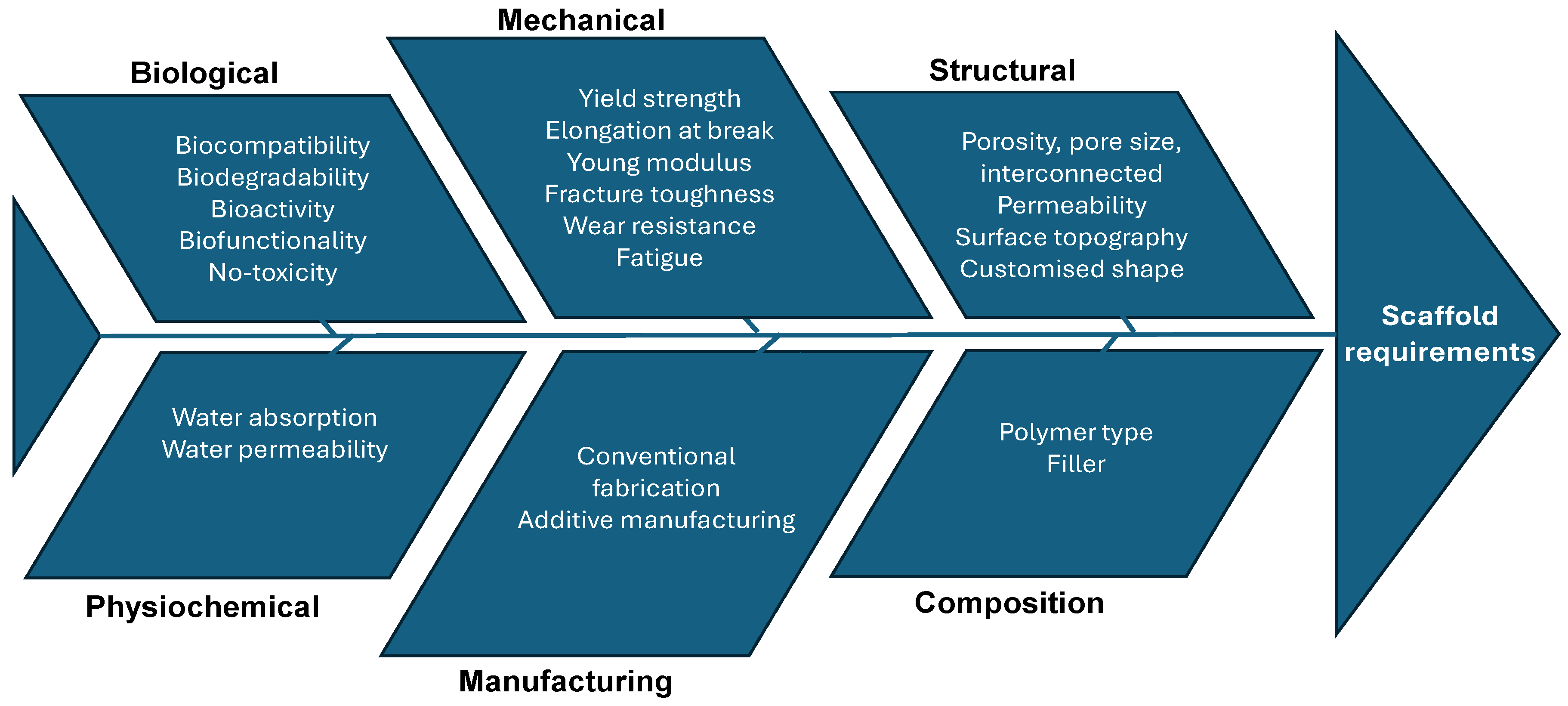
2.1. Bio-Scaffolds Requirements
- Biological requirements
- Structural requirements
- Material requirements
- Manufacturing technologies requirements
2.2. Bio-Scaffolds Design and Development
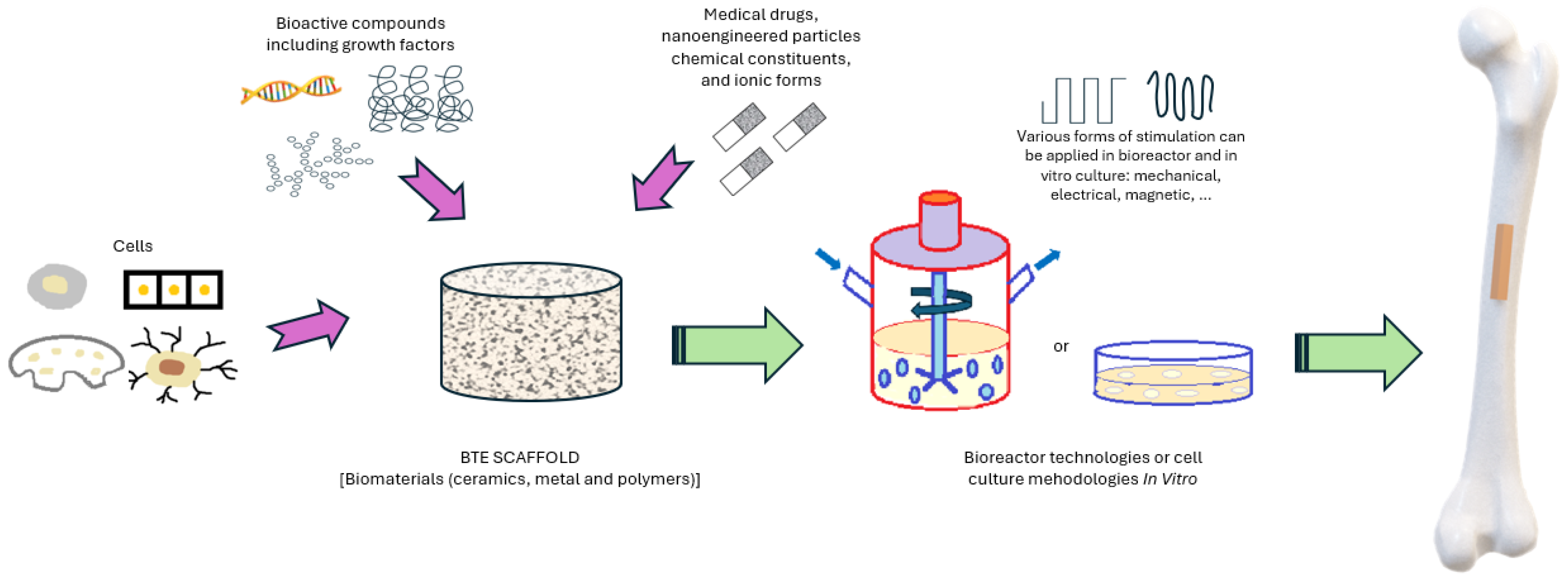
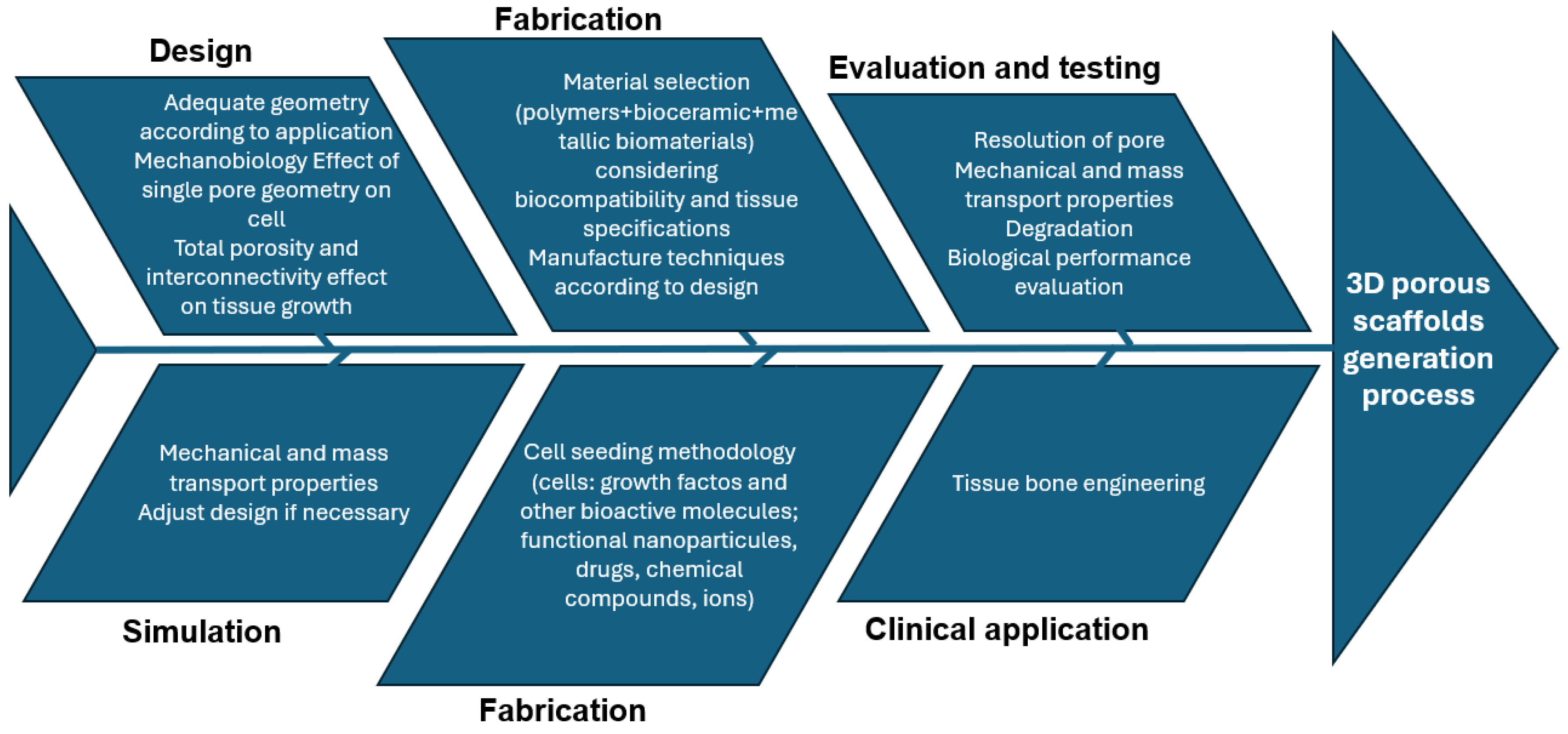
2.2.1. BTE Scaffold-Based Strategy Development
2.2.2. Scaffold Design Cause–Effect Diagram
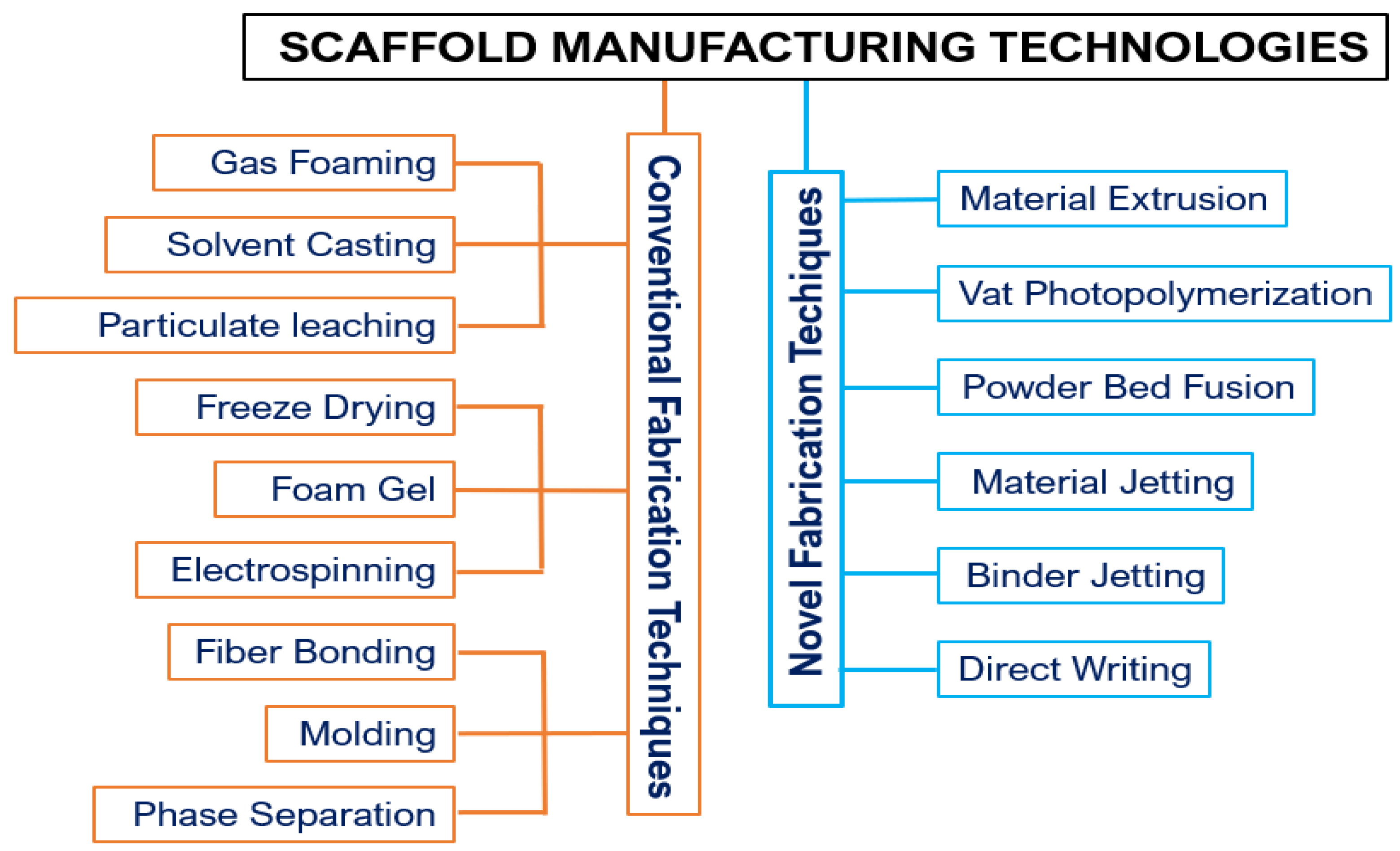
3. Manufacturing of Scaffolds by FDM
3.1. Additive Manufacturing
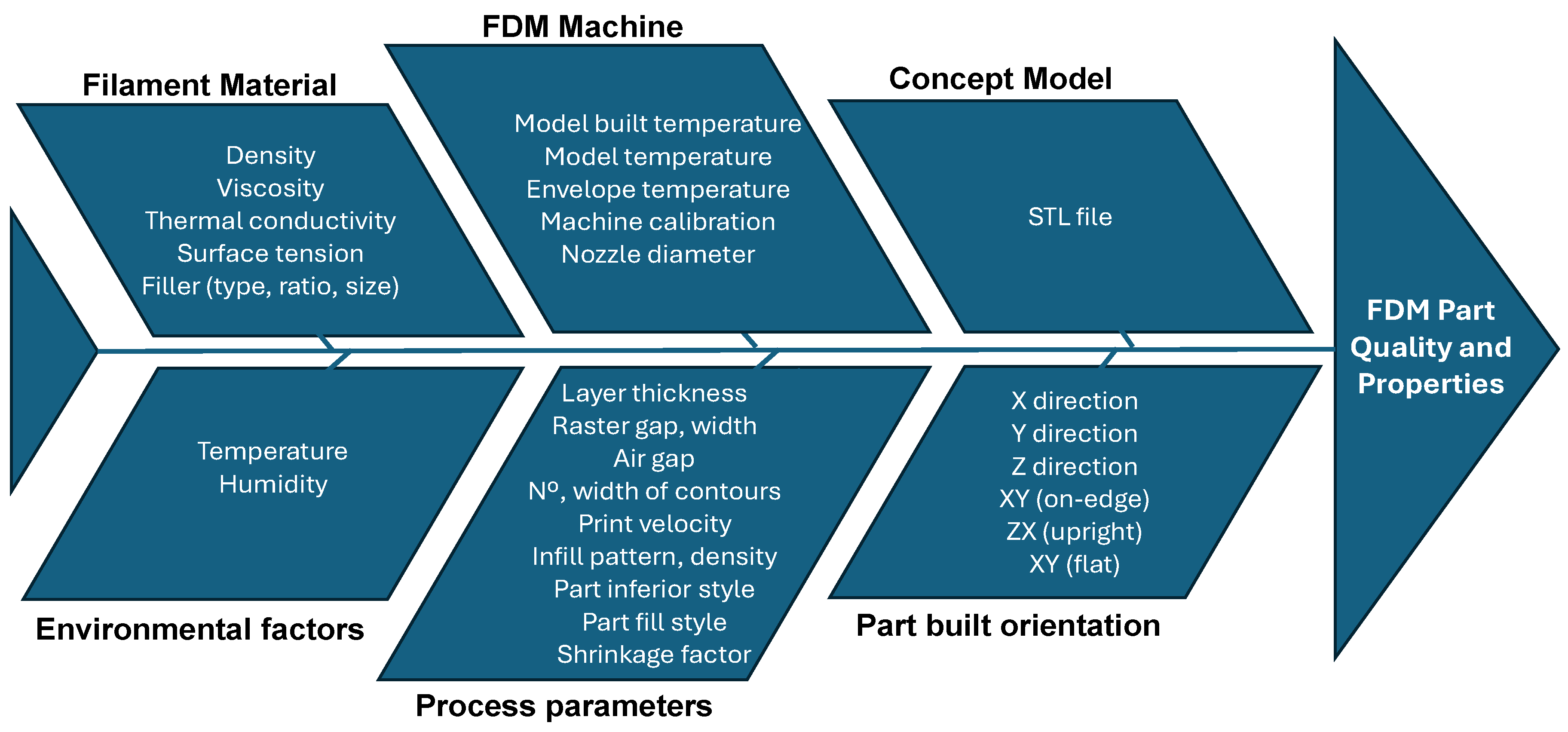
3.2. FDM Process
4. Materials for Printed Bio-Scaffolds
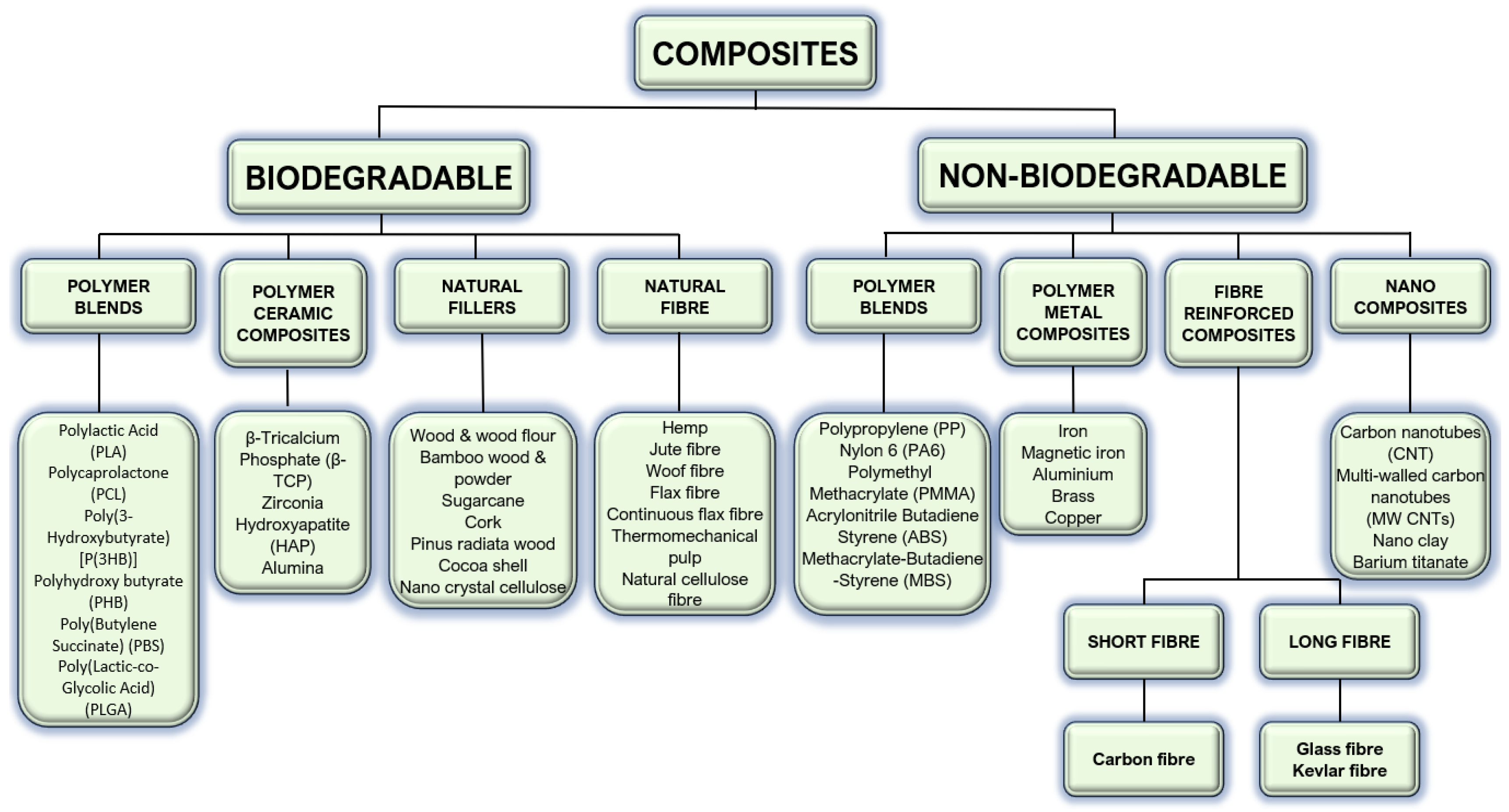
4.1. Polymers
4.2. Hybrids and Main Functional Fillers
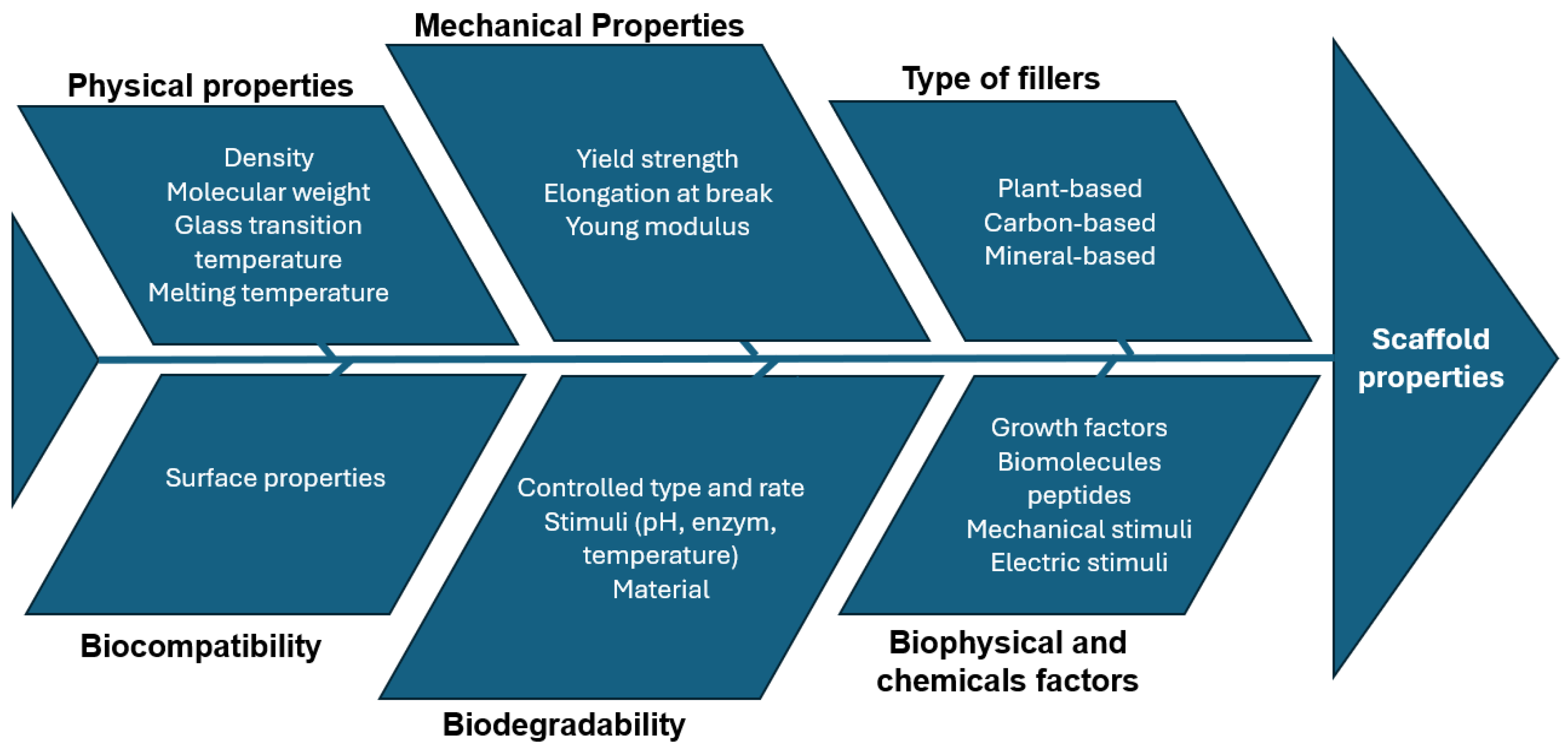
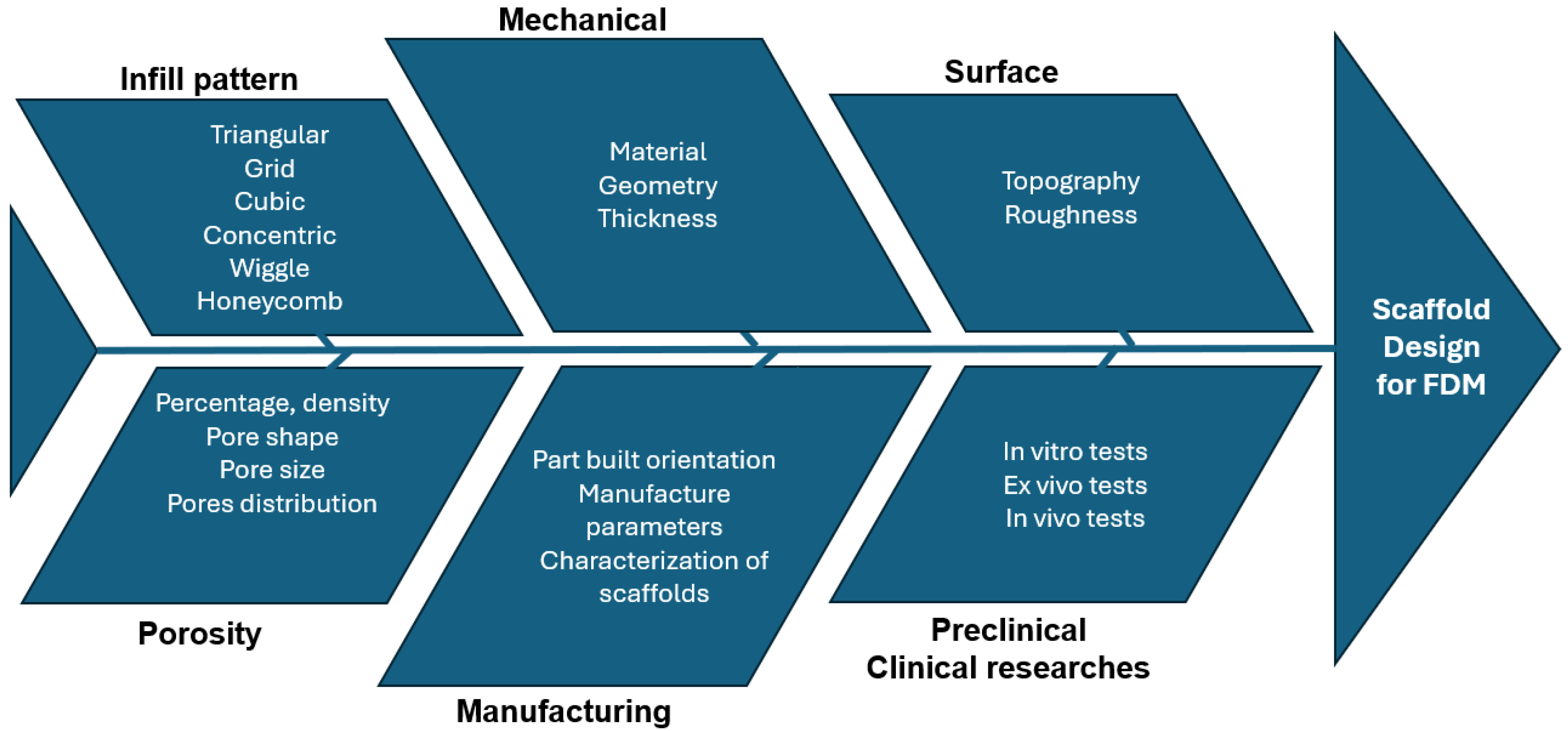
4.3. Material Cause–Effect Diagram
5. Scaffold Design
5.1. Porosity
5.2. Scaffold Design Cause–Effect Diagram
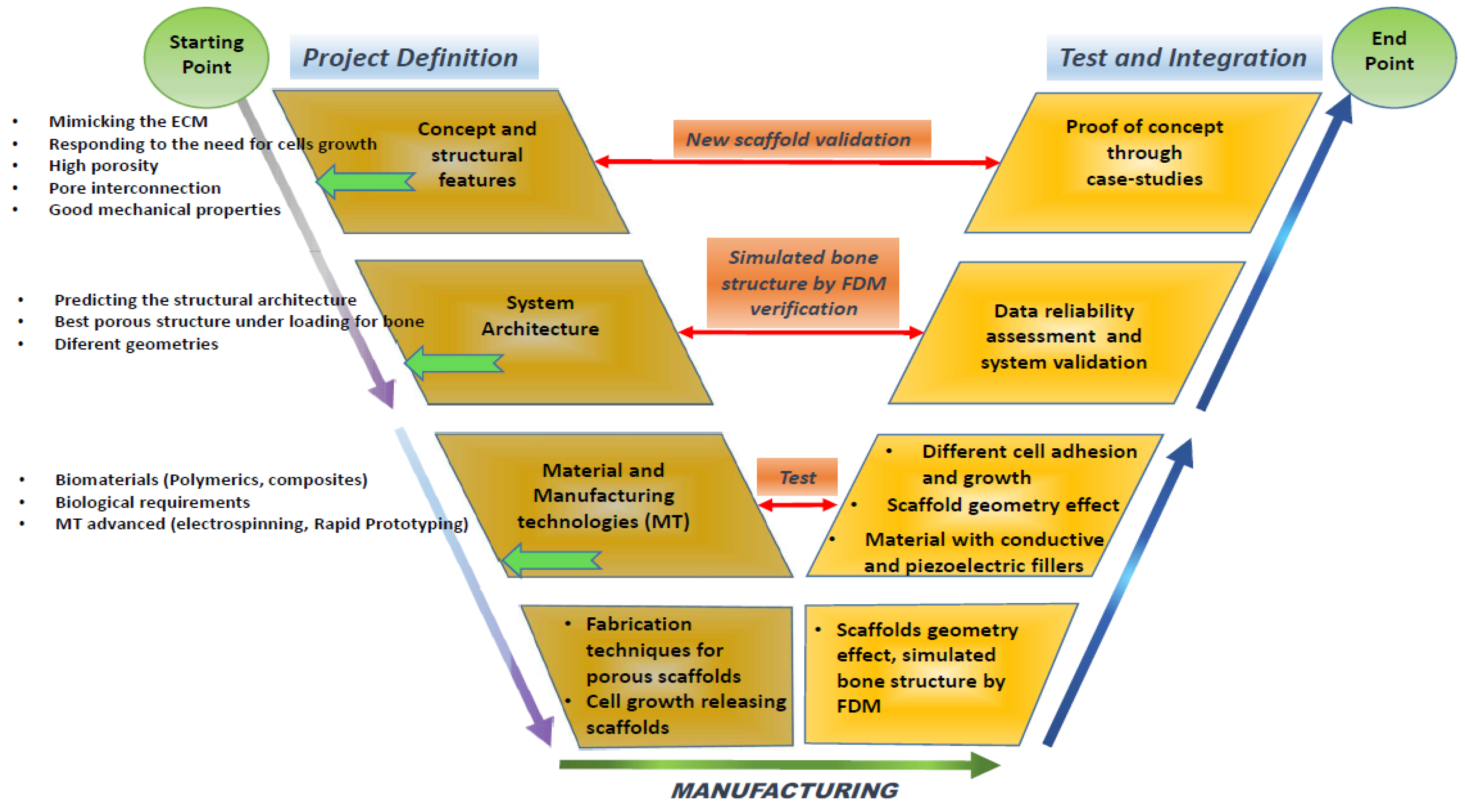
5.3. Scaffold Design Approach
- Project definition: This initial phase involves defining the specific requirements and objectives of the scaffold, including its intended medical applications, target tissue type, and the essential properties it must possess;
- Material phase: In this stage, the selection and formulation of the materials take place. The materials chosen are typically biocompatible and biodegradable to ensure they do not evoke an adverse reaction in the body and can degrade at a rate compatible with tissue regeneration. The development will focus on biopolymers that integrate well with biological systems without necessitating subsequent surgical removal;
- Structural design: This phase involves detailed modelling on the scaffold’s architecture. Critical parameters such as pore size, shape, interconnectivity, and the overall three-dimensional structure are designed to meet the specific mechanical and biological needs of the target tissue. Advanced CAD software may be used to achieve precise design specifications and optimize the scaffold’s structural integrity;
- The scaffold manufacturing phase: Utilising technologies like 3D printing—specifically, fused deposition modelling—the scaffold is fabricated. This phase focuses on accurately replicating the design specifications in the physical model, ensuring that the scaffold maintains its functional integrity under physiological conditions;
- The testing and integration of the scaffold through a case study: The final phase involves the rigorous testing of the scaffold in both in vitro and in vivo settings to evaluate its biocompatibility, mechanical stability, and efficacy in promoting tissue regeneration. Integration trials through case studies or clinical trials assess the scaffold’s performance in a real-world medical scenario, confirming its readiness for surgical use and integration within the human body.
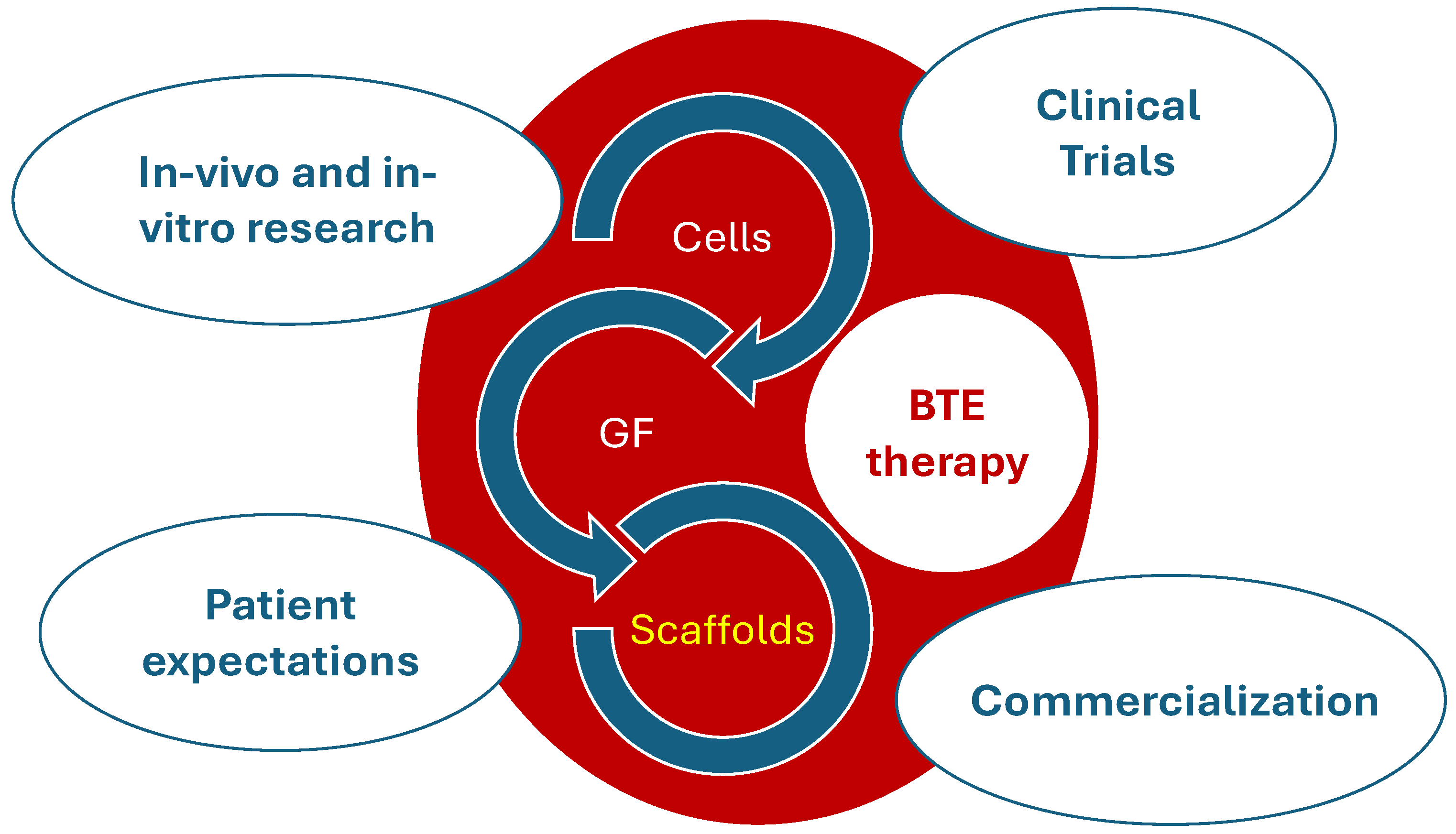
6. Concluding Remark
- The integration of materials and technologies: There is a pressing need to integrate diverse materials and technologies to develop scaffolds that effectively facilitate human bone healing. This includes the combination of different biopolymers, ceramics, and bioactive substances that should aim to replicate the intricate structure and functionality of natural bone;
- The prioritisation of low-cost, sustainable, and biocompatible materials: Developing scaffolds using materials that are not only cost-effective and sustainable but also biocompatible is vital. These materials should promote tissue compatibility without eliciting any adverse immune responses, thus making treatments accessible and safe for a broader population;
- Selecting the adequate composite for the optimal scaffold: The choice of the right composite is critical. These composites should possess the necessary mechanical strength, support cell attachment and growth, and degrade at a rate congruent with new tissue formation, thereby providing structural integrity during the healing process;
- Designing the scaffold in order to optimize the structural properties, degradation, and healing: The structural design of the scaffold must be optimized to enhance its mechanical properties, control degradation rates, and support the healing process. This involves the precise engineering of the scaffold’s architecture, such as pore size, shape, and interconnectivity, which are crucial for vascularisation and nutrient diffusion;
- The in situ cell growth: Encouraging cell growth directly within the scaffold at the site of implantation represents a significant frontier in BTE. Developing scaffolds that can not only support but also stimulate in situ cell proliferation and differentiation is essential for successful integration and healing.
Funding
Conflicts of Interest
References
- Gautam, G.; Kumar, S.; Kumar, K. Processing of biomaterials for bone tissue engineering: State of the art. Mater. Today Proc. 2022, 50, 2206–2217. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Ruston, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspective. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D Printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Hedayati, S.K.; Behravesh, A.H.; Hasannia, S.; Kordi, O.; Pourghaumi, M.; Saed, A.B.; Gashtasbi, F. Additive manufacture of PCL/nHA scaffolds reinforced with biodegradable continuous fibers: Mechanical properties, in-vitro degradation profile and cell study. Eur. Polym. J. 2022, 162, 110876. [Google Scholar] [CrossRef]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Rodrigues da Silva Sasso, G.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function and factors that influence bone cells. BioMed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspective. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Bair, H.C.; Sun, L.; Kohanski, R.A. Balanced regulation of proliferation, growth, differentiation and degradation in skeletal cells. Ann. N. Y. Acad. Sci. 2007, 1116, 165–173. [Google Scholar]
- Das, M.; Sharabani-Yosef, O.; Eliaz, N.; Mandler, D. Hydrogel-integrated 3D-printed poly(lactic acid) scaffolds for bone tissue engineering. J. Mater. Res. 2021, 36, 3833–3842. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Kundu, B.; NasKar, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yaszemski, M.J.; Payne, R.G.; Haynes, W.C.; Langer, R.; Mikos, A.G. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Jodati, H.; Yilmaz, B.; Elvis, Z. A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features. Ceram. Int. 2020, 46, 15725–15739. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A poly(D,L-lactide|resin for the preparation of tissue engineering scaffolds by stereolithography. Biomaterials 2009, 30, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, S467–S479. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, H.; Deng, X.; Wu, L.; Yi, T.; Gu, L.; Zhou, C.; Fan, Y.; Zhang, X. Scaffold structural microenvironmental cues to guide tissue regeneration in bone tissue applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef] [PubMed]
- Bouet, G.; Marchat, D.; Cruel, M.; Malaval, L. In vitro three-dimensional bone tissue models: From cells to controlled and dynamic environment. Tissue Eng. B Rev. 2015, 21, 133–156. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Y.; Arif, Z.U.; Noroozi, R.; Zolfagharian, A.; Bodaghi, M. 4D printing of shape memory polymer composites: A review on fabrication techniques, applications, and future perspectives. J. Manuf. Process. 2022, 81, 759–797. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Mahfouzi, S.H.; Safiabadi, T.S.H.; Amoabediny, G. 3D bioprinting for lung and tracheal tissue engineering: Criteria, advances, challenges, and future directions. Bioprinting 2021, 21, e00124. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019, 207, 297–316. [Google Scholar] [CrossRef]
- Dhania, S.; Bernela, M.; Rani, R.; Parsad, M.; Grewal, S.; Kumari, S.; Thakur, R. Scaffolds the backbone of tissue engineering: Advancements in use of polyhydroxyalkanoates (PHA). Int. J. Biol. Macromol. 2022, 208, 243–259. [Google Scholar] [CrossRef]
- Saghebasl, S.; Akbarzadeh, A.; Gorabi, A.M.; Nikzamir, N.; SeyedSadjadi, M.; Mostafavi, E. Biodegradable functional macromolecules as promising scaffolds for cardiac tissue engineering. Polym. Adv. Technol. 2022, 33, 2044–2068. [Google Scholar] [CrossRef]
- Litowezenko, J.; Wozmiak-Budych, M.J.; Staszak, K.; Wieszczycka, K.; Jurga, S.; Tylkowski, B. Milestones and current achievements in development of multifunctional bioscaffolds for medical applications. Bioact. Mater. 2021, 6, 2412–2438. [Google Scholar] [CrossRef]
- Top, N.; Sahin, I.; Gökce, H. Computer-aided design and additive manufacturing of bone scaffolds for tissue engineering: State of the art. J. Mater. Res. 2021, 36, 3725–3745. [Google Scholar] [CrossRef]
- Rajan, K.; Samykano, M.; Kadirgama, K.; Harun, W.S.W.; Rahman, M.M. Fused deposition modeling: Process, materials, parameters, properties, and applications. Int. J. Adv. Manuf. Technol. 2022, 120, 1531–1570. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Liang, H.; Gao, C.; Peng, S.; Shen, L.; Shuai, C. Additive manufacturing of bone scaffolds. Int. J. Bioprinting 2019, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.P.G.; Ruben, R.B.; Gonçalves, S.B.; Pinheiro, J.; Guedes, J.M.; Fernandes, P.R. Numerical and experimental evaluation of TPMS gyroid scaffolds for bone tissue engineering. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Dias, M.; Vorndran, E.; Gbureck, U.; Fernandes, P.; Pires, I.; Gouveia, B.; Armés, H.; Pires, E.; Rodrigues, J. Application of a 3D printed customized implant for a canine cruciate ligament treatment by tibial tuberosity advancement. Biofabrication 2014, 6, 025005. [Google Scholar] [CrossRef]
- Kohad, A.; Dalu, R. Optimization of Process Parameters in Fused Deposition Modeling: A Review. Int. J. 2017, 6, 505–511. [Google Scholar]
- Mishra, V.; Negi, S.; Kar, S.; Sharma, A.K.; Rajbahadur, Y.N.K.; Kumar, A. Recent advances in fused deposition of thermoplastic composite structures: A review. J. Thermoplast. Compos. Mater. 2022, 36, 1–39. [Google Scholar]
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Optimization of fused deposition modelling process parameters: A review of current research and future prospects. Adv. Manuf. 2015, 3, 42–53. [Google Scholar] [CrossRef]
- Zaneldin, E.; Ahmed, W.; Mansour, A.; Hassan, A.E. Dimensional stability of 3D printed objects made from plastic waste using FDM: Potential construction applications. Buildings 2021, 11, 516. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly(lactid acid)-based biomaterials for orthopaedic. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef]
- Flores-Jiménez, M.S.; Gardia-Gonzalez, A.; Fuentes-Aguilar, R.Q. Review on porous scaffolds generation process: A tissue engineering approach. ACS Appl. Bio Mater. 2023, 6, 1–23. [Google Scholar] [CrossRef]
- Dinis, J.C.; Morais, T.F.; Amorim, P.H.J.; Ruben, R.B.; Almeida, H.A.; Inforçati, P.N.; Bártolo, P.J.; Silva, J.V.L. Open source software for the automatic design of scaffold structures for tissue engineering applications. Procedia Technol. 2014, 16, 1542–1547. [Google Scholar] [CrossRef]
- Kumar, A.; Jacob, A. Techniques in scaffold fabrication process for tissue engineering applications: A review. J. Appl. Biol. Biotechnol. 2022, 10, 163–176. [Google Scholar] [CrossRef]
- Brézulier, D.; Chaigneau, L.; Jeanne, S.; Lebullenger, R. The challenge of 3D bioprinting of composite natural polymers PLA/Bioglass: Trends and benefits in cleft palate surgery. Biomedicines 2021, 9, 1553. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, H.C.; Ruben, R.B.; Viana, J.C. On the Fused Deposition Modelling of Personalised Bio-Scaffolds: Materials, Design, and Manufacturing Aspects. Bioengineering 2024, 11, 769. https://doi.org/10.3390/bioengineering11080769
Sousa HC, Ruben RB, Viana JC. On the Fused Deposition Modelling of Personalised Bio-Scaffolds: Materials, Design, and Manufacturing Aspects. Bioengineering. 2024; 11(8):769. https://doi.org/10.3390/bioengineering11080769
Chicago/Turabian StyleSousa, Helena Cardoso, Rui B. Ruben, and Júlio C. Viana. 2024. "On the Fused Deposition Modelling of Personalised Bio-Scaffolds: Materials, Design, and Manufacturing Aspects" Bioengineering 11, no. 8: 769. https://doi.org/10.3390/bioengineering11080769





