Controlled Lateral Pressure on Cortical Bone Using Blade-Equipped Implants: An Experimental Study in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Study Design
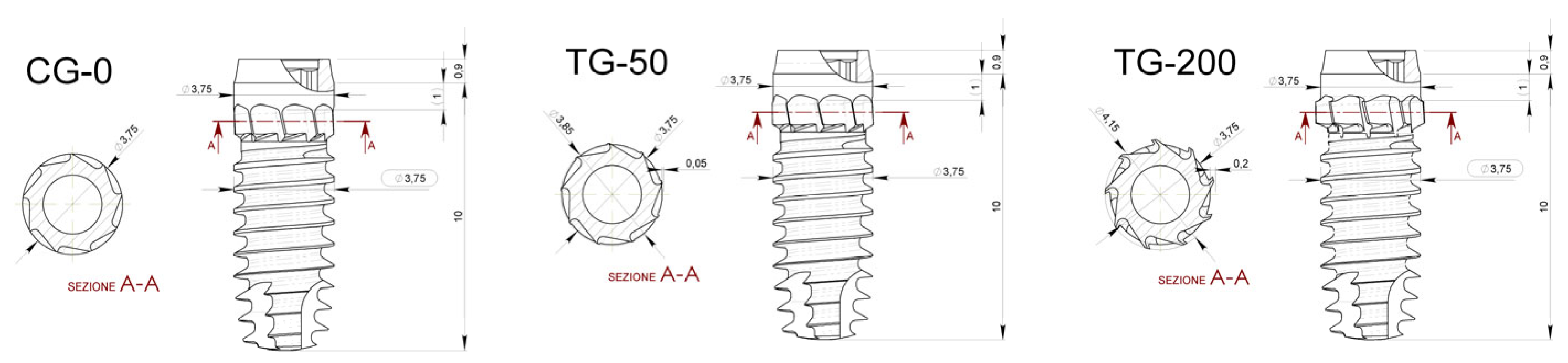
- Control Group (CG-0): Neutral blade (0.0 mm)/no radial difference;
- Test Group 1 (TG-50): Blades with a radial difference of +0.05 mm, resulting in mild bone decompression;
- Test Group 2 (TG-200): Blades with a radial difference of +0.2 mm, resulting in greater bone decompression.
2.3. Experimental Animals and Sample Size
2.4. Randomization and Allocation Concealment
2.5. Implant Characteristics
2.6. Anesthesia and Medication Procedures
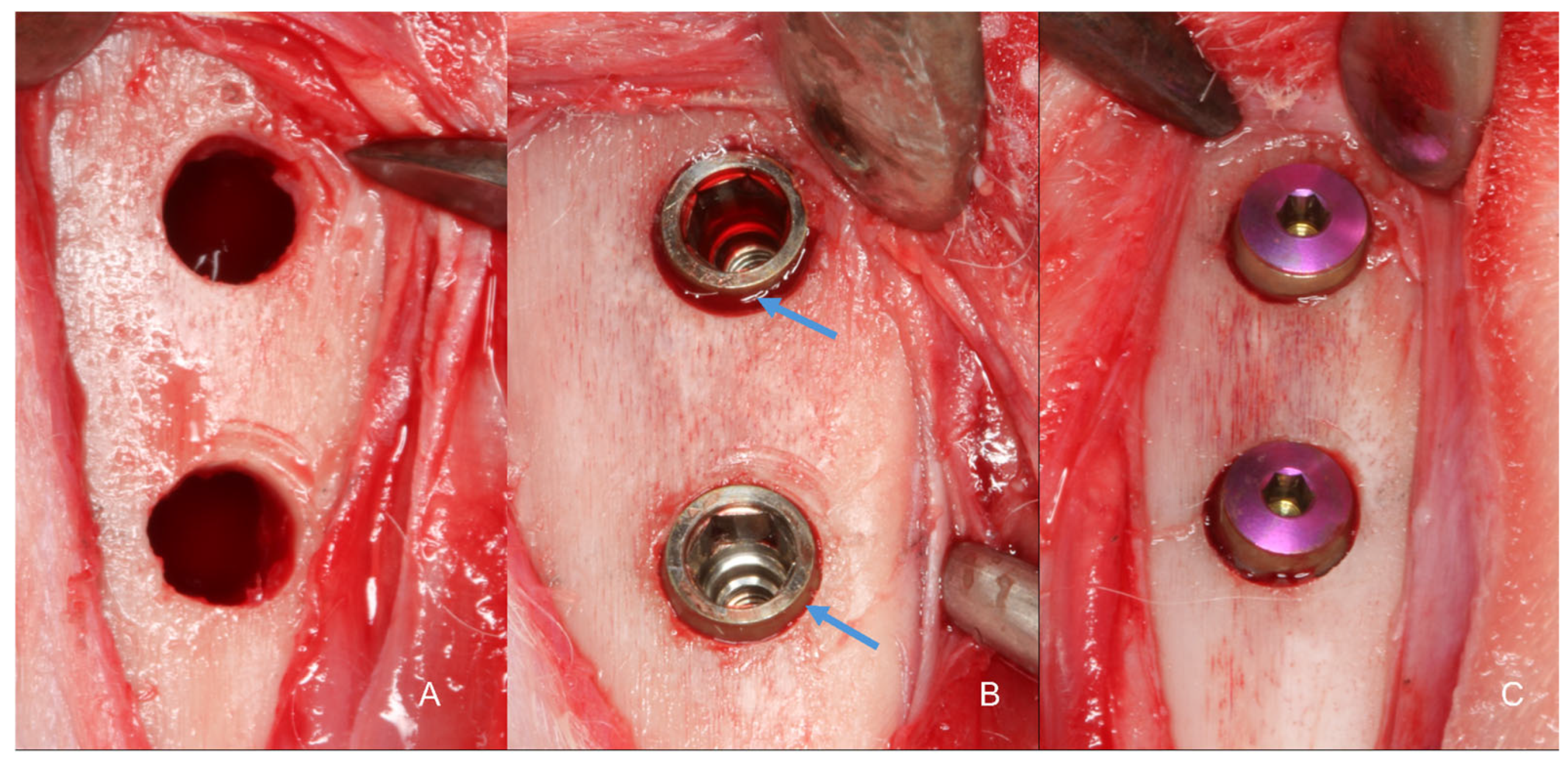
2.7. Surgical Procedure
2.8. Animal Maintenance
2.9. Euthanasia
2.10. Histological Processing
2.11. Histological Evaluation
2.12. Statistical Analysis
3. Results
3.1. Clinical Outcomes
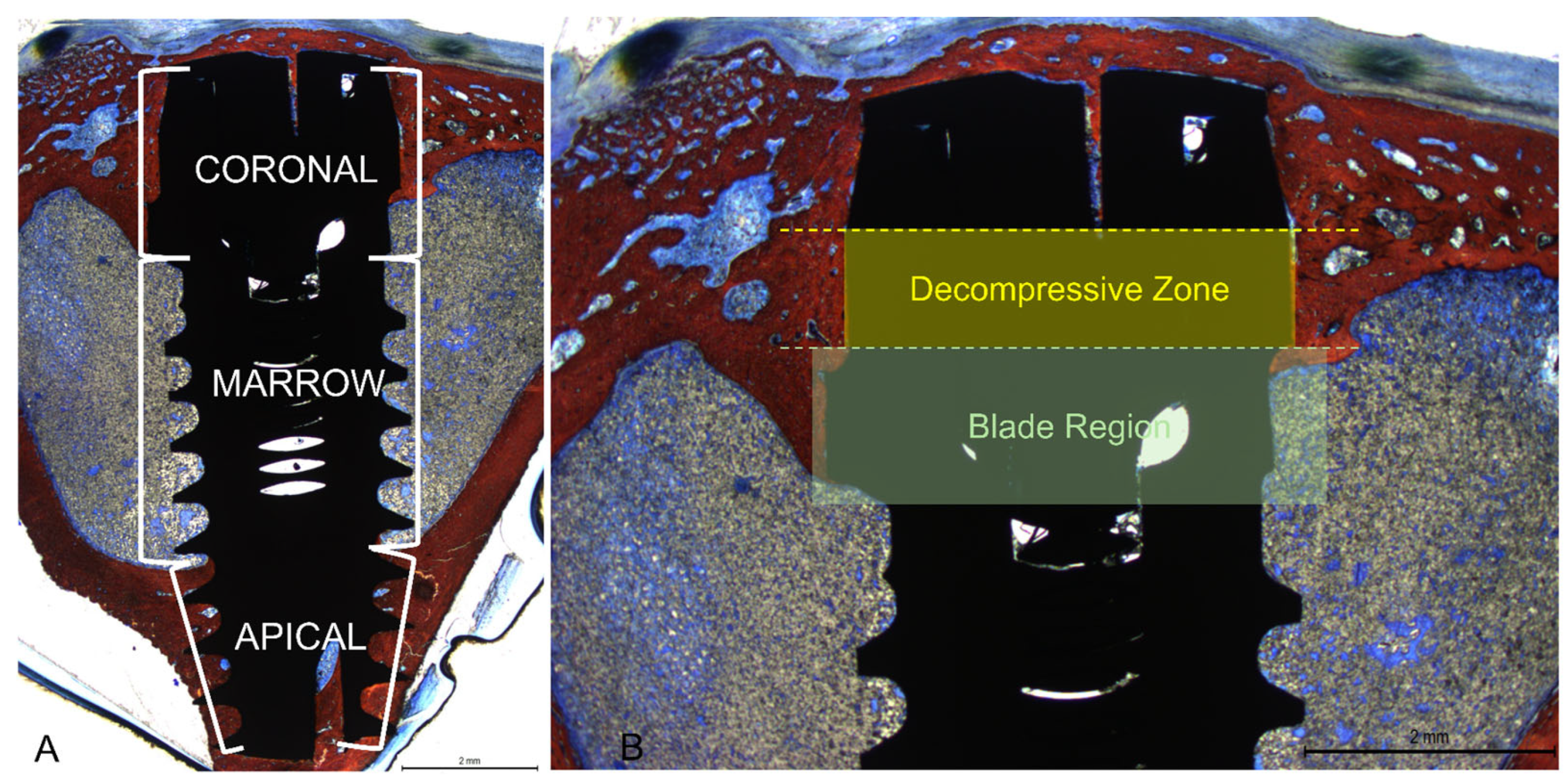
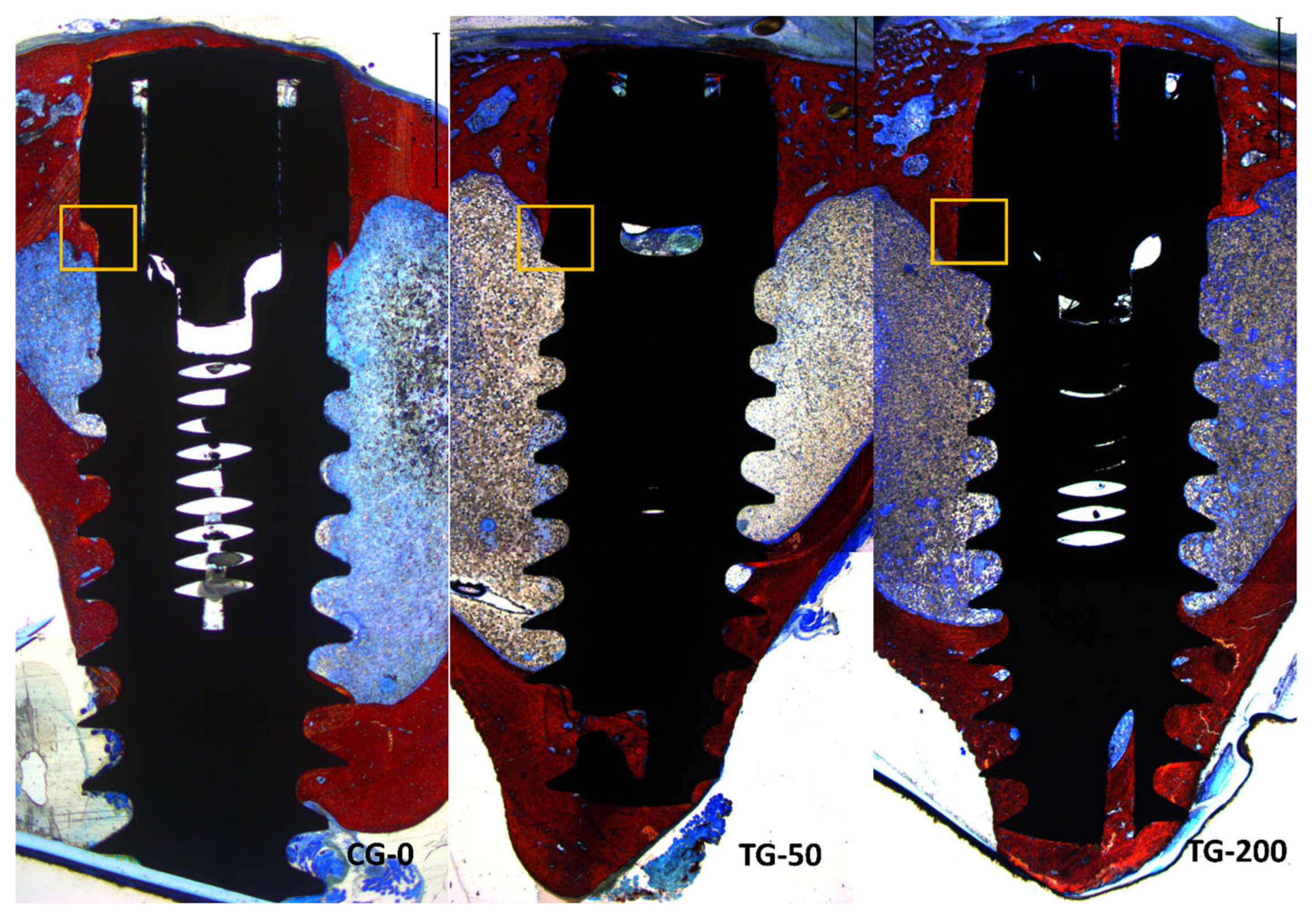
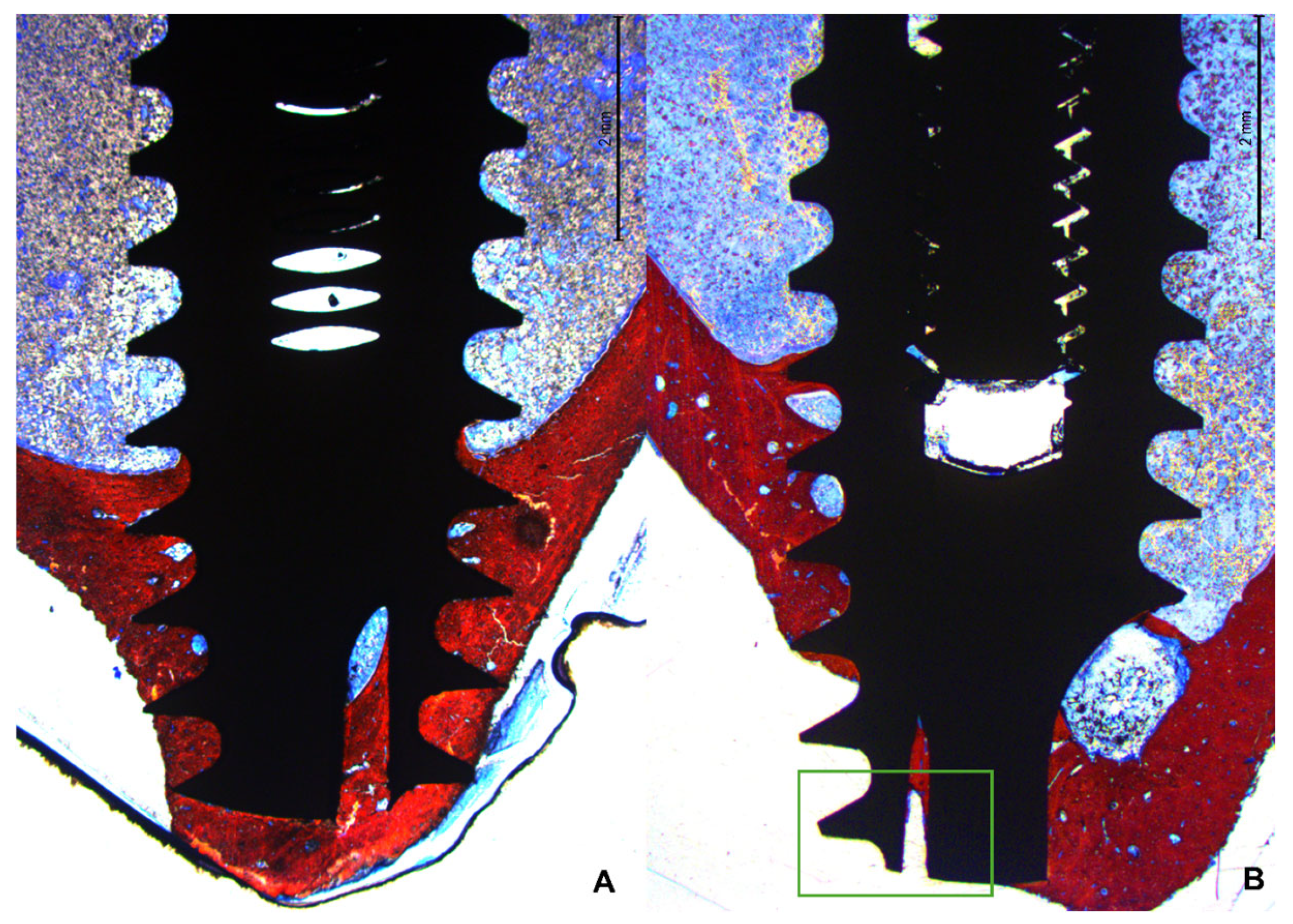
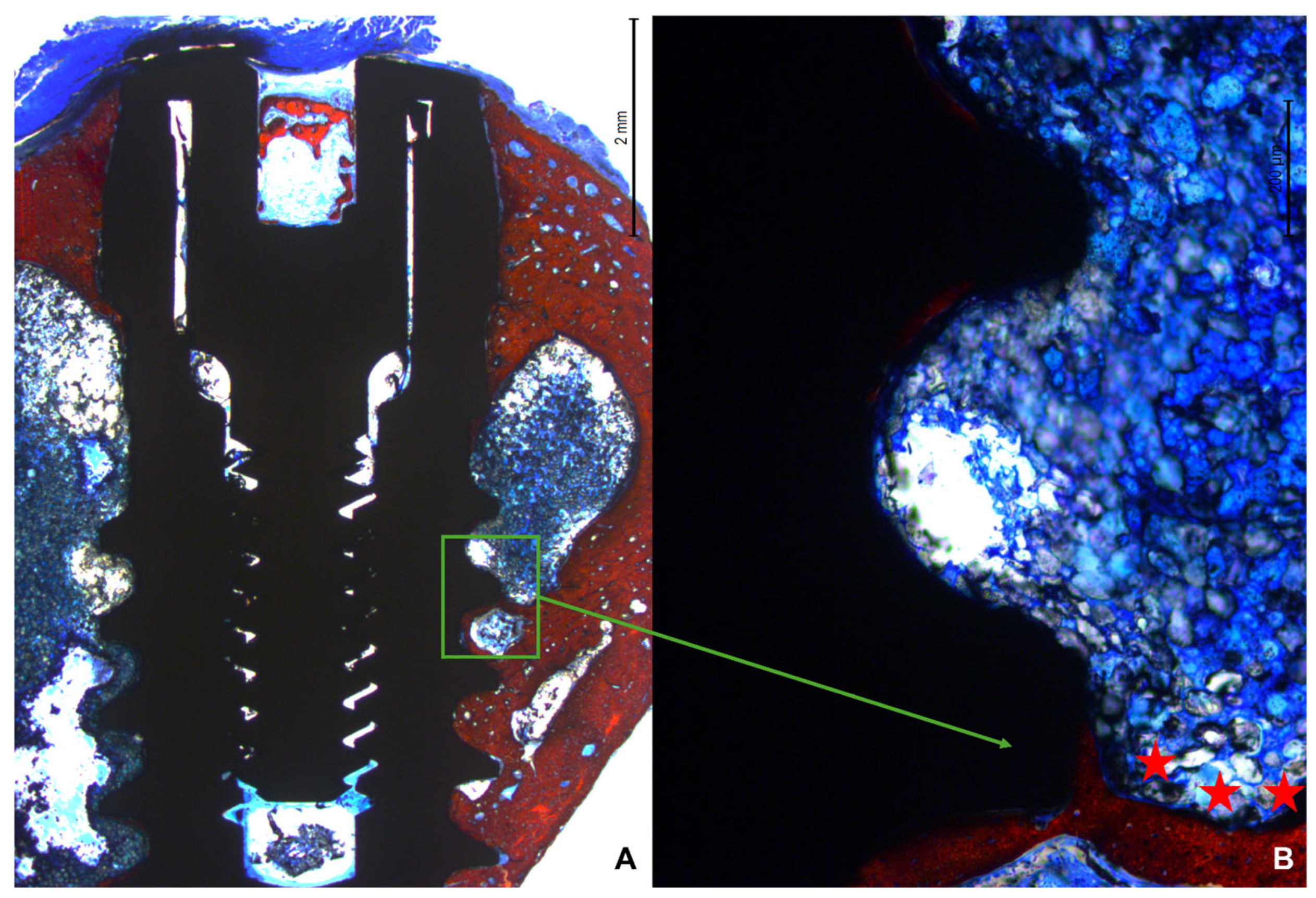
3.2. Descriptive Histological Evaluation
3.3. Histomorphometric Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gehrke, S.A.; Prados-Frutos, J.C.; Prados-Privado, M.; Calvo-Guirado, J.L.; Aramburú Júnior, J.; Pérez-Díaz, L.; Mazón, P.; Aragoneses, J.M.; De Aza, P.N. Biomechanical and Histological Analysis of Titanium (Machined and Treated Surface) Versus Zirconia Implant Materials: An In Vivo Animal Study. Materials 2019, 12, 856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanawa, T. Titanium-Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elias, C.N.; Fernandes, D.J.; Resende, C.R.; Roestel, J. Mechanical properties, surface morphology and stability of a modified commercially pure high strength titanium alloy for dental implants. Dent. Mater. 2015, 31, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant Dent. Relat. Res. 2019, 21 (Suppl. S1), 4–7. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Investig. Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Granato, R.; Marin, C.; Teixeira, H.S.; Suzuki, M.; Valverde, G.B.; Janal, M.N.; Lilin, T.; Bonfante, E.A. The effect of different implant macrogeometries and surface treatment in early biomechanical fixation: An experimental study in dogs. J. Mech. Behav. Biomed. Mater. 2011, 4, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Eliers Treichel, T.L.; Pérez-Díaz, L.; Calvo-Guirado, J.L.; Aramburú Júnior, J.; Mazón, P.; de Aza, P.N. Impact of Different Titanium Implant Thread Designs on Bone Healing: A Biomechanical and Histometric Study with an Animal Model. J. Clin. Med. 2019, 8, 777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Negri, B.; Calvo-Guirado, J.L.; Maté Sánchez de Val, J.E.; Delgado Ruiz, R.A.; Ramírez Fernández, M.P.; Gómez Moreno, G.; Aguilar Salvatierra, A.; Guardia, J.; Muñoz Guzón, F. Biomechanical and bone histomorphological evaluation of two surfaces on tapered and cylindrical root form implants: An experimental study in dogs. Clin. Implant Dent. Relat. Res. 2013, 15, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Perfetti, G.; Baldoni, E.; Berardi, D.; Colagiovanni, M.; Scogna, G. Implant micromotion is related to peak insertion torque and bone density. Clin. Oral Implant. Res. 2009, 20, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Martinez, H.; Davarpanah, M.; Missika, P.; Celletti, R.; Lazzara, R. Optimal implant stabilization in low density bone. Clin. Oral Implant. Res. 2001, 12, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Eskan, M.A.; Uzel, G.; Yilmaz, S. A fixed reconstruction of fully edentulous patients with immediate function using an apically tapered implant design: A retrospective clinical study. Int. J. Implant Dent. 2020, 6, 77. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Júnior, J.A.; Treichel, T.L.E.; do Prado, T.D.; Dedavid, B.A.; de Aza, P.N. Effects of insertion torque values on the marginal bone loss of dental implants installed in sheep mandibles. Sci. Rep. 2022, 12, 538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trisi, P.; Todisco, M.; Consolo, U.; Travaglini, D. High versus low implant insertion torque: A histologic, histomorphometric, and biomechanical study in the sheep mandible. Int. J. Oral Maxillofac. Implant. 2011, 26, 837–849. [Google Scholar] [PubMed]
- Scarano, A.; Piattelli, A.; Assenza, B.; Sollazzo, V.; Lucchese, A.; Carinci, F. Assessment of pain associated with insertion torque of dental implants. A prospective, randomizedcontrolled study. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. S2), 65–69. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R. The Influence of Low Insertion Torque on Primary Stability, Implant Survival, and Maintenance of Marginal Bone Levels: A Closed-Cohort Prospective Study. Int. J. Oral Maxillofac. Implant. 2017, 32, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Aldahlawi, S.; Demeter, A.; Irinakis, T. The effect of implant placement torque on crestal bone remodeling after 1 year of loading. Clin. Cosmet. Investig. Dent. 2018, 10, 203–209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campos, F.E.; Jimbo, R.; Bonfante, E.A.; Barbosa, D.Z.; Oliveira, M.T.; Janal, M.N.; Coelho, P.G. Are insertion torque and early osseointegration proportional? A histologic evaluation. Clin. Oral. Implant. Res. 2015, 26, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Kotsu, M.; Urbizo Velez, J.; Bengazi, F.; Tumedei, M.; Fujiwara, S.; Kato, S.; Botticelli, D. Healing at implants installed from ~70- to <10-Ncm insertion torques: An experimental study in dogs. Oral Maxillofac. Surg. 2021, 25, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Tovar, N.; Anchieta, R.B.; Machado, L.S.; Marin, C.; Teixeira, H.S.; Coelho, P.G. The combined effects of undersized drilling and implant macrogeometry on bone healing around dental implants: An experimental study. Int. J. Oral Maxillofac. Surg. 2014, 43, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Berardini, M.; Trisi, P.; Sinjari, B.; Rutjes, A.W.; Caputi, S. The Effects of High Insertion Torque Versus Low Insertion Torque on Marginal Bone Resorption and Implant Failure Rates: A Systematic Review with Meta-Analyses. Implant Dent. 2016, 25, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Berglundh, T.; Linder, E.; Lang, N.P.; Lindhe, J. Early bone formation adjacent to rough and turned endosseous implant surfaces. An experimental study in the dog. Clin. Oral Implant. Res. 2004, 15, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Stübinger, S.; Dard, M. The rabbit as experimental model for research in implant dentistry and related tissue regeneration. J. Investig. Surg. 2013, 26, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Caneva, M.; Lang, N.P.; Calvo Guirado, J.L.; Spriano, S.; Iezzi, G.; Botticelli, D. Bone healing at bicortically installed implants with different surface configurations. An experimental study in rabbits. Clin. Oral Implant. Res. 2015, 26, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Soto-Peñaloza, D.; Caneva, M.; Viña-Almunia, J.; Martin-de-Llano, J.J.; García-Mira, B.; Peñarrocha-Oltra, D.; Botticelli, D.; Peñarrocha-Diago, M. Effect on osseointegration of two implant macro-designs: A histomorphometric analysis of bicortically installed implants in different topographic sites of rabbit’s tibiae. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e502–e510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanayama, M.; Ferri, M.; Guzon, F.M.M.; Asano, A.; Alccayhuaman, K.A.A.; Rossi, E.F.; Botticelli, D. Influence on marginal bone levels at implants equipped with blades aiming to control the lateral pressure on the cortical bone. An experimental study in dogs. Oral Maxillofac. Surg. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Pereira, M.D.; Smith, A.A.; Houschyar, K.S.; Yin, X.; Mouraret, S.; Brunski, J.B.; Helms, J.A. Multiscale analyses of the bone-implant interface. J. Dent. Res. 2015, 94, 482–490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, J.E. Mechanisms of endosseous integration. Int. J. Prosthodont. 1998, 11, 391–401. [Google Scholar] [PubMed]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef]
- Berglundh, T.; Abrahamsson, I.; Lang, N.P.; Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants. Clin. Oral Implant. Res. 2003, 14, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Berglundh, T.; Buser, D.; Lindhe, J. Appositional bone formation in marginal defects at implants. Clin. Oral Implant. Res. 2003, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Berglundh, T.; Buser, D.; Lindhe, J. The jumping distance revisited: An experimental study in the dog. Clin. Oral Implant. Res. 2003, 14, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Botticelli, D.; Pantani, F.; Pereira, F.P.; Salata, L.A.; Lang, N.P. Bone healing pattern in surgically created circumferential defects around submerged implants: An experimental study in dog. Clin. Oral Implant. Res. 2012, 23, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Berglundh, T.; Persson, L.G.; Lindhe, J. Bone regeneration at implants with turned or rough surfaces in self-contained defects. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Sivolella, S.; Bressan, E.; Salata, L.A.; Urrutia, Z.A.; Lang, N.P.; Botticelli, D. Osteogenesis at implants without primary bone contact—An experimental study in dogs. Clin. Oral Implant. Res. 2012, 23, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.; Röstlund, T.; Albrektsson, B.; Albrektsson, T. Implant fixation improved by close fit. Cylindrical implant-bone interface studied in rabbits. Acta Orthop. Scand. 1988, 59, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Lang, N.P. The role of bone debris in early healing adjacent to hydrophilic and hydrophobic implant surfaces in man. Clin. Oral Implant. Res. 2011, 22, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Lang, N.P.; De Santis, E.; Morelli, F.; Favero, G.; Botticelli, D. Bone-healing pattern at the surface of titanium implants: An experimental study in the dog. Clin. Oral Implant. Res. 2014, 25, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ivanoff, C.J.; Sennerby, L.; Lekholm, U. Influence of mono- and bicortical anchorage on the integration of titanium implants. A study in the rabbit tibia. Int. J. Oral Maxillofac. Surg. 1996, 25, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Bhave, S.M.; Chand, S.; Yadav, L.; Pal, U.S.; Mohammad, S.; Singh, V.; Singh, G.; Maurya, H. Comparative evaluation of dental implants in posterior maxilla placed using unicortical and bicortical anchorage—A split-mouth prospective study. Natl. J. Maxillofac. Surg. 2023, 14, 109–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomé, G.; Caldas, W.; Bernardes, S.R.; Cartelli, C.A.; Gracher, A.H.P.; Trojan, L.C. Implant and prosthesis survival rates of full-arch immediate prostheses supported by implants with and without bicortical anchorage: Up to 2 years of follow-up retrospective study. Clin. Oral Implant. Res. 2021, 32, 37–43. [Google Scholar] [CrossRef] [PubMed]



| Groups | Coronal | Decompressive | Blades | Marrow | Apical | Total |
|---|---|---|---|---|---|---|
| CG-0 | 57.0 ± 15.0 | 60.6 ± 16.5 | 53.4 ± 23.3 * | 9.4 ± 4.4 | 71.8 ± 12.8 | 38.6 ± 7.3 |
| TG-50 | 48.6 ± 10.3 | 63.6 ± 18.0 | 33.6 ± 14.7 * | 10.0 ± 6.2 | 77.8 ± 11.4 | 40.7 ± 6.5 |
| TG-200 | 47.8 ± 12.5 | 61.2 ± 16.2 | 34.4 ± 15.5 * | 10.4 ± 11.5 | 78.8 ± 7.2 | 42.0 ± 8.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira Balan, V.; Ferri, M.; Pires Godoy, E.; Artioli, L.G.; Botticelli, D.; Silva, E.R.; Xavier, S.P. Controlled Lateral Pressure on Cortical Bone Using Blade-Equipped Implants: An Experimental Study in Rabbits. Bioengineering 2024, 11, 835. https://doi.org/10.3390/bioengineering11080835
Ferreira Balan V, Ferri M, Pires Godoy E, Artioli LG, Botticelli D, Silva ER, Xavier SP. Controlled Lateral Pressure on Cortical Bone Using Blade-Equipped Implants: An Experimental Study in Rabbits. Bioengineering. 2024; 11(8):835. https://doi.org/10.3390/bioengineering11080835
Chicago/Turabian StyleFerreira Balan, Vitor, Mauro Ferri, Eduardo Pires Godoy, Leticia Gabriela Artioli, Daniele Botticelli, Erick Ricardo Silva, and Samuel Porfirio Xavier. 2024. "Controlled Lateral Pressure on Cortical Bone Using Blade-Equipped Implants: An Experimental Study in Rabbits" Bioengineering 11, no. 8: 835. https://doi.org/10.3390/bioengineering11080835
APA StyleFerreira Balan, V., Ferri, M., Pires Godoy, E., Artioli, L. G., Botticelli, D., Silva, E. R., & Xavier, S. P. (2024). Controlled Lateral Pressure on Cortical Bone Using Blade-Equipped Implants: An Experimental Study in Rabbits. Bioengineering, 11(8), 835. https://doi.org/10.3390/bioengineering11080835









