Supercritical Fluids: An Innovative Strategy for Drug Development
Abstract
1. Introduction
2. Supercritical Procedures for Particle Formation and Dispersion
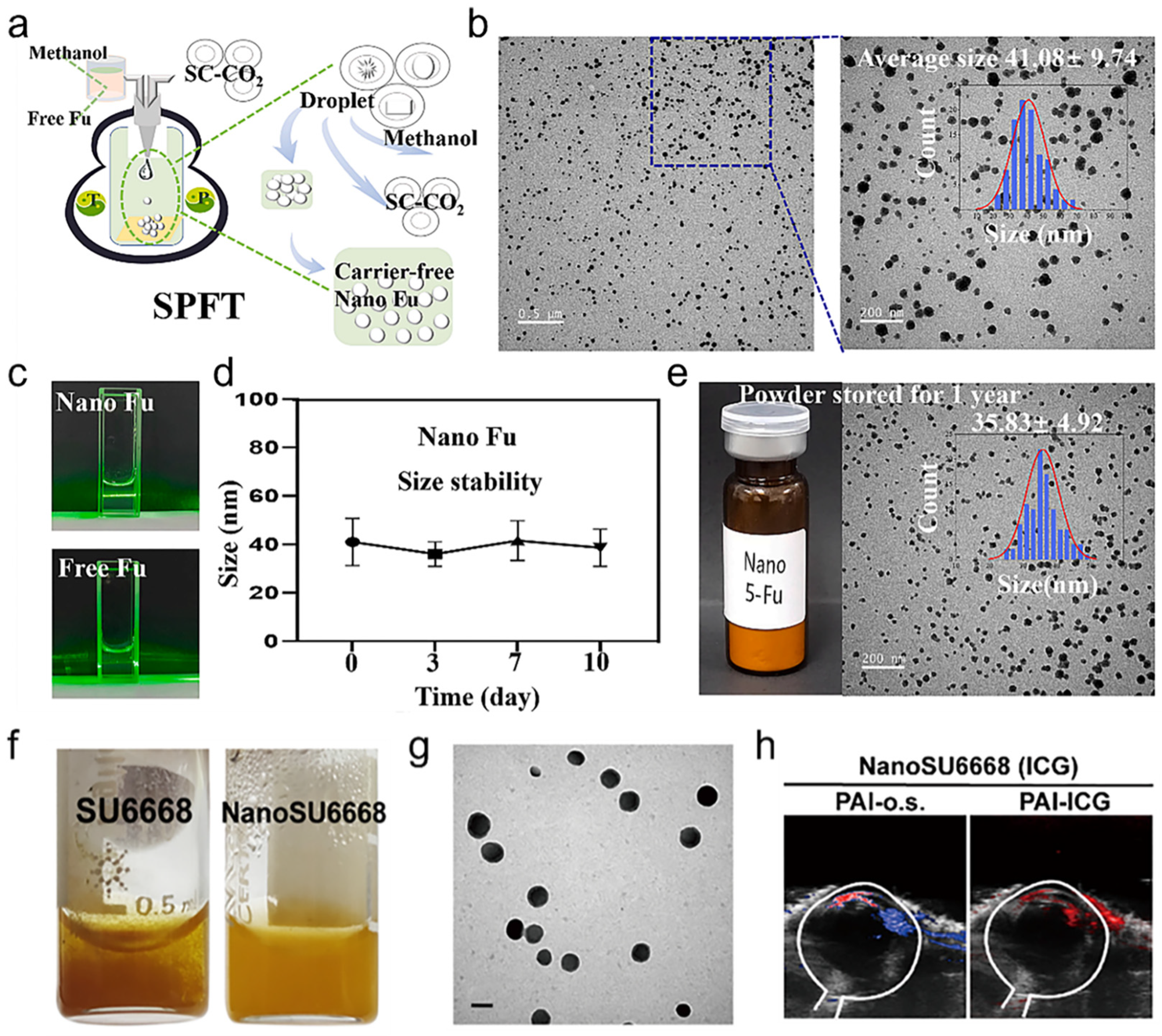
3. Supercritical Fluid-Assisted Drug Dispersion Technology
4. Supercritical Fluid-Assisted Drug Crystallization
5. Clinical Applications of Supercritical Fluid Technology
5.1. Fluorescent Surgery Navigation
5.2. Transcatheter Arterial Chemoembolization
5.3. Body Skin Scarring
5.4. Corneal Neovascularization
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SHIFT | Super-stable homogeneous intermix formulating technology |
| SPFT | Super-table pure-nanomedicine formulation technology |
| EPR effect | enhanced permeability and retention effect |
| SCF | Supercritical fluid |
| RESS | Rapid expansion process of supercritical solution |
| PGSS | Precipitation from gas saturated solution |
| SAS | Supercritical anti-solvent |
| HCC | Hepatocellular carcinoma |
| ICG | Indocyanine green |
| TARE | Transarterial radioembolization |
| DOX | Doxorubicin |
| TAE | Transcatheter arterial embolization |
| DSA | Digital subtraction angiography |
| TACE | Transarterial chemoembolization |
| 5-Fu | Fluorouracil |
| SU6668 | Orantinib |
References
- Campbell, N.; Deane, C.; Murphy, P. The sounds of nanotechnology. Nat. Nanotechnol. 2017, 12, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, M.; Fang, Z.; Zheng, L.; Huang, X.; Liu, K. Applications and challenges of ultra-small particle size nanoparticles in tumor therapy. J. Control. Release 2023, 353, 699–712. [Google Scholar] [CrossRef]
- Anane-Adjei, A.B.; Jacobs, E.; Nash, S.C.; Askin, S.; Soundararajan, R.; Kyobula, M.; Booth, J.; Campbell, A. Amorphous solid dispersions: Utilization and challenges in preclinical drug development within AstraZeneca. Int. J. Pharm. 2022, 614, 121387. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Ha, E.S.; Park, H.; Lee, S.K.; Sim, W.Y.; Jeong, J.S.; Baek, I.H.; Kim, M.S. Pure Trans-Resveratrol Nanoparticles Prepared by A Supercritical Antisolvent Process Using Alcohol and Dichloromethane Mixtures: Effect of Particle Size on Dissolution and Bioavailability in Rats. Antioxidants 2020, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, J.; Wang, W.; Winstead, D.A. Formation and characterization of solid dispersions of piroxicam and polyvinylpyrrolidone using spray drying and precipitation with compressed antisolvent. J. Pharm. Sci. 2009, 98, 2422–2431. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Supercritical Antisolvent Process for Pharmaceutical Applications: A Review. Processes 2020, 8, 938. [Google Scholar] [CrossRef]
- da Fonseca Machado, A.P.; Alves Rezende, C.; Alexandre Rodrigues, R.; Fernández Barbero, G.; de Tarso Vieira e Rosa, P.; Martínez, J. Encapsulation of anthocyanin-rich extract from blackberry residues by spray-drying, freeze-drying and supercritical antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Tran, P.; Park, J.-S. Application of supercritical fluid technology for solid dispersion to enhance solubility and bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2021, 610, 121247. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Zeng, Z. The antisolvent coprecipitation method for enhanced bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2022, 626, 122043. [Google Scholar] [CrossRef]
- Knez, Ž.; Pantić, M.; Cör, D.; Novak, Z.; Knez Hrnčič, M. Are supercritical fluids solvents for the future? Chem. Eng. Process. Process Intensif. 2019, 141, 107532. [Google Scholar] [CrossRef]
- Badens, E.; Masmoudi, Y.; Mouahid, A.; Crampon, C. Current situation and perspectives in drug formulation by using supercritical fluid technology. J. Supercrit. Fluids 2018, 134, 274–283. [Google Scholar] [CrossRef]
- Kang, X.; Mao, L.; Shi, J.; Liu, Y.; Zhai, B.; Xu, J.; Jiang, Y.; Lichtfouse, E.; Jin, H.; Guo, L. Supercritical carbon dioxide systems for sustainable and efficient dissolution of solutes: A review. Environ. Chem. Lett. 2024, 22, 815–839. [Google Scholar] [CrossRef]
- Martín, A.; Cocero, M.J. Micronization processes with supercritical fluids: Fundamentals and mechanisms. Adv. Drug Deliv. Rev. 2008, 60, 339–350. [Google Scholar] [CrossRef]
- Bagheri, H.; Hashemipour, H.; Ghader, S. Population balance modeling: Application in nanoparticle formation through rapid expansion of supercritical solution. Comput. Part. Mech. 2019, 6, 721–737. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, H.; Dai, Q.; Cheng, Y.; Zhang, Y.; Li, D.; Sun, Y.; Mao, J.; Ren, K.; Chu, C.; et al. A superstable homogeneous lipiodol-ICG formulation for locoregional hepatocellular carcinoma treatment. J. Control. Release 2020, 323, 635–643. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Quílez, C.; Barros, J.; Velasco, D.; Alvarez-Lorenzo, C.; Jorcano, J.L.; Monteiro, F.J.; García-González, C.A. Lidocaine-Loaded Solid Lipid Microparticles (SLMPs) Produced from Gas-Saturated Solutions for Wound Applications. Pharmaceutics 2020, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.L.; Rebelatto, E.A.; Lanza, M.; Ferreira, S.R.S. Production of quercetin-proline cocrystals by means of supercritical CO2 antisolvent. Adv. Powder Technol. 2023, 34, 104222. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Duarte, A.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals—A comprehensive review. Adv. Drug Deliv. Rev. 2018, 131, 22–78. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Tsay, R.-Y. Drug Release from a Spherical Matrix: Theoretical Analysis for a Finite Dissolution Rate Affected by Geometric Shape of Dispersed Drugs. Pharmaceutics 2020, 12, 582. [Google Scholar] [CrossRef]
- Attia, M.S.; Elshahat, A.; Hamdy, A.; Fathi, A.M.; Emad-Eldin, M.; Ghazy, F.-E.S.; Chopra, H.; Ibrahim, T.M. Soluplus® as a solubilizing excipient for poorly water-soluble drugs: Recent advances in formulation strategies and pharmaceutical product features. J. Drug Deliv. Sci. Technol. 2023, 84, 104519. [Google Scholar] [CrossRef]
- Rossi, G.; Tarasconi, A.; Baiocchi, G.; De’ Angelis, G.L.; Gaiani, F.; Di Mario, F.; Catena, F.; Dalla Valle, R. Fluorescence guided surgery in liver tumors: Applications and advantages. Acta Biomed. 2018, 89, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Goumard, C.; Komatsu, S.; Brustia, R.; Fartoux, L.; Soubrane, O.; Scatton, O. Technical feasibility and safety of laparoscopic right hepatectomy for hepatocellular carcinoma following sequential TACE–PVE: A comparative study. Surg. Endosc. 2017, 31, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, J.; Seifert, P.; Gühne, F.; Winkens, T.; Rauchfuß, F.; Settmacher, U.; Freesmeyer, M.; Drescher, R. Transarterial Radioembolization (TARE) in Patients with Hepatocellular Carcinoma: A Comparison of Palliative with Bridging-to-Transplant Concepts. Cancers 2024, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Teng, M.; Zhang, H.; Liang, X.; Cheng, H.; Liu, G. Advanced radionuclides in diagnosis and therapy for hepatocellular carcinoma. Chin. Chem. Lett. 2022, 33, 3371–3383. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Sabet, A.; Wilhelm, K.; Biersack, H.J.; Risse, J. Iodine-131-Lipiodol therapy in hepatic tumours. Methods 2011, 55, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.; Bubenheim, M.; Gardin, I.; Huet, E.; Riachi, G.; Clavier, E.; Goria, O.; Vera, P.; Scotté, M. Adjuvant I-131 Lipiodol After Resection or Radiofrequency Ablation for Hepatocellular Carcinoma. World J. Surg. 2016, 40, 1941–1950. [Google Scholar] [CrossRef]
- Montes, A.; Wehner, L.; Pereyra, C.; Martínez de la Ossa, E.J. Precipitation of submicron particles of rutin using supercritical antisolvent process. J. Supercrit. Fluids 2016, 118, 1–10. [Google Scholar] [CrossRef]
- He, P.; Xiong, Y.; Ye, J.; Chen, B.; Cheng, H.; Liu, H.; Zheng, Y.; Chu, C.; Mao, J.; Chen, A.; et al. A clinical trial of super-stable homogeneous lipiodol-nanoICG formulation-guided precise fluorescent laparoscopic hepatocellular carcinoma resection. J. Nanobiotechnol. 2022, 20, 250. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Pan, X.; Lu, W.; Zhang, J. Development and Challenge of Fluorescent Probes for Bioimaging Applications: From Visualization to Diagnosis. Top. Curr. Chem. 2022, 380, 22. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, H.; Chen, H.; Xu, P.; Ren, E.; Jiang, Y.; Li, D.; Gao, X.; Zheng, Y.; He, P.; et al. A pure nanoICG-based homogeneous lipiodol formulation: Toward precise surgical navigation of primary liver cancer after long-term transcatheter arterial embolization. Eur. J. Nuclear Med. Mol. Imaging 2022, 49, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Huang, Y.; Zhang, X.; Ouyang, Y.; Pan, P.; Lan, Y.; Zhong, Z.; Ping, L.; Lu, T.; Chen, Z.; et al. Supercritical fluid coating of flavonoids on excipients enhances drug release and antioxidant activity. Int. J. Pharm. 2023, 632, 122593. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; Saadati Ardestani, N.; Alwi, R.S.; Rojas, A.; Garlapati, C.; Sajadian, S.A. Solubility measurement of verapamil for the preparation of developed nanomedicines using supercritical fluid. Sci. Rep. 2023, 13, 17089. [Google Scholar] [CrossRef]
- Zhong, Z.; Lan, Y.; Chen, J.; Ping, L.; Li, X.; Wang, Q.; Zhuang, X.; Qiu, Z.; Yuan, T.; Guo, Q.; et al. Optimizing Paclitaxel Oral Absorption and Bioavailability: TPGS Co-Coating via Supercritical Anti-Solvent Fluidized Bed Technology. Pharmaceuticals 2024, 17, 412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, A.; Xie, Y.; Wen, H.; Kankala, R.K.; Huang, J.; Zhang, A.; Wang, Q.; Chen, B.; Dong, H.; et al. Nanocarrier of Pin1 inhibitor based on supercritical fluid technology inhibits cancer metastasis by blocking multiple signaling pathways. Regen. Biomater. 2023, 10, rbad014. [Google Scholar] [CrossRef] [PubMed]
- De Marco, I. Supercritical Fluids and Nanoparticles in Cancer Therapy. Micromachines 2022, 13, 1449. [Google Scholar] [CrossRef] [PubMed]
- Campardelli, R.; Trucillo, P.; Reverchon, E. Supercritical assisted process for the efficient production of liposomes containing antibiotics for ocular delivery. J. CO2 Util. 2018, 25, 235–241. [Google Scholar] [CrossRef]
- He, P.; Ren, E.; Chen, B.; Chen, H.; Cheng, H.; Gao, X.; Liang, X.; Liu, H.; Li, J.; Li, B.; et al. A super-stable homogeneous Lipiodol-hydrophilic chemodrug formulation for treatment of hepatocellular carcinoma. Theranostics 2022, 12, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yi, S.; Zhang, J.; Chu, C.; Peng, X.; Li, C.; Sun, X.; Zhang, Y.; Cheng, H.; Xiong, X.; et al. Carrier-free 5-Fu nanoparticle-mediated domestication therapy for scar treatment: A preclinical and first-in-human study. Chem. Eng. J. 2023, 475, 146061. [Google Scholar] [CrossRef]
- Wu, H.; Ye, J.; Zhang, M.; Zhang, L.; Lin, S.; Li, Q.; Liu, Y.; Han, Y.; Huang, C.; Wu, Y.; et al. A SU6668 pure nanoparticle-based eyedrops: Toward its high drug Accumulation and Long-time treatment for corneal neovascularization. J. Nanobiotechnol. 2024, 22, 290. [Google Scholar] [CrossRef]
- Wang, Z.-D.; Peng, H.-H.; Guan, Y.-X.; Yao, S.-J. Supercritical CO2 assisted micronization of curcumin-loaded oil-in-water emulsion promising in colon targeted delivery. J. CO2 Util. 2022, 59, 101966. [Google Scholar] [CrossRef]
- Amani, M.; Saadati Ardestani, N.; Majd, N.Y. Utilization of supercritical CO2 gas antisolvent (GAS) for production of Capecitabine nanoparticles as anti-cancer drug: Analysis and optimization of the process conditions. J. CO2 Util. 2021, 46, 101465. [Google Scholar] [CrossRef]
- Xiong, Y.; He, P.; Zhang, Y.; Chen, H.; Peng, Y.; He, P.; Tian, J.; Cheng, H.; Liu, G.; Li, J. Superstable homogeneous lipiodol-ICG formulation: Initial feasibility and first-in-human clinical application for ruptured hepatocellular carcinoma. Regen. Biomater. 2023, 10, rbac106. [Google Scholar] [CrossRef] [PubMed]
- Khudaida, S.H.; Yen, Y.-T.; Su, C.-S. Cocrystal screening of anticancer drug p-toluenesulfonamide and preparation by supercritical antisolvent process. J. Supercrit. Fluids 2024, 204, 106106. [Google Scholar] [CrossRef]
- Li, H.; Kim, Y.; Jung, H.; Hyun, J.Y.; Shin, I. Near-infrared (NIR) fluorescence-emitting small organic molecules for cancer imaging and therapy. Chem. Soc. Rev. 2022, 51, 8957–9008. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-F.; Al-Taher, M.; Yu, C.-Y.; Liu, Y.-W.; Liu, Y.-Y.; Marescaux, J.; Cheng, Y.-F.; Diana, M.; Wang, C.-C. Super-Selective Intra-Arterial Indocyanine Green Administration for Near-Infrared Fluorescence-Based Positive Staining of Hepatic Segmentation: A Feasibility Study. Surg. Innov. 2021, 28, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xu, D.; Cheng, H.; Chu, C.; Liu, G. Recent Advances in Interventional Fluorescence Imaging: Toward the Precise Visualization of Transarterial Mini-Invasive Delivery Systems. Acc. Mater. Res. 2023, 4, 251–263. [Google Scholar] [CrossRef]
- He, P.; Xiong, Y.; Luo, B.; Liu, J.; Zhang, Y.; Xiong, Y.; Su, S.; Fang, C.; Peng, Y.; Cheng, H.; et al. An exploratory human study of superstable homogeneous lipiodol-indocyanine green formulation for precise surgical navigation in liver cancer. Bioeng. Transl. Med. 2023, 8, e10404. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Cheng, H.; Liu, H.; Zhang, Y.; Liu, G. Super-stable homogeneous embolic agents advance the treatment of hepatocellular carcinoma. iRADIOLOGY 2023, 1, 190–194. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, X.; Liu, G. Superstable homogeneous iodinated formulation technology: Revolutionizing transcatheter arterial chemoembolization. Sci. Bull. 2020, 65, 1685–1687. [Google Scholar] [CrossRef]
- Vorstandlechner, V.; Laggner, M.; Copic, D.; Klas, K.; Direder, M.; Chen, Y.; Golabi, B.; Haslik, W.; Radtke, C.; Tschachler, E.; et al. The serine proteases dipeptidyl-peptidase 4 and urokinase are key molecules in human and mouse scar formation. Nat. Commun. 2021, 12, 6242. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. 5-Fluorouracil in Dermatology: The Diverse Uses Beyond Malignant and Premalignant Skin Disease. Dermatol. Surg. 2021, 47, e66–e70. [Google Scholar] [CrossRef]
- Liu, W.; Wu, X.; Gao, Z.; Xia, L. Minimally Invasive Technologies for Treatment of HTS and Keloids: Low-Dose 5-Fluorouracil. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; pp. 251–262. [Google Scholar]
- Liang, Y.; Ding, A.; Lyu, D.; Wang, D.; Zhou, R. Comparison of Ultrasound-Assisted Low-Dose Versus Medium-Dose 5-Fluorouracil and Triamcinolone Acetonide in the Treatment of Hypertrophic Scar. Dermatol. Ther. 2023, 2023, 5245805. [Google Scholar] [CrossRef]
- Khalid, R.; Mahmood, S.; Mohamed Sofian, Z.; Hilles, A.R.; Hashim, N.M.; Ge, Y. Microneedles and Their Application in Transdermal Delivery of Antihypertensive Drugs-A Review. Pharmaceutics 2023, 15, 2029. [Google Scholar] [CrossRef]
- Malek-Khatabi, A.; Sadat Razavi, M.; Abdollahi, A.; Rahimzadeghan, M.; Moammeri, F.; Sheikhi, M.; Tavakoli, M.; Rad-Malekshahi, M.; Faraji Rad, Z. Recent progress in PLGA-based microneedle-mediated transdermal drug and vaccine delivery. Biomater. Sci. 2023, 11, 5390–5409. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhang, Y.S.; Wang, S.B.; Lee, C.H.; Chen, A.Z. Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications. Adv. Healthc. Mater. 2017, 6, 1700433. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, S.; Wang, Z.; Chen, X.; Dana, R.; Annabi, N. Advanced nanodelivery platforms for topical ophthalmic drug delivery. Drug Discovery Today 2021, 26, 1437–1449. [Google Scholar] [CrossRef]
- Nicholas, M.P.; Mysore, N. Corneal neovascularization. Exp. Eye Res. 2021, 202, 108363. [Google Scholar] [CrossRef]
- Onugwu, A.L.; Nwagwu, C.S.; Onugwu, O.S.; Echezona, A.C.; Agbo, C.P.; Ihim, S.A.; Emeh, P.; Nnamani, P.O.; Attama, A.A.; Khutoryanskiy, V.V. Nanotechnology based drug delivery systems for the treatment of anterior segment eye diseases. J. Control. Release 2023, 354, 465–488. [Google Scholar] [CrossRef]
- Sheikholeslami, B.; Lam, N.W.; Dua, K.; Haghi, M. Exploring the impact of physicochemical properties of liposomal formulations on their in vivo fate. Life Sci. 2022, 300, 120574. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, I.; Bettini, R. Are pharmaceutics really going supercritical? Int. J. Pharm. 2008, 364, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.S.; Pathare, S.R.; More, M.A.; Waykhinde, N.A.; Laddha, U.D.; Salunkhe, K.S.; Kshirsagar, S.J.; Patil, S.S.; Ramteke, K.H. Dry Powder Inhaler with the technical and practical obstacles, and forthcoming platform strategies. J. Control. Release 2023, 355, 292–311. [Google Scholar] [CrossRef]
- Jash, A.; Krueger, A.; Rizvi, S.S.H. Venturi-based rapid expansion of supercritical solution (Vent-RESS): Synthesis of liposomes for pH-triggered delivery of hydrophilic and lipophilic bioactives. Green Chem. 2022, 24, 5326–5337. [Google Scholar] [CrossRef] [PubMed]





| Active Compound | Solvent | Pressure (MPa) | Temperature (°C) | Time (hours) | Size (nm) | Reference |
|---|---|---|---|---|---|---|
| ICG | EtOH/DCM | 10 | 40 | 0.5 | 40.7 ± 4.5 | [32] |
| DOX | MeOH | 10 | 45 | 0.5 | 155 ± 29 | [39] |
| 5-Fu | MeOH | 20 | / | 1 | 41.08 ± 9.74 | [40] |
| SU6668 | EtOH/DMSO | / | / | / | 135 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Liang, X.; Peng, Y.; Liu, G.; Cheng, H. Supercritical Fluids: An Innovative Strategy for Drug Development. Bioengineering 2024, 11, 788. https://doi.org/10.3390/bioengineering11080788
Liu H, Liang X, Peng Y, Liu G, Cheng H. Supercritical Fluids: An Innovative Strategy for Drug Development. Bioengineering. 2024; 11(8):788. https://doi.org/10.3390/bioengineering11080788
Chicago/Turabian StyleLiu, Hui, Xiaoliu Liang, Yisheng Peng, Gang Liu, and Hongwei Cheng. 2024. "Supercritical Fluids: An Innovative Strategy for Drug Development" Bioengineering 11, no. 8: 788. https://doi.org/10.3390/bioengineering11080788
APA StyleLiu, H., Liang, X., Peng, Y., Liu, G., & Cheng, H. (2024). Supercritical Fluids: An Innovative Strategy for Drug Development. Bioengineering, 11(8), 788. https://doi.org/10.3390/bioengineering11080788









