Long-Term Survival and Regeneration Following Transplantation of 3D-Printed Biodegradable PCL Tracheal Grafts in Large-Scale Porcine Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Preparation of PCL Tracheal Grafts
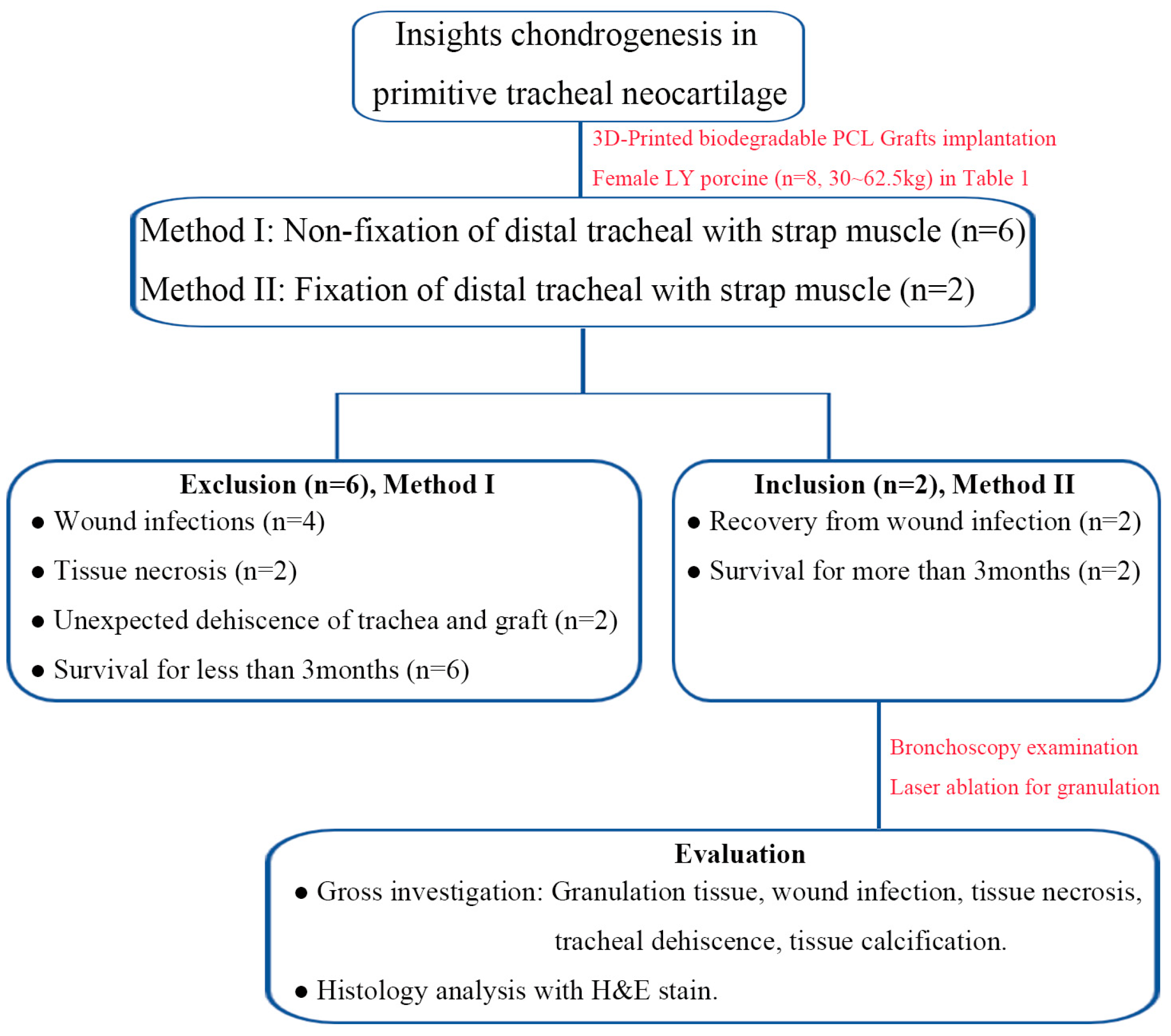
2.2. Methods Design for In Vivo Experiments
2.3. Anesthesia, Surgical Intervention and Postoperative Care
2.4. Scheduled Postoperative Bronchoscopic Evaluation and Laser Ablation for Granulation Tissue
2.5. Tracheal Tissue Harvested and Gross Examination
2.6. Regenerated Tubular Tissue outside Graft by Histology Analysis
2.7. Statistical Analyses
3. Results
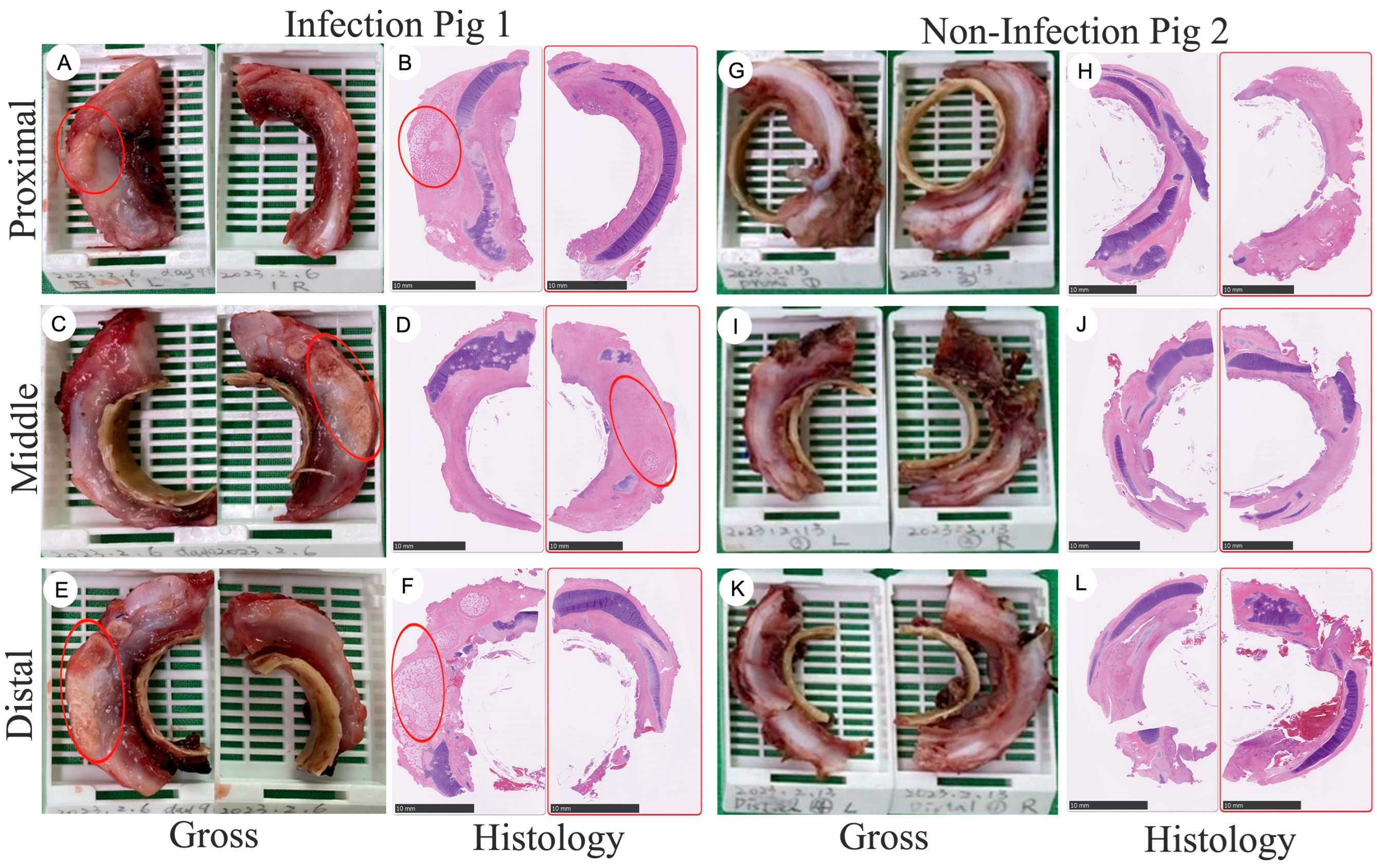
3.1. Gross Specimen of Tissue Regeneration Following Long-Term Implantation into Large-Scale Porcines
3.2. Tracheal Tissue Regeneration Followed by Tracheal Graft Implantation
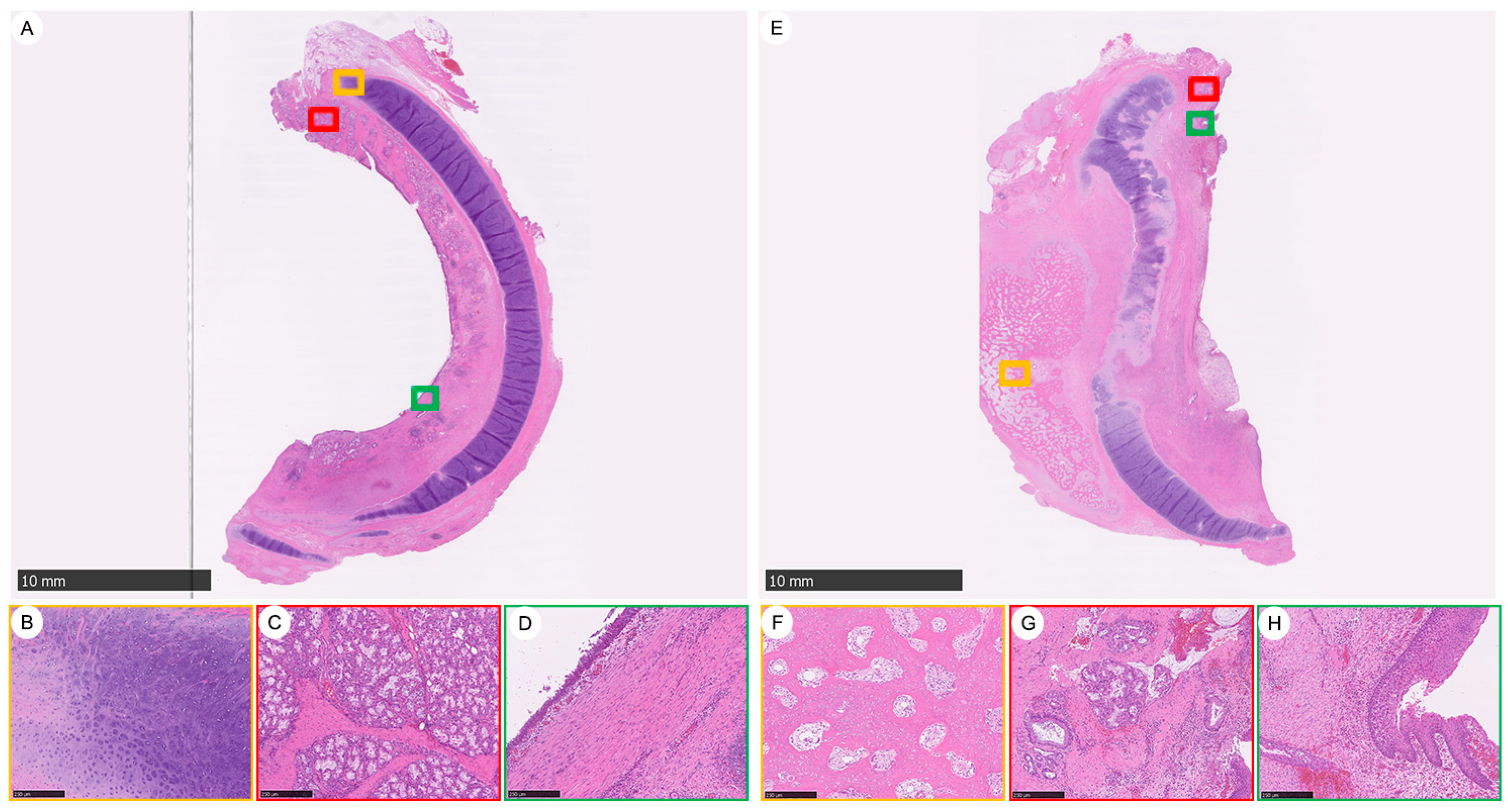
3.3. The Impact of Bacterial Infection-Induced Inflammation after Tracheal Graft Transplantation and Heterotopic Ossification (HO) Observed 92 Days Post-Implantation
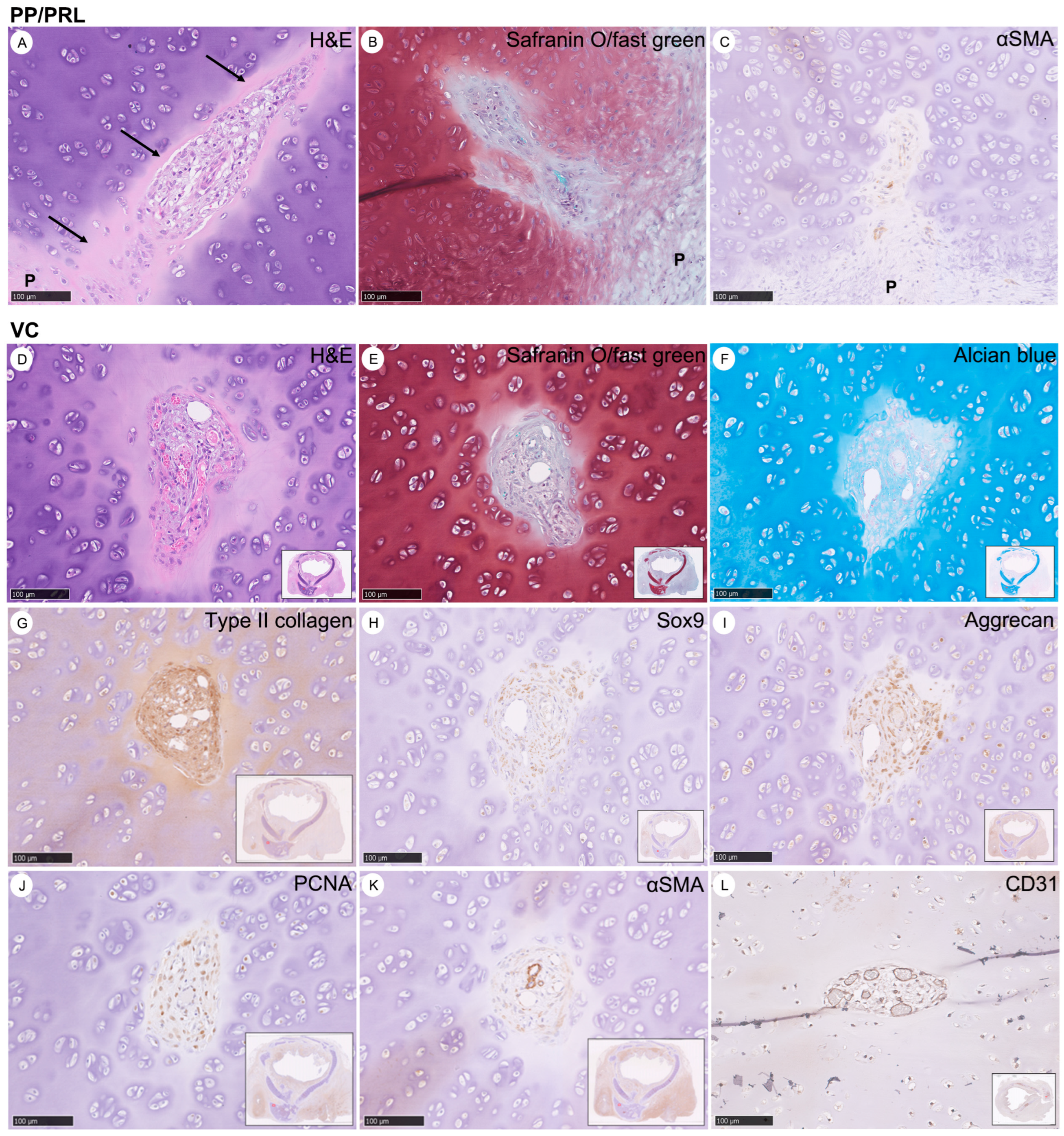
3.4. Characteristics of Vascular Canals (VCs) in the Evolution of Chondrogenesis: Derived from Perichondrium as Chondro-Modulators in the Maturation of Cartilage to Prune Excessive Chondrocytes
3.5. Concise Four-Stage Developmental Model to Elucidate the Intricate Process of Tracheal Cartilage Regeneration Based on the Occurrence of Key Chondrogenesis Features
3.6. Types of Cartilage Evolution during Chondrogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakanishi, R. Cryopreservation of the tracheal grafts: Review and perspective. Organogenesis 2009, 5, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lan, N.; Pang, C.; Tong, X. Bone marrow-derived mesenchymal stem cells enhance cryopreserved trachea allograft epithelium regeneration and vascular endothelial growth factor expression. Transplantation 2011, 92, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Martinod, E.; Seguin, A.; Radu, D.M.; Boddaert, G.; Chouahnia, K.; Fialaire-Legendre, A.; Dutau, H.; Vénissac, N.; Marquette, C.H.; Baillard, C.; et al. Airway transplantation: A challenge for regenerative medicine. Eur. J. Med. Res. 2013, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Candas, F.; Gorur, R.; Haholu, A.; Yildizhan, A.; Yucel, O.; Ay, H.; Memis, A.; Isitmangil, T. Is Tracheal Transplantation Possible With Cryopreserved Tracheal Allograft and Hyperbaric Oxygen Therapy? An Experimental Study. Ann. Thorac. Surg. 2016, 101, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayoubi, A.M.; Rehmani, S.S.; Sinclair, C.F.; Lebovics, R.S.; Bhora, F.Y. Reconstruction of Anterior Tracheal Defects Using a Bioengineered Graft in a Porcine Model. Ann. Thorac. Surg. 2017, 103, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Ott, L.M.; Weatherly, R.A.; Detamore, M.S. Overview of tracheal tissue engineering: Clinical need drives the laboratory approach. Ann. Biomed. Eng. 2011, 39, 2091–2113. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liao, Z.; Yang, Z.; Gao, C.; Fu, L.; Li, P.; Zhao, T.; Cao, F.; Chen, W.; Yuan, Z.; et al. 3D Printed Poly(ε-Caprolactone)/Meniscus Extracellular Matrix Composite Scaffold Functionalized With Kartogenin-Releasing PLGA Microspheres for Meniscus Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 662381. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jung, S.Y.; Lee, S.J.; Lee, H.J.; Truong, M.D.; Kim, H.S. Fabrication and characterization of 3D-printed elastic auricular scaffolds: A pilot study. Laryngoscope 2019, 129, 351–357. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, J.; Richards, G.; Lu, S.; Eglin, D. Optimization of electrospray fabrication of stem cell-embedded alginate-gelatin microspheres and their assembly in 3D-printed poly(ε-caprolactone) scaffold for cartilage tissue engineering. J. Orthop. Transl. 2019, 18, 128–141. [Google Scholar] [CrossRef]
- Wang, C.; Dong, J.; Liu, F.; Liu, N.; Li, L. 3D-printed PCL@BG scaffold integrated with SDF-1α-loaded hydrogel for enhancing local treatment of bone defects. J. Biol. Eng. 2024, 18, 1. [Google Scholar] [CrossRef]
- Huber, F.; Vollmer, D.; Vinke, J.; Riedel, B.; Zankovic, S.; Schmal, H.; Seidenstuecker, M. Influence of 3D Printing Parameters on the Mechanical Stability of PCL Scaffolds and the Proliferation Behavior of Bone Cells. Materials 2022, 15, 2091. [Google Scholar] [CrossRef]
- de Wit, R.J.J.; van Dis, D.J.; Bertrand, M.E.; Tiemessen, D.; Siddiqi, S.; Oosterwijk, E.; Verhagen, A. Scaffold-based tissue engineering: Supercritical carbon dioxide as an alternative method for decellularization and sterilization of dense materials. Acta Biomater. 2023, 155, 323–332. [Google Scholar] [CrossRef]
- Guimaraes, A.B.; Correa, A.T.; Pego-Fernandes, P.M.; Maizato, M.J.; Cestari, I.A.; Cardoso, P.F. Biomechanical Properties of the Porcine Trachea before and after Decellularization for Airway Transplantation. J. Heart Lung Transplant. 2021, 40 (Suppl. S4), S385–S386. [Google Scholar] [CrossRef]
- V, G.R.; Wilson, J.; L, V.T.; Nair, P.D. Assessing the 3D Printability of an Elastomeric Poly(caprolactone-co-lactide) Copolymer as a Potential Material for 3D Printing Tracheal Scaffolds. ACS Omega 2022, 7, 7002–7011. [Google Scholar]
- Wang, H.; Wang, Z.; Liu, H.; Liu, J.; Li, R.; Zhu, X.; Ren, M.; Wang, M.; Liu, Y.; Li, Y.; et al. Three-Dimensional Printing Strategies for Irregularly Shaped Cartilage Tissue Engineering: Current State and Challenges. Front. Bioeng. Biotechnol. 2021, 9, 777039. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, B. Understanding cellular mechanisms underlying airway epithelial repair: Selecting the most appropriate animal models. Sci. World J. 2012, 2012, 961684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sundaram, S.; Le, A.V.; Huang, A.H.; Zhang, J.; Hatachi, G.; Beloiartsev, A.; Caty, M.G.; Yi, T.; Leiby, K.; et al. Engineered Tissue-Stent Biocomposites as Tracheal Replacements. Tissue Eng. Part A 2016, 22, 1086–1097. [Google Scholar] [CrossRef]
- Weidenbecher, M.; Tucker, H.M.; Gilpin, D.A.; Dennis, J.E. Tissue-engineered trachea for airway reconstruction. Laryngoscope 2009, 119, 2118–2123. [Google Scholar] [CrossRef]
- Komura, M.; Komura, H.; Kanamori, Y.; Tanaka, Y.; Suzuki, K.; Sugiyama, M.; Nakahara, S.; Kawashima, H.; Hatanaka, A.; Hoshi, K.; et al. An animal model study for tissue-engineered trachea fabricated from a biodegradable scaffold using chondrocytes to augment repair of tracheal stenosis. J. Pediatr. Surg. 2008, 43, 2141–2146. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Y.; Zhang, Z.; Tao, R.; Liu, Y.; He, A.; Yin, Z.; Li, D.; Zhang, W.; Liu, W.; et al. Long-term functional reconstruction of segmental tracheal defect by pedicled tissue-engineered trachea in rabbits. Biomaterials 2013, 34, 3336–3344. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, H.; Dong, W.; Bai, J.; Gao, B.; Xia, D.; Feng, B.; Chen, M.; He, X.; Yin, M.; et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci. Rep. 2017, 7, 5246. [Google Scholar] [CrossRef]
- Martinod, E.; Chouahnia, K.; Radu, D.M.; Joudiou, P.; Uzunhan, Y.; Bensidhoum, M.; Santos Portela, A.M.; Guiraudet, P.; Peretti, M.; Destable, M.D.; et al. Feasibility of Bioengineered Tracheal and Bronchial Reconstruction Using Stented Aortic Matrices. JAMA 2018, 319, 2212–2222. [Google Scholar] [CrossRef]
- Martinod, E.; Paquet, J.; Dutau, H.; Radu, D.M.; Bensidhoum, M.; Abad, S.; Uzunhan, Y.; Vicaut, E.; Petite, H. In Vivo Tissue Engineering of Human Airways. Ann. Thorac. Surg. 2017, 103, 1631–1640. [Google Scholar] [CrossRef]
- Grillo, H.C. Tracheal replacement: A critical review. Ann. Thorac. Surg. 2002, 73, 1995–2004. [Google Scholar] [CrossRef]
- Delaere, P.R.; Vranckx, J.J.; Meulemans, J.; Vander Poorten, V.; Segers, K.; Van Raemdonck, D.; De Leyn, P.; Decaluwé, H.; Dooms, C.; Verleden, G. Learning curve in tracheal allotransplantation. Am. J. Transpl. 2012, 12, 2538–2545. [Google Scholar] [CrossRef] [PubMed]
- Ekser, B.; Ezzelarab, M.; Hara, H.; van der Windt, D.J.; Wijkstrom, M.; Bottino, R.; Trucco, M.; Cooper, D.K. Clinical xenotransplantation: The next medical revolution? Lancet 2012, 379, 672–683. [Google Scholar] [CrossRef]
- Fanous, N.; Husain, S.A.; Ruzmetov, M.; Rodefeld, M.D.; Turrentine, M.W.; Brown, J.W. Anterior pericardial tracheoplasty for long-segment tracheal stenosis: Long-term outcomes. J. Thorac. Cardiovasc. Surg. 2010, 139, 18–23; discussion 23–25. [Google Scholar] [CrossRef]
- Fishman, J.M.; Lowdell, M.; Birchall, M.A. Stem cell-based organ replacements-airway and lung tissue engineering. Semin. Pediatr. Surg. 2014, 23, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Nakamura, T.; Kanemaru, S.; Asato, R.; Yamashita, M.; Tanaka, S.; Magrufov, A.; Ito, J.; Shimizu, Y. Regenerative medicine of the trachea: The first human case. Ann. Otol. Rhinol. Laryngol. 2005, 114, 429–433. [Google Scholar] [CrossRef]
- Kim, I.G.; Wu, Y.; Park, S.A.; Cho, H.; Choi, J.J.; Kwon, S.K.; Shin, J.W.; Chung, E.J. Tissue-Engineered Esophagus via Bioreactor Cultivation for Circumferential Esophageal Reconstruction. Tissue Eng. Part A 2019, 25, 1478–1492. [Google Scholar] [CrossRef]
- Shai, S.E.; Lai, Y.L.; Hung, Y.W.; Hsieh, C.W.; Huang, B.J.; Su, K.C.; Wang, C.H.; Hung, S.C. De novo cartilage growth after implantation of a 3-D-printed tracheal graft in a porcine model. Am. J. Transl. Res. 2020, 12, 3728–3740. [Google Scholar] [PubMed]
- Liu, Y.; Zhang, J.; Long, J.; Qiu, X.; Wang, T.; Wang, J. The Effects of Rapamycin on the Proliferation, Migration, and Apoptosis of Human Tracheal Fibroblasts (HTrF) and Human Tracheal Epithelial Cells (HTEpiC). J. Clin. Med. 2022, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, M.; Motz, K.; Murphy, M.; Feeley, M.; Ding, D.; Lee, A.; Elisseeff, J.H.; Hillel, A.T. Engineering an immunomodulatory drug-eluting stent to treat laryngotracheal stenosis. Biomater. Sci. 2019, 7, 1863–1874. [Google Scholar] [CrossRef] [PubMed]


| Item | Animals | |
|---|---|---|
| Porcine 1 | Porcine 2 | |
| Weight, kg | 60 | 62.5 |
| Number of bronchoscopy | 9 | 10 |
| Number of laser ablation for granulation tissue | 8 | 7 |
| Complication | Granulation Infection (soft tissue) Calcification | Granulation |
| Weight gain, kg | 100 | 101 |
| Survival day | 92 | 92 |
| Cause of death (no. of animal) | Sacrifice | Sacrifice |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shai, S.-E.; Lai, Y.-L.; Hung, Y.-W.; Hsieh, C.-W.; Su, K.-C.; Wang, C.-H.; Chao, T.-H.; Chiu, Y.-T.; Wu, C.-C.; Hung, S.-C. Long-Term Survival and Regeneration Following Transplantation of 3D-Printed Biodegradable PCL Tracheal Grafts in Large-Scale Porcine Models. Bioengineering 2024, 11, 832. https://doi.org/10.3390/bioengineering11080832
Shai S-E, Lai Y-L, Hung Y-W, Hsieh C-W, Su K-C, Wang C-H, Chao T-H, Chiu Y-T, Wu C-C, Hung S-C. Long-Term Survival and Regeneration Following Transplantation of 3D-Printed Biodegradable PCL Tracheal Grafts in Large-Scale Porcine Models. Bioengineering. 2024; 11(8):832. https://doi.org/10.3390/bioengineering11080832
Chicago/Turabian StyleShai, Sen-Ei, Yi-Ling Lai, Yi-Wen Hung, Chi-Wei Hsieh, Kuo-Chih Su, Chun-Hsiang Wang, Te-Hsin Chao, Yung-Tsung Chiu, Chia-Ching Wu, and Shih-Chieh Hung. 2024. "Long-Term Survival and Regeneration Following Transplantation of 3D-Printed Biodegradable PCL Tracheal Grafts in Large-Scale Porcine Models" Bioengineering 11, no. 8: 832. https://doi.org/10.3390/bioengineering11080832
APA StyleShai, S.-E., Lai, Y.-L., Hung, Y.-W., Hsieh, C.-W., Su, K.-C., Wang, C.-H., Chao, T.-H., Chiu, Y.-T., Wu, C.-C., & Hung, S.-C. (2024). Long-Term Survival and Regeneration Following Transplantation of 3D-Printed Biodegradable PCL Tracheal Grafts in Large-Scale Porcine Models. Bioengineering, 11(8), 832. https://doi.org/10.3390/bioengineering11080832









