Stable and Thin-Polymer-Based Modification of Neurovascular Stents with 2-Methacryloyloxyethyl Phosphorylcholine Polymer for Antithrombogenicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating of Stent and Flat Metallic Plate with 2-Methacryloyloxyethyl Phosphorylcholine Polymer
2.2. Surface Characterization
2.2.1. X-ray Photoelectron Spectroscopy
2.2.2. Atomic Force Microscopic Observation
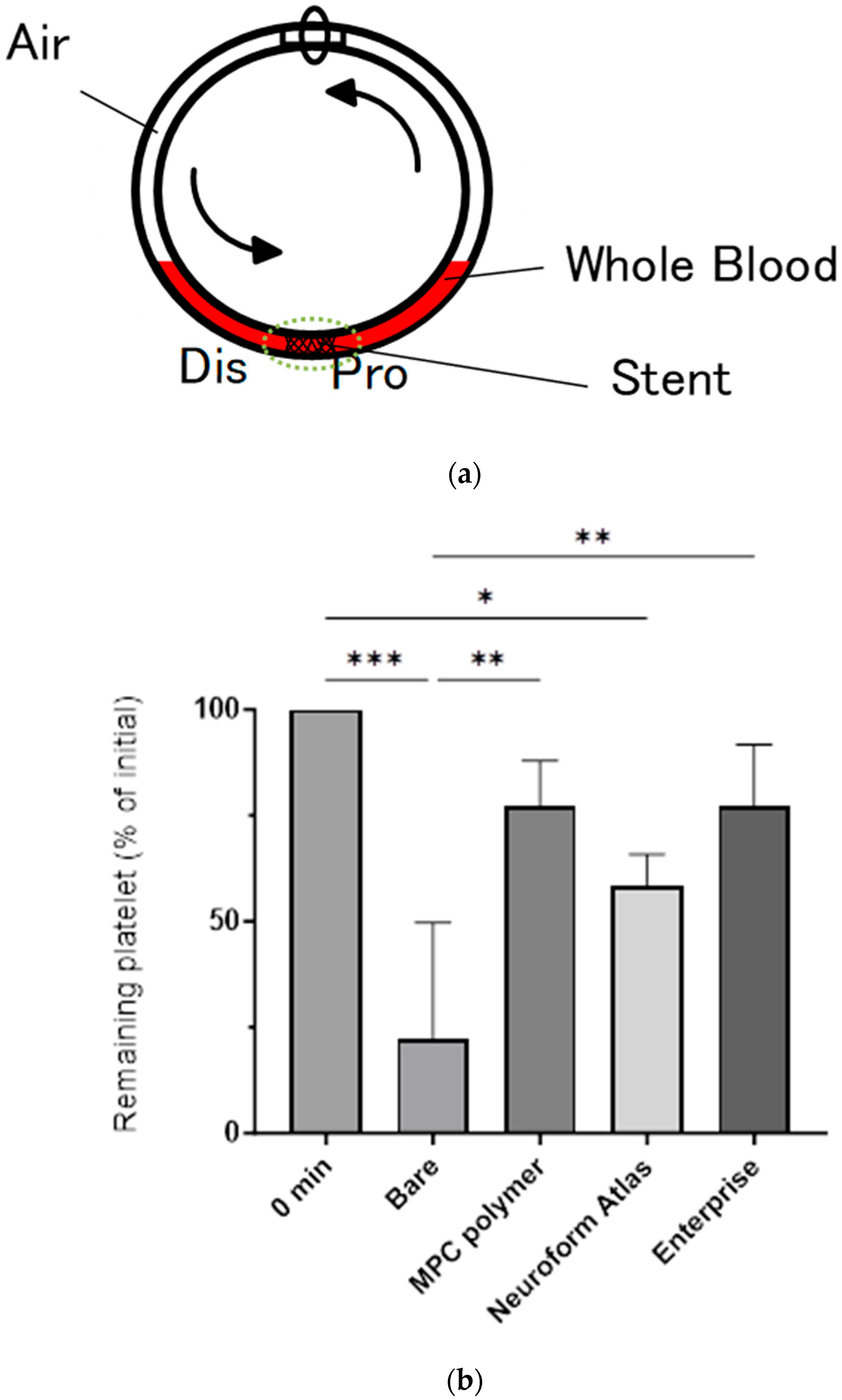
2.3. Blood Compatibility Assay Using Chandler Loop Model
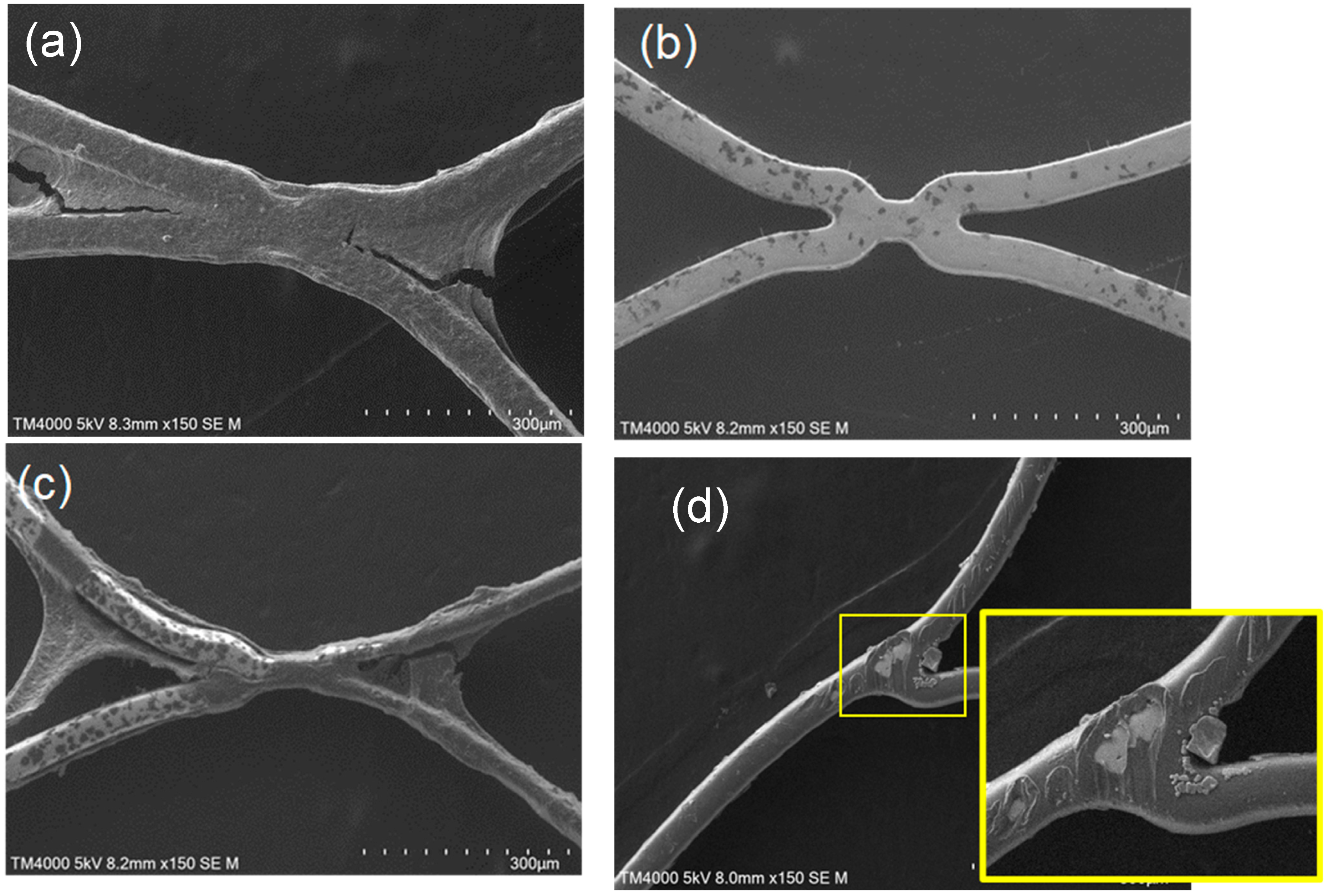
2.4. Scanning Electron Microscopic Observation
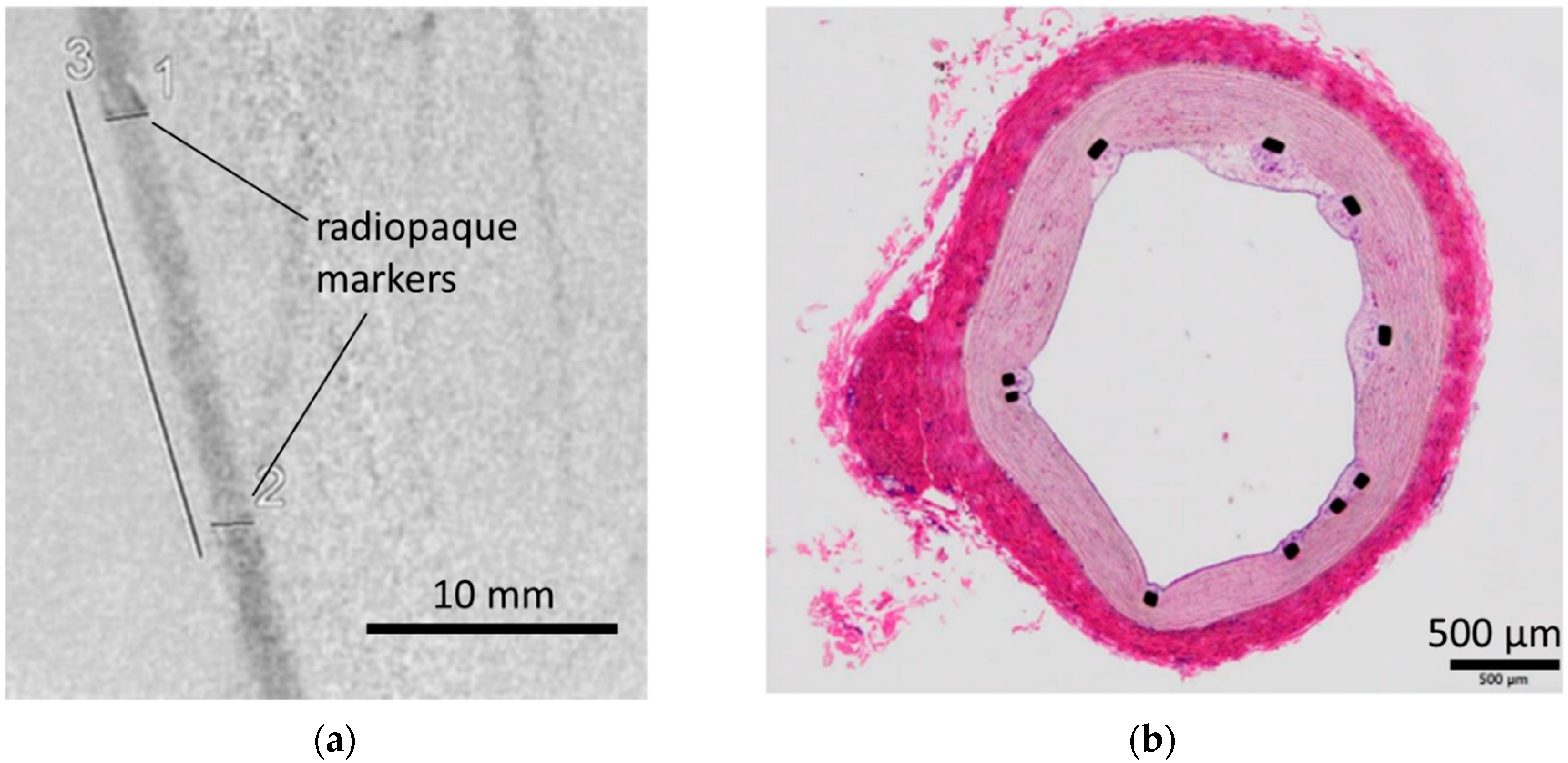
2.5. In Vivo Implantation of Coated Stents in Pigs
2.6. Statistical Analysis
3. Results and Discussion
3.1. Surface Characterization
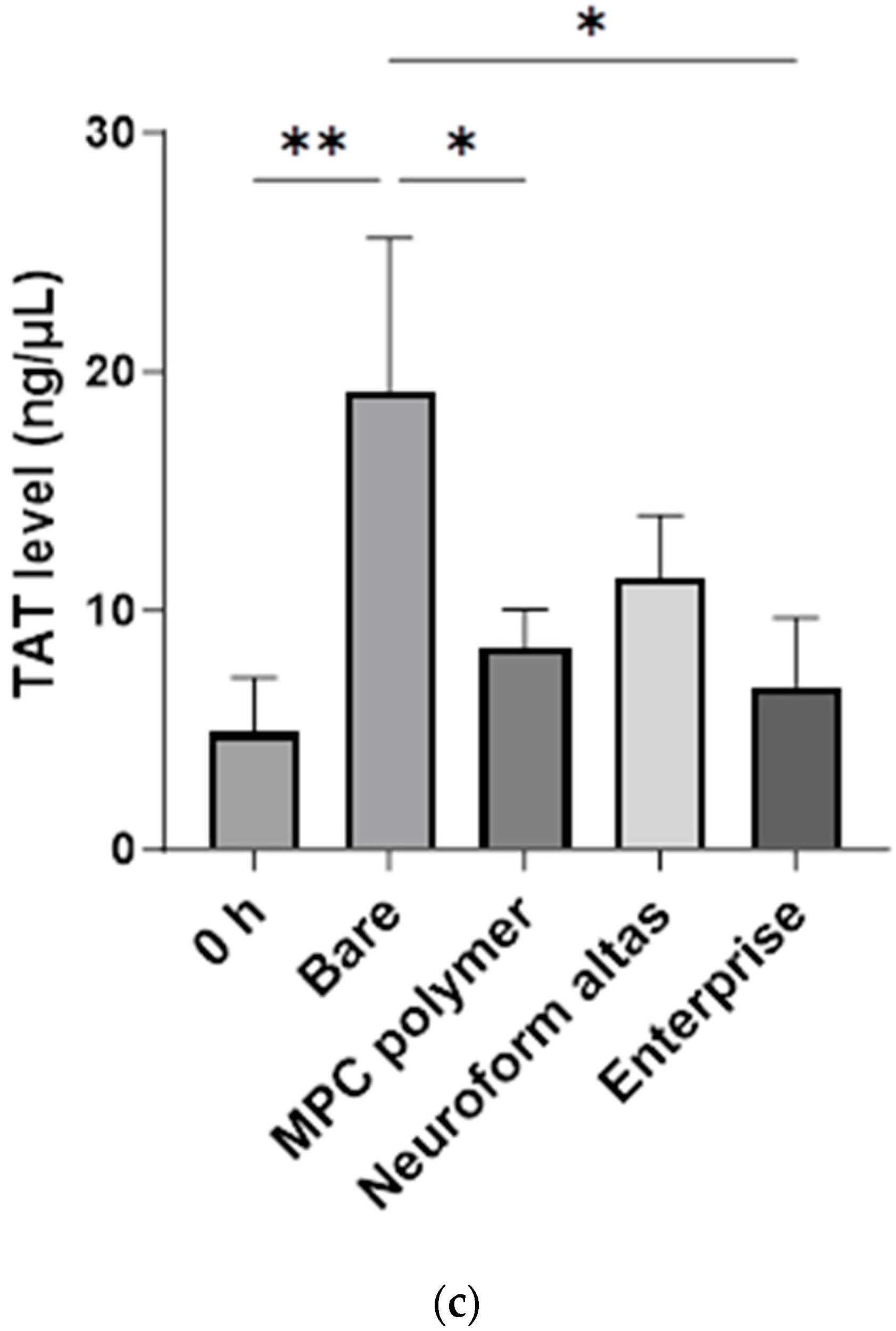
3.2. In Vitro Evaluation: Whole-Blood Test Using Chandler Loop
3.3. In Vivo Evaluation: Stent Placement in Internal Thoracic Artery of a Pig
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hop, J.W.; Rinkel, G.J.; Algra, A.; Van Gijn, J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: A systematic review. Stroke 1997, 28, 660–664. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, J.; Rinkel, G.J.E. Subarachnoid haemorrhage: Diagnosis, causes and management. Brain 2001, 124, 249–278. [Google Scholar] [CrossRef] [PubMed]
- Van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, C.; Kaier, K.; Shah, M.; Mühlen, C.v.Z.; Beck, J.; Urbach, H.; Meckel, S. Cerebral aneurysms: Germany-wide real-world outcome data of endovascular or neurosurgical treatment from 2007 to 2019. J. NeuroInterventional Surg. 2024, 16, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Golnari, P.; Nazari, P.; Garcia, R.M.; Weiss, H.; Shaibani, A.; Hurley, M.C.; Ansari, S.A.; Potts, M.B.; Jahromi, B.S. Volumes, outcomes, and complications after surgical versus endovascular treatment of aneurysms in the United States (1993–2015): Continued evolution versus steady-state after more than 2 decades of practice. J. Neurosurg. 2021, 134, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Medical Advisory Secretariat Ministry of Health and Long-Term Care. Coil embolization for intracranial aneurysms: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2006, 6, 1–114. [Google Scholar]
- Park, H.S.; Kwon, S.C.; Park, E.S.; Park, J.B.; Kim, M.S. A new definition for wide-necked cerebral aneurysms. J. Cerebrovasc. Endovasc. Neurosurg. 2019, 21, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Chalouhi, N.; Jabbour, P.; Singhal, S.; Drueding, R.; Starke, R.M.; Dalyai, R.T.; Tjoumakaris, S.; Gonzalez, L.F.; Dumont, A.S.; Rosenwasser, R.; et al. Stent-Assisted Coiling of Intracranial Aneurysms Predictors of Complications, Recanalization, and Outcome in 508 Cases. Stroke 2013, 44, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wang, Y.-J.; Deng, Z.; Wu, Q.; Zhang, J.-M. Stent-Assisted Coiling versus Coiling in Treatment of Intracranial Aneurysm: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e82311. [Google Scholar] [CrossRef]
- Quintana, E.M.; Valdes, P.V.; Deza, E.M.; García, A.G.; Rodríguez, M.C.; Pérez, J.M.J.; Chaviano, J.; Morales, J.C.G.; Batista, K.P.; García, F.A. Initial experience and one-year follow-up with Neuroform Atlas Stent System for the treatment of brain aneurysms. Interv. Neuroradiol. 2019, 25, 521–529. [Google Scholar] [CrossRef]
- Burkhardt, J.-K.; Srinivasan, V.; Srivatsan, A.; Albuquerque, F.; Ducruet, A.; Hendricks, B.; Gross, B.; Jankowitz, B.; Thomas, A.; Ogilvy, C.; et al. Multicenter Postmarket Analysis of the Neuroform Atlas Stent for Stent-Assisted Coil Embolization of Intracranial Aneurysms. Am. J. Neuroradiol. 2020, 4, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodayan, Y.; Rhodes, N.; Blackburn, S.; Derdeyn, C.P.; Cross, D.T.; Moran, C.J. Comparison of Enterprise With Neuroform Stent-Assisted Coiling of Intracranial Aneurysms. Am. J. Roentgenol. 2013, 200, 872–878. [Google Scholar] [CrossRef]
- Ge, H.; Lv, X.; Yang, X.; He, H.; Jin, H.; Li, Y. LVIS Stent Versus Enterprise Stent for the Treatment of Unruptured Intracranial Aneurysms. World Neurosurg. 2016, 91, 365–370. [Google Scholar] [CrossRef]
- Alkhalili, K.; Hannallah, J.; Cobb, M.; Chalouhi, N.; Philips, J.L.; Echeverria, A.B.; Jabbour, P.; Babiker, M.H.; Frakes, D.H.; Gonzalez, L.F. The Effect of Stents in Cerebral Aneurysms: A Review. Asian J. Neurosurg. 2018, 13, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Iida, O.; Ohki, T.; Soga, Y.; Suematsu, N.; Nakama, T.; Yamaoka, T.; Tobita, K.; Ichihashi, S. Twelve-Month Outcomes From the Japanese Post-Market Surveillance Study of the Viabahn Endoprosthesis. J. Endovasc. Ther. 2022, 29, 855–865. [Google Scholar] [CrossRef]
- Ishihara, K.; Ueda, T.; Nakabayashi, N. Preparation of Phospholipid Polymers and Their Properties as Polymer Hydrogel Membranes. Polym J. 1990, 22, 355–360. [Google Scholar] [CrossRef]
- Rice, H.; Galdámez, M.M.; Holtmannspötter, M.; Spelle, L.; Lagios, K.; Ruggiero, M.; Vega, P.; Sonwalkar, H.; Chapot, R.; Lamin, S. Periprocedural to 1-year safety and efficacy outcomes with the Pipeline Embolization Device with Shield technology for intracranial aneurysms. NeuroIntervent Surg. 2020, 12, 1107–1112. [Google Scholar] [CrossRef]
- Ishihara, K. Revolutionary advances in 2-methacryloyloxyethyl phosphorylcholine polymers as biomaterials. J. Biomed. Mater. Res. Part A 2019, 107, 933–943. [Google Scholar] [CrossRef]
- Asif, S.; Asawa, K.; Inoue, Y.; Ishihara, K.; Lindell, B.; Holmgren, R.; Nilsson, B.; Rydén, A.; Jensen-Waern, M.; Teramura, Y.; et al. Validation of an MPC Polymer Coating to Attenuate Surface-Induced Crosstalk between the Complement and Coagulation Systems in Whole Blood in In Vitro and In Vivo Models. Macromol. Biosci. 2019, 19, e1800485. [Google Scholar] [CrossRef]
- Inuzuka, N.; Shobayashi, Y.; Tateshima, S.; Sato, Y.; Ohba, Y.; Ekdahl, K.N.; Nilsson, B.; Teramura, Y. Stent coating containing a charged silane coupling agent that regulates protein adsorption to confer antithrombotic and cell-adhesion properties. Sci. Rep. 2024, 14, 15178. [Google Scholar] [CrossRef]
- Kyomoto, M.; Moro, T.; Saiga, K.-I.; Miyaji, F.; Kawaguchi, H.; Takatori, Y.; Nakamura, K.; Ishihara, K. Lubricity and stability of poly(2-methacryloyloxyethyl phosphorylcholine) polymer layer on Co–Cr–Mo surface for hemi-arthroplasty to prevent degeneration of articular cartilage. Biomaterials 2010, 31, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Ferez, L.; Thami, T.; Akpalo, E.; Flaud, V.; Tauk, L.; Janot, J.M.; Déjardin, P. Interface of Covalently Bonded Phospholipids with a Phosphorylcholine Head: Characterization, Protein Nonadsorption, and Further Functionalization. Langmuir 2011, 27, 11536–11544. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K. Blood compatible surfaces with phosphorylcholine-based polymers for cardiovascular medical devices. Langmuir 2019, 5, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Snyder, T.A.; Tsukui, H.; Kihara, S.; Akimoto, T.; Litwak, K.N.; Kameneva, M.V.; Yamazaki, K.; Wagner, W.R. Preclinical biocompatibility assessment of the EVAHEART ventricular assist device: Coating comparison and platelet activation. J. Biomed. Mater. Res. Part A 2006, 81, 85–92. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. 510(k) Premarket Notification Summary–K173200; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- Manning, N.W.; Cheung, A.; Phillips, T.J.; Wenderoth, J.D. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: Multicentre experience. J. NeuroInterventinal Surg. 2019, 11, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Jin, L.; Dong, J.; Fu, Z.; Liu, E.; Yin, S.; Jian, L.; Luo, P.; Liu, B.; Huang, W.; et al. Clinical outcomes of pipeline embolization devices with shield technology for treating intracranial aneurysms. Front. Neurol. 2022, 13, 971664. [Google Scholar] [CrossRef]
- Yeomans, J.; Sandu, L.; Sastry, A. Pipeline Flex embolisation device with Shield Technology for the treatment of patients with intracranial aneurysms: Periprocedural and 6 month outcomes. Neuroradiol. J. 2020, 33, 471–478. [Google Scholar] [CrossRef]
- Kawamura, A.; Toda, K.; Sawa, Y. First clinical experience with the double cuff tipless inflow cannula in the EVAHEART left ventricular assist system: Case report. Artif. Organs 2020, 44, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Alkarithi, G.; Duval, C.; Shi, Y.; Macrae, F.L.; Ariëns, R.A. Thrombus Structural Composition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2370–2383. [Google Scholar] [CrossRef]
- Torrado, J.; Buckley, L.; Durán, A.; Trujillo, P.; Toldo, S.; Valle Raleigh, J.; Abbate, A.; Biondi-Zoccai, G.; Guzmán, L.A. Restenosis, Stent Thrombosis, and Bleeding Complications. J. Am. Coll. Cardiol. 2018, 71, 1676–1695. [Google Scholar] [CrossRef]
- Reininger, A.J.; Bernlochner, I.; Penz, S.M.; Ravanat, C.; Smethurst, P.; Farndale, R.W.; Gachet, C.; Brandl, R.; Siess, W. A 2-Step Mechanism of Arterial Thrombus Formation Induced by Human Atherosclerotic Plaques. J. Am. Coll. Cardiol. 2010, 55, 1147–1158. [Google Scholar] [CrossRef]
- Cho, S.H.; Jo, W.I.; Jo, Y.E.; Yang, K.H.; Park, J.C.; Lee, D.H. Bench-top Comparison of Physical Properties of 4 Commercially-Available Self-Expanding Intracranial Stents. Neurointervention 2017, 12, 31–39. [Google Scholar] [CrossRef]
- Lewis, A.L.; Tolhurst, L.A.; Stratford, P.W. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre- and post-implantation. Biomaterials 2002, 7, 1697–1706. [Google Scholar] [CrossRef]



| Score | Evaluation Criteria |
|---|---|
| Level 1 | Almost no thrombus formation on the stent surface, which remains flat. |
| Level 2 | Slight thrombus formation on the stent surface, but it is mostly flat. |
| Level 3 | Thin thrombus formation observed in localized areas of the stent surface. |
| Level 4 | Wide areas of the stent surface covered with a thin thrombus, but overall, it is flat. |
| Level 5 | Stent surface is predominantly flat, but localized, raised thrombi are observed. |
| Level 6 | Wide areas of the stent surface are covered with a thin thrombus, with localized, raised thrombi. |
| Level 7 | Stent surface is predominantly covered with a thin thrombus, with some areas having raised thrombi. |
| Level 8 | Entire stent surface is covered with thrombi, with numerous localized, raised thrombi. |
| Level 9 | Entire stent surface is covered with thick, raised thrombi, with some areas having significantly thick, multilayered thrombi. |
| Level 10 | Entire stent surface is completely covered with significantly thick, multilayered thrombi. |
| Stent Sample | Atomic Component (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| P(2p) | Si(2p) | C(1s) | N(1s) | Ti(2p) | O(1s) | Ni(2p3/2) | ||
| Bare | Mean | 0.0 | 0.0 | 35.5 | 0.6 | 27.6 | 32.3 | 3.3 |
| (n = 6) | SD | 0.0 | 0.0 | 12.6 | 0.7 | 9.9 | 3.8 | 2.1 |
| MPC polymer-coated | Mean | 4.4 | 0.1 | 46.3 | 1.2 | 13.3 | 32.2 | 0.9 |
| (n = 6) | SD | 1.3 | 0.2 | 11.6 | 0.3 | 6.2 | 4.9 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inuzuka, N.; Shobayashi, Y.; Tateshima, S.; Sato, Y.; Ohba, Y.; Ishihara, K.; Teramura, Y. Stable and Thin-Polymer-Based Modification of Neurovascular Stents with 2-Methacryloyloxyethyl Phosphorylcholine Polymer for Antithrombogenicity. Bioengineering 2024, 11, 833. https://doi.org/10.3390/bioengineering11080833
Inuzuka N, Shobayashi Y, Tateshima S, Sato Y, Ohba Y, Ishihara K, Teramura Y. Stable and Thin-Polymer-Based Modification of Neurovascular Stents with 2-Methacryloyloxyethyl Phosphorylcholine Polymer for Antithrombogenicity. Bioengineering. 2024; 11(8):833. https://doi.org/10.3390/bioengineering11080833
Chicago/Turabian StyleInuzuka, Naoki, Yasuhiro Shobayashi, Satoshi Tateshima, Yuya Sato, Yoshio Ohba, Kazuhiko Ishihara, and Yuji Teramura. 2024. "Stable and Thin-Polymer-Based Modification of Neurovascular Stents with 2-Methacryloyloxyethyl Phosphorylcholine Polymer for Antithrombogenicity" Bioengineering 11, no. 8: 833. https://doi.org/10.3390/bioengineering11080833






