Substantiation and Effectiveness of Remote Monitoring System Based on IoMT Using Portable ECG Device
Abstract
:1. Introduction
2. Materials and Methods
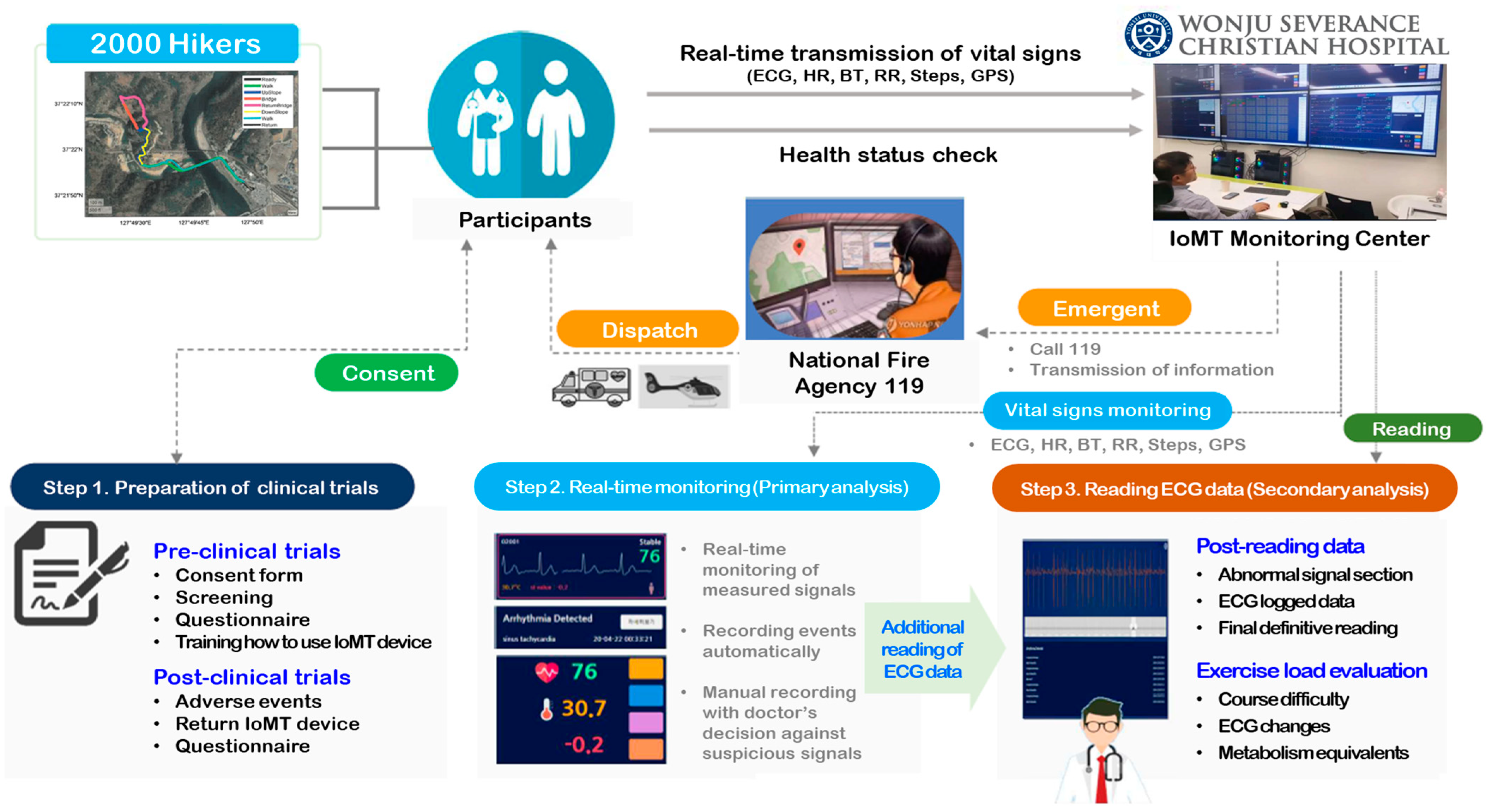
2.1. Participants
2.2. ECG Monitoring Device
2.3. Role of the Physician at the IoMT Monitoring Center
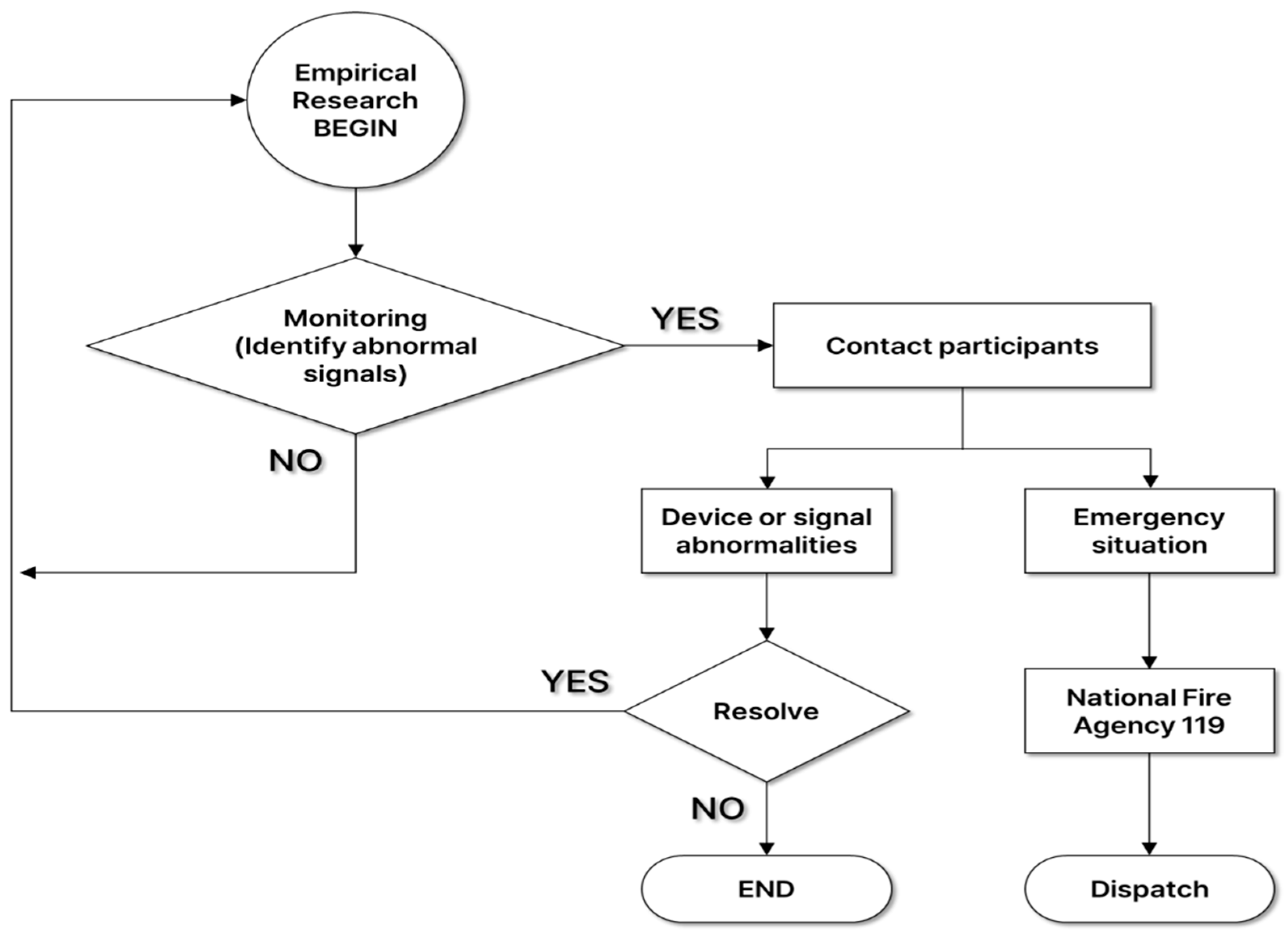
2.4. Empirical Research Scenarios
2.5. Follow-Up Management of Participants Who Were Recommended to Visit a Hospital
2.6. Satisfaction Survey
3. Results
3.1. General Characteristics
3.2. Detection of Abnormal Signals and Recommendation to a Hospital through Participant ECG Monitoring
3.3. Observations of Participants Who Were Recommended to Visit a Hospital
3.4. Satisfaction Survey after Completion of Empirical Research
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korean Law Information Center. Enforcement Decree of the Medical Service Act. Ministry of Government Legislation. Available online: https://www.law.go.kr/LSW/eng/engLsSc.do?y=0&x=0&menuId=2&query=medi-cal§ion=lawNm#liBgcolor29 (accessed on 6 August 2024).
- Lee, H.Y. Legal issues in telemedicine. Hanyang Law Assoc. 2021, 32, 3–29. [Google Scholar]
- Okuyama, M.; Kamiya, H.; Kamidani, S.; Saitoh, A. Adverse Event Following Immunization Monitoring System in Japan. Pediatr. Infect. Dis. J. 2024, 43, e52–e56. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, P.; Sianis, A.; Brown, J.; Ali, A.; Briasoulis, A. Chronic disease management in heart failure: Focus on telemedicine and remote monitoring. Rev. Cardiovasc. Med. 2021, 22, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Casariego-Vales, E.; Blanco-López, R.; Rosón-Calvo, B.; Suárez-Gil, R.; Santos-Guerra, F.; Dobao-Feijoo, M.J.; Ares-Rico, R.; Bal-Alvaredo, M.; on behalf of the TELEA-COVID Lugo Comanagement Team. Efficacy of telemedicine and telemonitoring in at-home monitoring of patients with COVID-19. J. Clin. Med. 2021, 10, 2893. [Google Scholar] [CrossRef]
- Congrete, S.; Metersky, M.L. Telemedicine and remote monitoring as an adjunct to medical management of bronchiectasis. Life 2021, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, M.R.L.; Levit, L.A.; Schenkel, C.; Kirkwood, K.; Fashoyin-Aje, L.A.; Bruinooge, S.S.; Kelley, M.J.; Mailman, J.A.; Magnuson, A.; Mirda, D.P.; et al. Researcher experience and comfort with telemedicine and remote patient monitoring in cancer treatment trials. Oncologist 2024, 29, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. The Impact of Regulatory Approaches on Entrepreneurship and Innovation: In the Context of the Growth of Entrepreneurship in South Korea. Asia-Pac. J. Bus. Ventur. Entrep. 2022, 17, 11067. [Google Scholar]
- Yoon, W.T.; Choi, E.S. Outcomes of Institutionalizing the Gangwon Digital Healthcare Regulatory Free Special Zone. J. Digit. Contents Soc. 2024, 25, 951–959. [Google Scholar] [CrossRef]
- Shim, W.; Park, J. Regulatory reform plans and strategies for the emerging digital healthcare industry. J. Regul. Stud. 2018, 27, 29–61. [Google Scholar]
- Lee, J.W.; Kim, S.H.; Kim, C.B.; Kim, K.K. A Comparative Study on the telehealth regulations between USA, Australia and Japan for developing the Korean telehealth system. Korean J. Med. Law 2010, 18, 79–104. [Google Scholar]
- Cho, E.S.; Yoon, W.T. A Study on the Diversity of Science and Technology Policy Issues and Policy Responsiveness. Korea Assoc. Public Manag. 2021, 35, 201–223. [Google Scholar]
- Zarama, V.; Arango-Granados, M.C.; Manzano-Nunez, R.; Sheppard, J.P.; Roberts, N.; Plüddemann, A. The diagnostic accuracy of cardiac ultrasound for acute myocardial ischemia in the emergency department: A systematic review and meta-analysis. Scand. J. Trauma Resusc. Emerg. Med. 2024, 32, 19. [Google Scholar] [CrossRef] [PubMed]
- Mincholé, A.; Rodriguez, B. Artificial intelligence for the electrocardiogram. Nat. Med. 2019, 25, 22–23. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Using Remote Patient Monitoring Technologies for Better Cardiovascular Disease Outcomes: Guidanc; American Heart Association: Dallas, TX, USA, 2019. [Google Scholar]
- Simovic, S.; Providencia, R.; Barra, S.; Kircanski, B.; Guerra, J.M.; Conte, G.; Duncker, D.; Marijon, E.; Anic, A.; Boveda, S. The use of remote monitoring of cardiac implantable devices during the COVID-19 pandemic: An EHRA physician survey. Europace 2022, 24, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kim, S.H.; Lee, W.; Kang, S.H.; Yoon, C.H.; Youn, T.J.; Chae, I.H. Comparison of continuous ECG monitoring by wearable patch device and conventional telemonitoring device. J. Korean Med. Sci. 2020, 35, e363. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Kim, D.; Park, H.J.; Ban, K.; Ahn, S.; Park, S.M. A wireless power transfer based implantable ECG monitoring device. Energies 2020, 13, 905. [Google Scholar] [CrossRef]
- Jatmiko, W.; Ma’sum, M.A.; Wisesa, H.A.; Sanabila, H.R. Developing smart Tele-ECG system for early detection and monitoring heart diseases based on ECG signal: Progress and challenges. Int. J. Smart Sens. Intell. Syst. 2019, 12, 1–28. [Google Scholar] [CrossRef]
- Wasimuddin, M.; Elleithy, K.; Abuzneid, A.S.; Faezipour, M.; Abuzaghleh, O. Stages-based ECG signal analysis from traditional signal processing to machine learning approaches: A survey. IEEE Access 2020, 8, 177782–177803. [Google Scholar] [CrossRef]
- Kaushal, C.; Islam, M.K.; Singla, A.; Al Amin, M. An IoMT-based smart remote monitoring system for healthcare. IoT-Enabled Smart Healthc. Syst. Serv. Appl. 2022, 177–198. [Google Scholar] [CrossRef]
- Lupton, D. How does health feel? Towards research on the affective atmospheres of digital health. Digit. Health 2017, 3, 2055207617701276. [Google Scholar] [CrossRef]
- Awotunde, J.B.; Ajagbe, S.A.; Florez, H. Internet of things with wearable devices and artificial intelligence for elderly uninterrupted healthcare monitoring systems. In International Conference on Applied Informatics; Springer International Publishing: Cham, Switzerland, 2022; pp. 278–291. [Google Scholar]
- Lee, H.Y.; Lee, K.H.; Lee, K.H.; Erdenbayar, U.; Hwang, S.; Lee, E.Y.; Lee, J.H.; Kim, H.J.; Park, S.B.; Park, J.W.; et al. Internet of medical things-based real-time digital health service for precision medicine: Empirical studies using MEDBIZ platform. Digit. Health 2023, 9, 20552076221149659. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.M.; Komatireddy, R.; Haaser, S.; Topol, S.; Sheard, J.; Encinas, J.; Fought, A.J.; Topol, E.J. Comparison of 24-hour Holter Monitoring with 14-day Novel Adhesive Patch Electrocardiographic Monitoring. Am. J. Med. 2014, 127, 95.e11–95.e17. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.M.; Riddell, F.; Madon, M.; Gleva, M.J. Comparison of diagnostic value using a small, single channel, P-wave centric sternal ECG monitoring patch with a standard 3-lead Holter system over 24 hours. Am. Heart J. 2017, 185, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Lueken, M.; Gramlich, M.; Leonhardt, S.; Marx, N.; Zink, M.D. Automated signal quality assessment of single-lead ecg recordings for early detection of silent atrial fibrillation. Sensors 2023, 23, 5618. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanaiah, B.; Kamala, J. ECG signal processing and KNN classifier-based abnormality detection by VH-doctor for remote cardiac healthcare monitoring. Soft Comput. 2020, 24, 17457–17466. [Google Scholar] [CrossRef]
- Suba, S.; Fleischmann, K.E.; Schell-Chaple, H.; Prasad, P.; Marcus, G.M.; Hu, X.; Pelter, M.M. Diagnostic and prognostic significance of premature ventricular complexes in community and hospital-based participants: A scoping review. PLoS ONE 2021, 16, e0261712. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, S.A.; Faranesh, A.Z.; Atlas, S.J.; McManus, D.D.; Singer, D.E.; Pagoto, S.; Pantelopoulos, A.; Foulkes, A.S. Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: The Fitbit heart study. Am. Heart J. 2021, 238, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Mwachiro, D.M.; Baron-Lee, J.; Kates, F.R. Impact of post-discharge follow-up calls on 30-day hospital Readmissions in Neurosurgery. Glob. J. Qual. Saf. Healthc. 2019, 2, 46–52. [Google Scholar] [CrossRef]
- Edwards, P. Questionnaires in clinical trials: Guidelines for optimal design and administration. Trials 2010, 11, 2. [Google Scholar] [CrossRef]
| Regulation Evidence | Substantiation |
|---|---|
| Medical service act |
|
| Article 34 (Remote Medical Treatment) | |
| Medical service act |
|
| Article 37 (Radiation Generating Devices for Diagnostic) | |
| Pharmaceutical affairs act |
|
| Article 23-3 (Establishment and Operation of Information System for Safe Use of Drugs) |
| Variables | Participants (Hikers) | ||
|---|---|---|---|
| Total (n = 2000) | Male (n = 811) | Female (n = 1189) | |
| Age (mean ± S.D) | 49.36 ± 14.65 | 49.35 ± 15.81 | 49.37 ± 13.81 |
| Age group (n, %) | |||
| Under 20 | 51 (2.55%) | 22 (2.71%) | 29 (2.43%) |
| 20s | 248 (12.40%) | 118 (14.54%) | 130 (10.93%) |
| 30s | 266 (13.30%) | 105 (12.94%) | 161 (13.54%) |
| 40s | 353 (17.65%) | 133 (16.39%) | 220 (18.50%) |
| 50s | 561 (28.05%) | 188 (23.18%) | 373 (31.37%) |
| 60s | 428 (21.40%) | 187 (23.05%) | 241 (20.26%) |
| More than 70 | 93 (4.65%) | 58 (7.15%) | 35 (2.94%) |
| Height (cm) | 164.16 ± 8.65 | 171.35 ± 7.14 | 159.26 ± 5.63 |
| Weight (kg) | 64.26 ± 11.80 | 73.04 ± 10.97 | 58.26 ± 7.98 |
| SBP (mmHg) | 130.89 ± 17.12 | 136.85 ± 14.36 | 126.82 ± 17.65 |
| DBP (mmHg) | 85.61 ± 10.59 | 88.79 ± 10.43 | 83.44 ± 10.15 |
| PR (bpm) | 74.87 ± 22.94 | 76.05 ± 33.77 | 74.07 ± 10.30 |
| Temperature (°C) | 36.72 ± 10.32 | 36.79 ± 11.49 | 36.67 ± 9.44 |
| Chronic disease (n, %) | |||
| None | 1325 (66.25%) | 518 (63.87%) | 807 (67.87%) |
| One | 422 (21.10%) | 183 (22.57%) | 239 (20.10%) |
| Two | 187 (9.35%) | 77 (9.49%) | 110 (9.25%) |
| More than three | 66 (3.30%) | 33 (4.07%) | 33 (2.78%) |
| Family history (n, %) | |||
| Yes | 906 (45.30%) | 297 (36.62%) | 609 (51.22%) |
| No | 1094 (54.70%) | 514 (63.38%) | 580 (48.78%) |
| Trials | Participants | Abnormal Signals | Recommendation to Visit a Hospital | Details about CD (n) | |
|---|---|---|---|---|---|
| Total | CD | ||||
| 1 | 21 | - | - | - | - |
| 2 | 10 | - | - | - | - |
| 3 | 25 | 3 | 3 | 2 | AF (1), SA (1) |
| 4 | 44 | 1 | 1 | 1 | PVC (1) |
| 5 | 58 | 4 | 2 | 1 | Arrhythmia (1) |
| 6 | 59 | 5 | 5 | 3 | AF (2), Tachycardia (1) |
| 7 | 37 | 13 | 11 | 2 | Arrhythmia (1), Bradycardia (1) |
| 8 | 65 | 8 | 7 | 5 | Arrhythmia (2), SA block (1), PAC (1), BBB (1) |
| 9 | 46 | 15 | 15 | 4 | AV block (2), Tachycardia (1), Bradycardia (1) |
| 10 | 26 | 6 | 4 | 1 | Tachycardia (1) |
| 11 | 8 | - | - | - | - |
| 12 | 141 | 25 | 22 | 2 | Arrhythmia (1), Tachycardia (1) |
| 13 | 144 | 40 | 36 | 21 | PVC (7), Arrhythmia (4), Tachycardia (3), AF (2), PAC (1), Chest pain (2), Palpitations (1), Abnormal rhythms (1) |
| 14 | 48 | 9 | 8 | 4 | PVC (2), Arrhythmia (2) |
| 15 | 48 | 9 | 7 | 3 | AF (1), Arrhythmia (1), PVC (1) |
| 16 | 48 | 5 | 5 | 4 | PVC (2), VF (1), BBB (1) |
| 17 | 48 | 10 | 10 | 9 | Arrhythmia (3), Bradycardia (2), AF (1), PVC (1) BBB (1), ST depression (1) |
| 18 | 144 | 23 | 23 | 17 | PVC (9), BBB (5), AF (2), Tachycardia (1) |
| 19 | 144 | 16 | 16 | 14 | PVC (10), Arrhythmia (2), BBB (1), Sinus arrhythmia (1) |
| 20 | 48 | 10 | 10 | 7 | BBB (3), Arrhythmia (2), PVC (1), PAC (1) |
| 21 | 48 | 9 | 9 | 5 | Arrhythmia (3), AF (1), PVC (1) |
| 22 | 82 | 17 | 15 | 7 | PVC (6), BBB (1) |
| 23 | 72 | 10 | 9 | 6 | Arrhythmia (3), PVC (2), BBB (1) |
| 24 | 72 | 8 | 8 | 6 | PAC (2), PVC (1), Arrhythmia (1), AF (1), Abnormal rhythms (1) |
| 25 | 48 | 12 | 12 | 7 | PVC (5), AF (1), PAC (1) |
| 26 | 72 | 13 | 13 | 12 | PVC (8), BBB (2), Arrhythmia (1), Tachycardia (1) |
| 27 | 168 | 22 | 22 | 22 | PVC (15), PAC (5), Arrhythmia (1), Tachycardia (1) |
| 28 | 48 | 4 | 4 | 3 | BBB (3) |
| 29 | 48 | 9 | 9 | 5 | PVC (2), Arrhythmia (2), PAC (1) |
| 30 | 130 | 12 | 10 | 9 | PVC (5), Arrhythmia (3), Tachycardia (1) |
| Total | 2000 | 318 | 296 | 182 | |
| Variables | Number of Patients |
|---|---|
| Diagnosis method (n, %) | |
| EKG | 17 (56.67) |
| EKG and ultrasound | 6 (20.00) |
| Ultrasound | 5 (16.67) |
| EKG and X-ray | 1 (3.33) |
| General treatment | 1 (3.33) |
| Timing of hospital visits (n, %) | |
| Within one month | 24 (80.00) |
| After one month | 6 (20.00) |
| Diagnosis results (n, %) | |
| Progress observation | 2 (6.67) |
| Stent procedure | 1 (3.33) |
| Arrhythmia | 1 (3.33) |
| AF and arrhythmia | 1 (3.33) |
| Heart medication prescription | 1 (3.33) |
| Panic disorder | 1 (3.33) |
| No abnormality | 23 (76.68) |
| Questions | Values | |
|---|---|---|
| Distribution (n, %) | Score (Mean ± S.D) | |
| Have you previously participated in any clinical studies? | ||
| Yes | 117 (5.85) | - |
| No | 1883 (94.15) | |
| Was the health information provided helpful? (e.g., ECG, heart rate, respiration, body temperature) | ||
| Yes | 1931 (96.55) | - |
| No | 69 (3.45) | |
| Do you believe that the devices used will be helpful for health management? | ||
| Yes | 1959 (97.95) | - |
| No | 41 (2.05) | |
| Did you feel any discomfort after attaching the device? (e.g., detachment, itchiness, restriction of movement) | ||
| Very Satisfied (1) | 802 (40.10) | 2.02 ± 1.35 |
| Satisfied (2) | 485 (24.25) | |
| Neutral (3) | 39 (1.95) | |
| Dissatisfied (4) | 123 (6.15) | |
| Very Dissatisfied (5) | 187 (9.35) | |
| Do you trust the health information provided? (e.g., ECG, heart rate, respiration, body temperature) | ||
| Very Satisfied (5) | 700 (35.00) | 3.87 ± 1.20 |
| Satisfied (4) | 810 (40.50) | |
| Neutral (3) | 174 (8.70) | |
| Dissatisfied (2) | 161 (8.05) | |
| Very Dissatisfied (1) | 155 (7.75) | |
| Are you interested in using the HiCardi (attached device) in the future? | ||
| Very Satisfied (5) | 328 (16.40) | 3.53 ± 1.13 |
| Satisfied (4) | 636 (31.80) | |
| Neutral (3) | 362 (18.10) | |
| Dissatisfied (2) | 205 (10.25) | |
| Very Dissatisfied (1) | 105 (5.25) | |
| Is it easy to access health information through the mobile application? (e.g., ECG, heart rate, respiration, body temperature) | ||
| Very Satisfied (5) | 673 (33.65) | 3.88 ± 1.15 |
| Satisfied (4) | 846 (42.30) | |
| Neutral (3) | 187 (9.35) | |
| Dissatisfied (2) | 163 (8.15) | |
| Very Dissatisfied (1) | 131 (6.55) | |
| How satisfied are you with this empirical research? | ||
| Very Satisfied (5) | 813 (40.65) | 3.98 ± 1.17 |
| Satisfied (4) | 760 (38.00) | |
| Neutral (3) | 164 (8.20) | |
| Dissatisfied (2) | 117 (5.85) | |
| Very Dissatisfied (1) | 146 (7.30) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Kim, Y.-J.; Lee, K.-H.; Lee, J.-H.; Cho, S.-P.; Park, J.; Park, I.-H.; Youk, H. Substantiation and Effectiveness of Remote Monitoring System Based on IoMT Using Portable ECG Device. Bioengineering 2024, 11, 836. https://doi.org/10.3390/bioengineering11080836
Lee H-Y, Kim Y-J, Lee K-H, Lee J-H, Cho S-P, Park J, Park I-H, Youk H. Substantiation and Effectiveness of Remote Monitoring System Based on IoMT Using Portable ECG Device. Bioengineering. 2024; 11(8):836. https://doi.org/10.3390/bioengineering11080836
Chicago/Turabian StyleLee, Hee-Young, Yoon-Ji Kim, Kang-Hyun Lee, Jung-Hun Lee, Sung-Pil Cho, Junghwan Park, Il-Hwan Park, and Hyun Youk. 2024. "Substantiation and Effectiveness of Remote Monitoring System Based on IoMT Using Portable ECG Device" Bioengineering 11, no. 8: 836. https://doi.org/10.3390/bioengineering11080836







