Bovine Placentome-Derived Extracellular Matrix: A Sustainable 3D Scaffold for Cultivated Meat
Abstract
1. Introduction
2. Materials and Methods
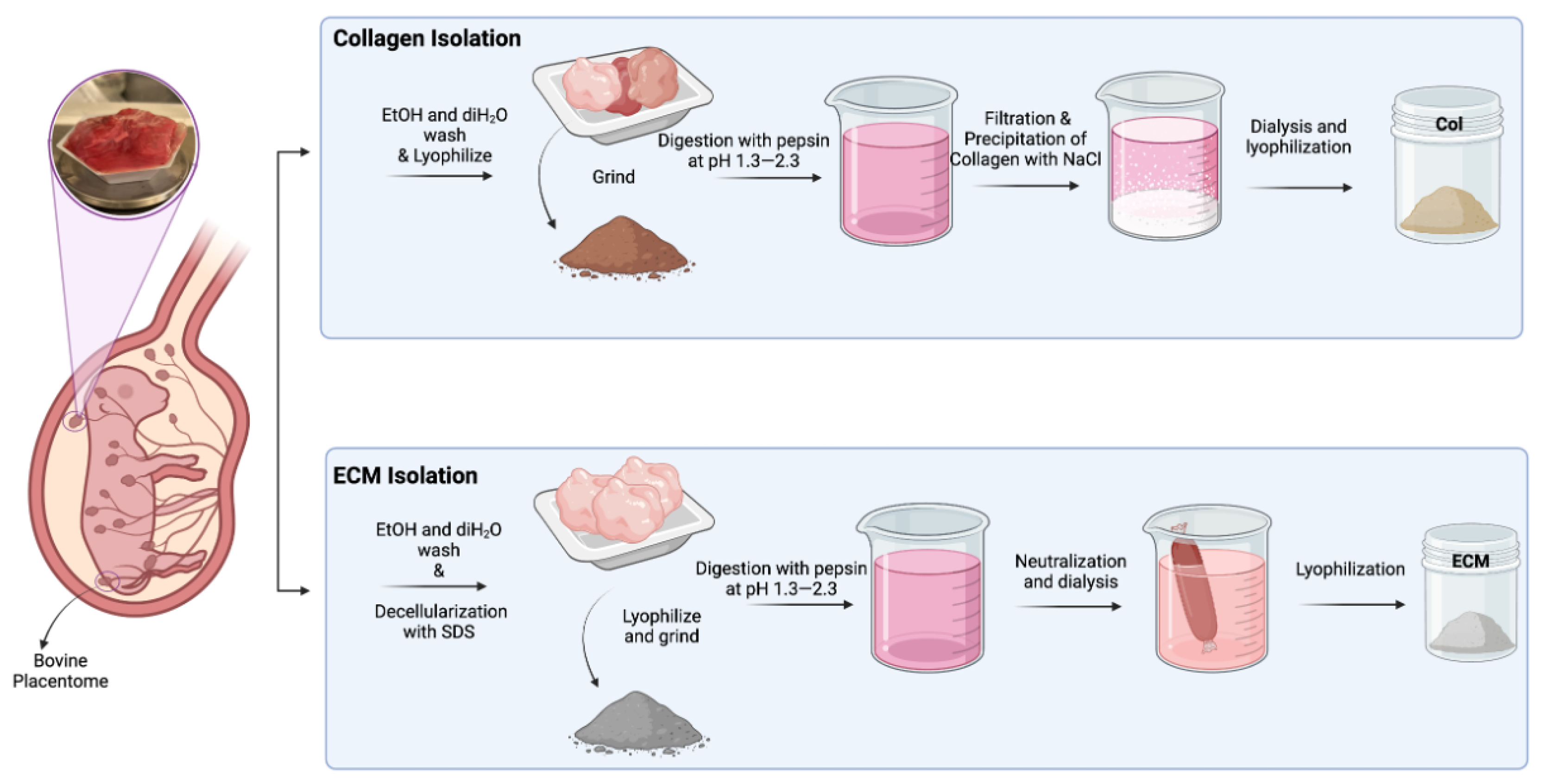
2.1. Preparation of Bovine Placentome Tissue for Extraction
2.2. Extraction of Collagen from Bovine Placental Tissues
2.3. Extraction of Extracellular Matrix (ECM) from Bovine Placental Tissues
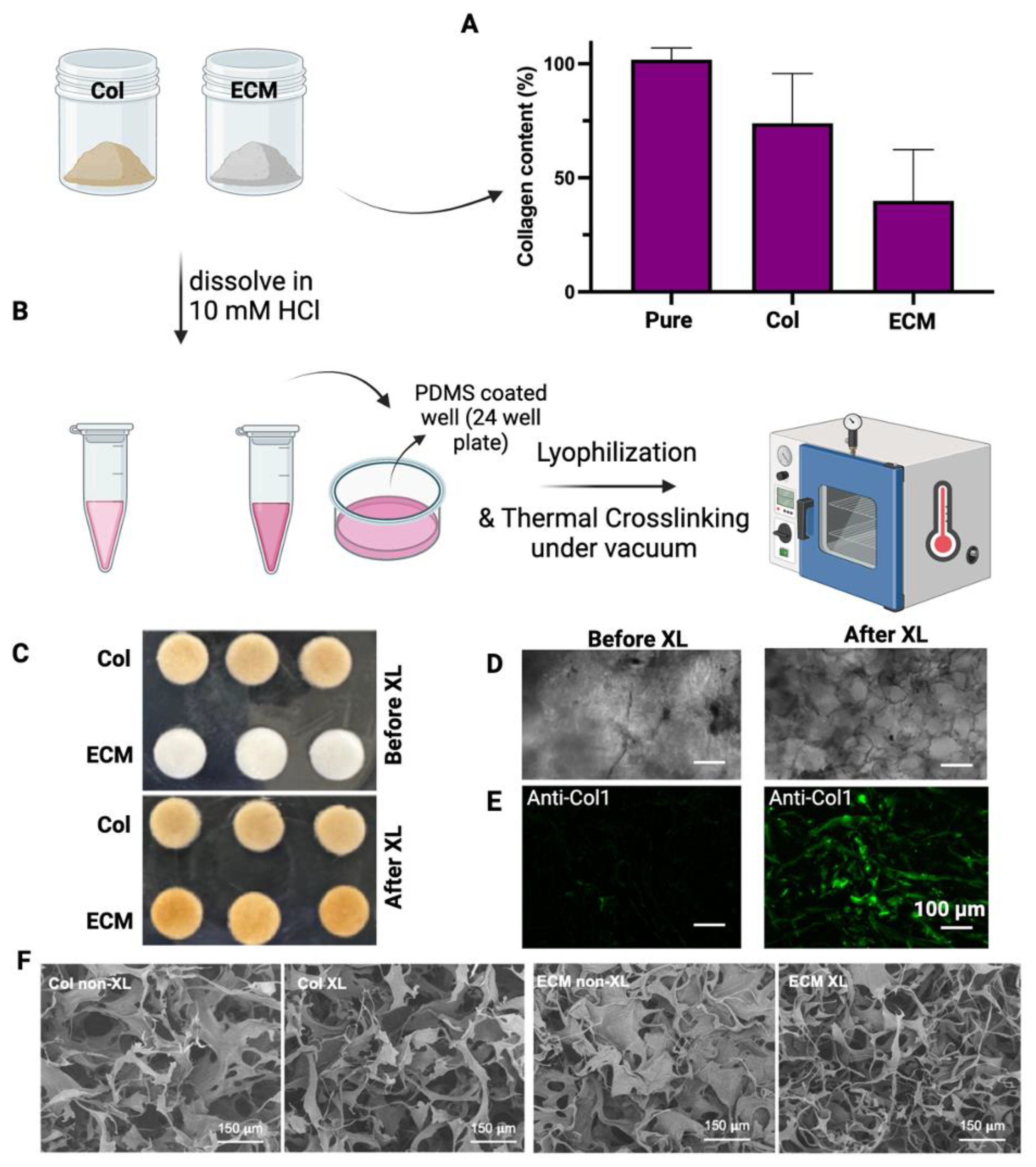
2.4. Characterization of Collagen & ECM
2.5. Production of Collagen and ECM Scaffolds
2.6. Scanning Electron Microscopy
2.7. Isolation and Characterization of Bovine Myoblasts
2.8. Culturing Bovine Myoblasts on Scaffolds
2.9. Monitoring Growth of Bovine Myoblasts in Scaffolds
2.10. Staining
2.10.1. Immunofluorescent Staining
2.10.2. CalceinAM Staining
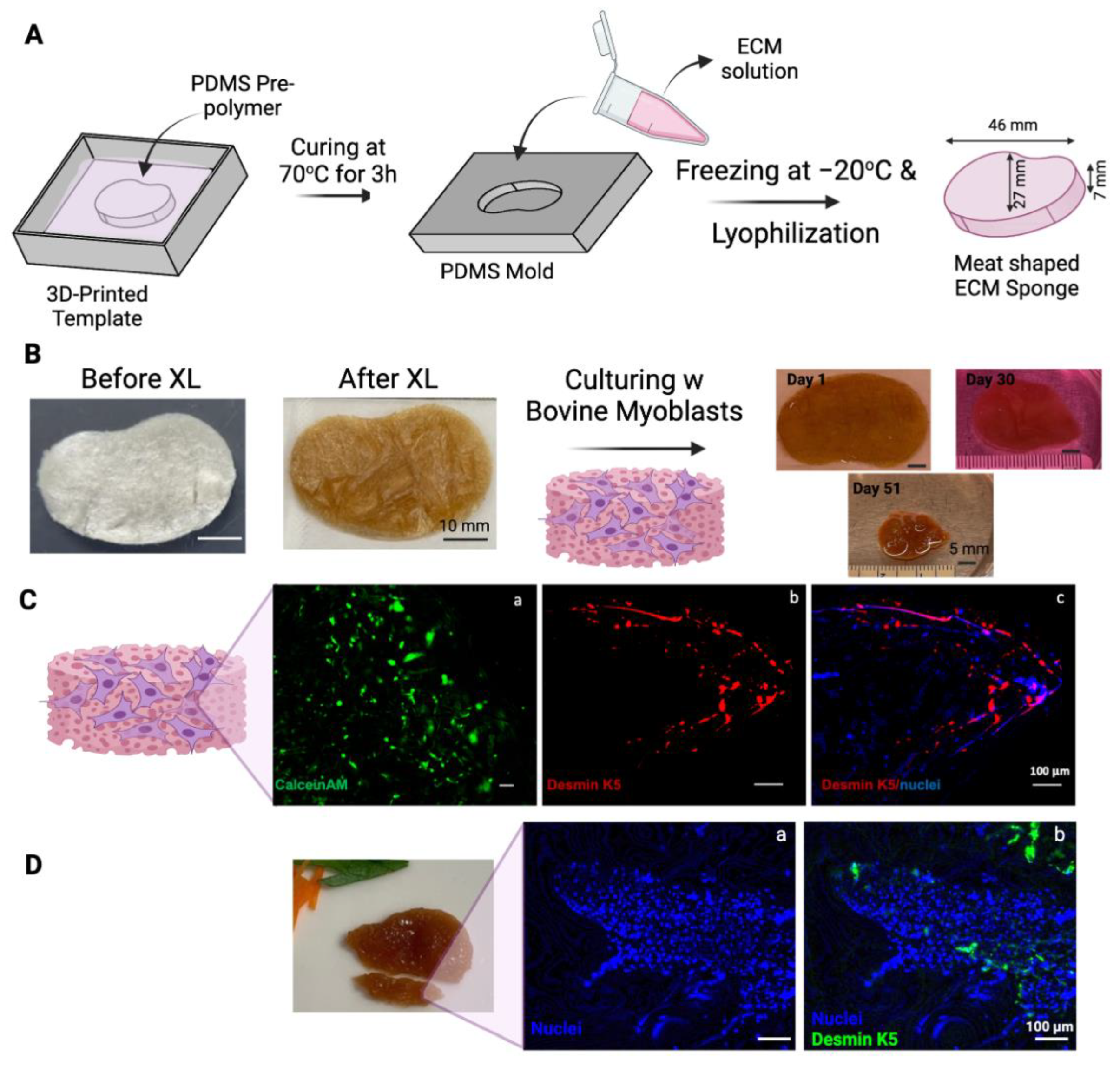
2.11. Design of Steak Construct
2.12. Preparing PDMS Mold from 3D Printed Steak-Shaped Mold
2.13. Preparing BP-ECM for Steak-Shaped Scaffold
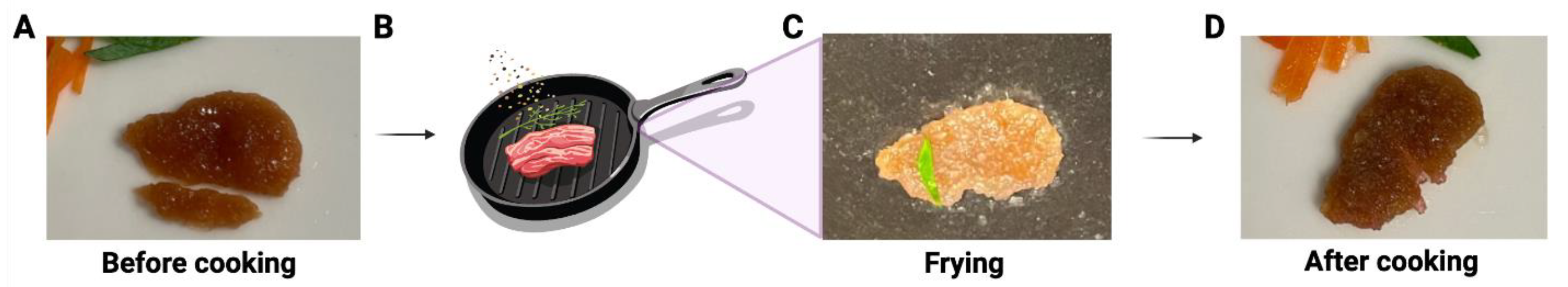
2.14. Pan Frying ECM Scaffolds Containing Bovine Myoblasts
2.15. Statistical Analysis
3. Results
3.1. Isolation of Collagen and ECM from Bovine Placentome
3.2. Myoblast Culture on Collagen and ECM Scaffolds
3.3. Making a Muscle Tissue Like Meat Construct
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lynch, J.; Pierrehumbert, R. Climate Impacts of Cultured Meat and Beef Cattle. Front. Sustain. Food Syst. 2019, 3, 5. [Google Scholar] [CrossRef]
- Reisinger, A.; Clark, H. How Much Do Direct Livestock Emissions Actually Contribute to Global Warming? Glob. Chang. Biol. 2018, 24, 1749–1761. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate Change and Livestock: Impacts, Adaptation, and Mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Monger, X.C.; Gilbert, A.-A.; Saucier, L.; Vincent, A.T. Antibiotic Resistance: From Pig to Meat. Antibiotics 2021, 10, 1209. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The Proximal Origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.; Ward, M.; van Bunnik, B.; Farrar, J. Antimicrobial Resistance in Humans, Livestock and the Wider Environment. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef]
- Benoit, M.; Mottet, A. Energy Scarcity and Rising Cost: Towards a Paradigm Shift for Livestock. Agric. Syst. 2023, 205, 103585. [Google Scholar] [CrossRef]
- Kumar, P.; Abubakar, A.A.; Verma, A.K.; Umaraw, P.; Ahmed, M.A.; Mehta, N.; Hayat, M.N.; Kaka, U.; Sazili, A.Q. New Insights in Improving Sustainability in Meat Production: Opportunities and Challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 11830–11858. [Google Scholar] [CrossRef]
- O’Neill, E.N.; Cosenza, Z.A.; Baar, K.; Block, D.E. Considerations for the Development of Cost-effective Cell Culture Media for Cultivated Meat Production. Compr. Rev. Food Sci. Food Saf. 2021, 20, 686–709. [Google Scholar] [CrossRef] [PubMed]
- Saied, A.A.; Chandran, D.; Chopra, H.; Dey, A.; Emran, T.B.; Dhama, K. Cultivated Meat Could Aid in Reducing Global Antimicrobial Resistance Burden – Producing Meat without Antibiotics as a Safer Food System for the Future. Int. J. Surg. 2023, 109, 189–190. [Google Scholar] [CrossRef]
- Reiss, J.; Robertson, S.; Suzuki, M. Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. Int. J. Mol. Sci. 2021, 22, 7513. [Google Scholar] [CrossRef] [PubMed]
- Pajčin, I.; Knežić, T.; Azoulay, I.S.; Vlajkov, V.; Djisalov, M.; Janjušević, L.; Grahovac, J.; Gadjanski, I. Bioengineering Outlook on Cultivated Meat Production. Micromachines 2022, 13, 402. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, H.L.; Teixeira de Mattos, M.J. Environmental Impacts of Cultured Meat Production. Environ. Sci. Technol. 2011, 45, 6117–6123. [Google Scholar] [CrossRef] [PubMed]
- Ozhava, D.; Bhatia, M.; Freman, J.; Mao, Y. Sustainable Cell Sources for Cultivated Meat. J. Biomed. Res. Environ. Sci. 2022, 3, 1382–1388. [Google Scholar] [CrossRef]
- David, S.; Tsukerman, A.; Safina, D.; Maor-Shoshani, A.; Lavon, N.; Levenberg, S. Co-Culture Approaches for Cultivated Meat Production. Nat. Rev. Bioeng. 2023, 1, 817–831. [Google Scholar] [CrossRef]
- Simsa, R.; Yuen, J.; Stout, A.; Rubio, N.; Fogelstrand, P.; Kaplan, D.L. Extracellular Heme Proteins Influence Bovine Myosatellite Cell Proliferation and the Color of Cell-Based Meat. Foods 2019, 8, 521. [Google Scholar] [CrossRef]
- Stout, A.J.; Mirliani, A.B.; Rittenberg, M.L.; Shub, M.; White, E.C.; Yuen, J.S.K.; Kaplan, D.L. Simple and Effective Serum-Free Medium for Sustained Expansion of Bovine Satellite Cells for Cell Cultured Meat. Commun. Biol. 2022, 5, 466. [Google Scholar] [CrossRef]
- Messmer, T.; Klevernic, I.; Furquim, C.; Ovchinnikova, E.; Dogan, A.; Cruz, H.; Post, M.J.; Flack, J.E. A Serum-Free Media Formulation for Cultured Meat Production Supports Bovine Satellite Cell Differentiation in the Absence of Serum Starvation. Nat. Food 2022, 3, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kolkmann, A.M.; Essen, A.V.; Post, M.J.; Moutsatsou, P. Development of a Chemically Defined Medium for in Vitro Expansion of Primary Bovine Satellite Cells. Front. Bioeng. Biotechnol. 2022, 10, 895289. [Google Scholar] [CrossRef]
- Li, L.; Chen, L.; Chen, X.; Chen, Y.; Ding, S.; Fan, X.; Liu, Y.; Xu, X.; Zhou, G.; Zhu, B.; et al. Chitosan-sodium Alginate-Collagen/Gelatin Three-Dimensional Edible Scaffolds for Building a Structured Model for Cell Cultured Meat. Int. J. Biol. Macromol. 2022, 209, 668–679. [Google Scholar] [CrossRef]
- Enrione, J.; Blaker, J.J.; Brown, D.I.; Weinstein-Oppenheimer, C.R.; Pepczynska, M.; Olguín, Y.; Sánchez, E.; Acevedo, C.A. Edible Scaffolds Based on Non-Mammalian Biopolymers for Myoblast Growth. Materials 2017, 10, 1404. [Google Scholar] [CrossRef]
- Ianovici, I.; Zagury, Y.; Redenski, I.; Lavon, N.; Levenberg, S. 3D-Printable Plant Protein-Enriched Scaffolds for Cultivated Meat Development. Biomaterials 2022, 284, 121487. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Chen, Y.; Zhu, H.-Z.; Li, C.-B.; Song, W.-J.; Ding, S.-J.; Zhou, G.-H. Production of Cultured Meat by Culturing Porcine Smooth Muscle Cells in Vitro with Food Grade Peanut Wire-Drawing Protein Scaffold. Food Res. Int. 2022, 159, 111561. [Google Scholar] [CrossRef]
- Xiang, N.; Yuen, J.S.K.; Stout, A.J.; Rubio, N.R.; Chen, Y.; Kaplan, D.L. 3D Porous Scaffolds from Wheat Glutenin for Cultured Meat Applications. Biomaterials 2022, 285, 121543. [Google Scholar] [CrossRef]
- Jones, J.D.; Rebello, A.S.; Gaudette, G.R. Decellularized Spinach: An Edible Scaffold for Laboratory-Grown Meat. Food Biosci. 2021, 41, 100986. [Google Scholar] [CrossRef]
- Bodiou, V.; Moutsatsou, P.; Post, M.J. Microcarriers for Upscaling Cultured Meat Production. Front. Nutr. 2020, 7, 10. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, V.; Singh, S.K.; Gupta, J.; Kumar, M.; Sarma, D.K.; Verma, V. Recent Advances in Bioengineered Scaffold for in Vitro Meat Production. Cell Tissue Res. 2023, 391, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2022, 9, 2102908. [Google Scholar] [CrossRef]
- Haeger, J.-D.; Hambruch, N.; Pfarrer, C. The Bovine Placenta in Vivo and in Vitro. Theriogenology 2016, 86, 306–312. [Google Scholar] [CrossRef]
- Seah, J.S.H.; Singh, S.; Tan, L.P.; Choudhury, D. Scaffolds for the Manufacture of Cultured Meat. Crit. Rev. Biotechnol. 2022, 42, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, L.; Liu, W.; Chen, X. An Overview of Recent Progress in Engineering Three-Dimensional Scaffolds for Cultured Meat Production. Foods 2023, 12, 2614. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Choi, J. Three-Dimensional Scaffolds, Materials, and Fabrication for Cultured Meat Applications: A Scoping Review and Future Direction. Food Hydrocoll. 2024, 152, 109881. [Google Scholar] [CrossRef]
- Alaribe, F.N.; Manoto, S.L.; Motaung, S.C.K.M. Scaffolds from Biomaterials: Advantages and Limitations in Bone and Tissue Engineering. Biologia 2016, 71, 353–366. [Google Scholar] [CrossRef]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M.G. Physical Crosslinking of Collagen Fibers: Comparison of Ultraviolet Irradiation and Dehydrothermal Treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, Z.; Zhang, Y.; Chen, F.; Zhou, Y.; An, Q. Dehydrothermally Crosslinked Collagen/Hydroxyapatite Composite for Enhanced in Vivo Bone Repair. Colloids Surf. B Biointerfaces 2018, 163, 394–401. [Google Scholar] [CrossRef]
- Bektas, C.K.; Kimiz, I.; Sendemir, A.; Hasirci, V.; Hasirci, N. A Bilayer Scaffold Prepared from Collagen and Carboxymethyl Cellulose for Skin Tissue Engineering Applications. J. Biomater. Sci., Polym. Ed. 2018, 29, 1764–1784. [Google Scholar] [CrossRef]
- Cissell, D.D.; Link, J.M.; Hu, J.C.; Athanasiou, K.A. A Modified Hydroxyproline Assay Based on Hydrochloric Acid in Ehrlich’s Solution Accurately Measures Tissue Collagen Content. Tissue Eng. Part C Methods 2017, 23, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kilic, C.; Girotti, A.; Rodriguez-Cabello, J.C.; Hasirci, V. A Collagen-Based Corneal Stroma Substitute with Micro-Designed Architecture. Biomater. Sci. 2013, 2, 318–329. [Google Scholar] [CrossRef]
- Spinazzola, J.M.; Gussoni, E. Isolation of Primary Human Skeletal Muscle Cells. Bio-Protoc. 2017, 7, e2591. [Google Scholar] [CrossRef]
- Lee, K.; Jackson, A.; John, N.; Zhang, R.; Ozhava, D.; Bhatia, M.; Mao, Y. Bovine Fibroblast-Derived Extracellular Matrix Promotes the Growth and Preserves the Stemness of Bovine Stromal Cells during In Vitro Expansion. J. Funct. Biomater. 2023, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Knitlova, J.; Doubkova, M.; Eckhardt, A.; Ostadal, M.; Musilkova, J.; Bacakova, L.; Novotny, T. Increased Collagen Crosslinking in Stiff Clubfoot Tissue: Implications for the Improvement of Therapeutic Strategies. Int. J. Mol. Sci. 2021, 22, 11903. [Google Scholar] [CrossRef]
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking Enzymes with Pleiotropic Functions. Nat. Rev. Mol. Cell Biol. 2003, 4, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Anang, D.M.; Fuseini, A. Production of Cultured Meat: Challenges and Opportunities. In Future Proteins; Elsevier: Amsterdam, The Netherlands, 2023; pp. 285–310. [Google Scholar]
- Sharma, S.; Thind, S.S.; Kaur, A. In Vitro Meat Production System: Why and How? J. Food Sci. Technol. 2015, 52, 7599–7607. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, N.; Sharma, S.; Mehta, N.; Verma, A.K.; Chemmalar, S.; Sazili, A.Q. In-Vitro Meat: A Promising Solution for Sustainability of Meat Sector. J. Anim. Sci. Technol. 2021, 63, 693–724. [Google Scholar] [CrossRef] [PubMed]
- Fasciano, S.; Wheba, A.; Ddamulira, C.; Wang, S. Recent Advances in Scaffolding Biomaterials for Cultivated Meat. Biomater. Adv. 2024, 162, 213897. [Google Scholar] [CrossRef]
- Rodríguez, M.I.A.; Barroso, L.G.R.; Sánchez, M.L. Collagen: A Review on Its Sources and Potential Cosmetic Applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Attupuram, N.M.; Kumaresan, A.; Narayanan, K.; Kumar, H. Cellular and Molecular Mechanisms Involved in Placental Separation in the Bovine: A Review. Mol. Reprod. Dev. 2016, 83, 287–297. [Google Scholar] [CrossRef]
- Schlafer, D.H.; Fisher, P.J.; Davies, C.J. The Bovine Placenta before and after Birth: Placental Development and Function in Health and Disease. Anim. Reprod. Sci. 2000, 60, 145–160. [Google Scholar] [CrossRef]
- McCabe, M.C.; Schmitt, L.R.; Hill, R.C.; Dzieciatkowska, M.; Maslanka, M.; Daamen, W.F.; van Kuppevelt, T.H.; Hof, D.J.; Hansen, K.C. Evaluation and Refinement of Sample Preparation Methods for Extracellular Matrix Proteome Coverage. Mol. Cell. Proteom. 2021, 20, 100079. [Google Scholar] [CrossRef]
- Hashimoto, A.; Hirose, T.; Hashimoto, K.; Mizumoto, S.; Nitahara-Kasahara, Y.; Saka, S.; Yoshizawa, T.; Okada, T.; Yamada, S.; Kosho, T.; et al. Collagen Network Formation in In Vitro Models of Musculocontractural Ehlers–Danlos Syndrome. Genes 2023, 14, 308. [Google Scholar] [CrossRef]
- Freyman, T.M.; Yannas, I.V.; Pek, Y.-S.; Yokoo, R.; Gibson, L.J. Micromechanics of Fibroblast Contraction of a Collagen–GAG Matrix. Exp. Cell Res. 2001, 269, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.; Yen, F.-C.; Baruch, L.; Machluf, M. Scaffolding Technologies for the Engineering of Cultured Meat: Towards a Safe, Sustainable, and Scalable Production. Trends Food Sci. Technol. 2022, 126, 13–25. [Google Scholar] [CrossRef]
- Yen, F.-C.; Glusac, J.; Levi, S.; Zernov, A.; Baruch, L.; Davidovich-Pinhas, M.; Fishman, A.; Machluf, M. Cultured Meat Platform Developed through the Structuring of Edible Microcarrier-Derived Microtissues with Oleogel-Based Fat Substitute. Nat. Commun. 2023, 14, 2942. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Kim, H.J.; Won, S.; Choi, S.M.; Han, S.S. Effect of Grape Seed Extract on Gelatin-Based Edible 3D-Hydrogels for Cultured Meat Application. Gels 2023, 9, 65. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent Progress in the Fabrication Techniques of 3D Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2020, 110, 110716. [Google Scholar] [CrossRef]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Freeze-Drying as a Novel Biofabrication Method for Achieving a Controlled Microarchitecture within Large, Complex Natural Biomaterial Scaffolds. Adv. Healthc. Mater. 2017, 6, 1700598. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Lou, Y.-Y.; Li, T.-H.; Liu, B.-Z.; Chen, K.; Zhang, D.; Li, T. Cross-Linking Methods of Type I Collagen-Based Scaffolds for Cartilage Tissue Engineering. Am. J. Transl. Res. 2021, 14, 1146–1159. [Google Scholar]
- Yannas, I.V.; Tobolsky, A.V. Cross-Linking of Gelatine by Dehydration. Nature 1967, 215, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, B.; Comín, R.; Salvatierra, N.A.; Cid, M.P. Three-Dimensional Printing of Collagen and Hyaluronic Acid Scaffolds with Dehydrothermal Treatment Crosslinking. Compos. Commun. 2020, 19, 1–5. [Google Scholar] [CrossRef]
- Štiglic, A.D.; Kargl, R.; Beaumont, M.; Strauss, C.; Makuc, D.; Egger, D.; Plavec, J.; Rojas, O.J.; Kleinschek, K.S.; Mohan, T. Influence of Charge and Heat on the Mechanical Properties of Scaffolds from Ionic Complexation of Chitosan and Carboxymethyl Cellulose. ACS Biomater. Sci. Eng. 2021, 7, 3618–3632. [Google Scholar] [CrossRef]
- Kučerová, V.; Lagaňa, R.; Výbohová, E.; Hýrošová, T. The Effect of Chemical Changes during Heat Treatment on the Color and Mechanical Properties of Fir Wood. BioResources 2016, 11, 9079–9094. [Google Scholar] [CrossRef]
- Weadock, K.S.; Miller, E.J.; Keuffel, E.L.; Dunn, M.G. Effect of Physical Crosslinking Methods on Collagen-fiber Durability in Proteolytic Solutions. J. Biomed. Mater. Res. 1996, 32, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.A.; Opazo-Navarrete, M.; Meurs, M.; Minor, M.; Sala, G.; van Boekel, M.; Stieger, M.; Janssen, A.E.M. Denaturation and in Vitro Gastric Digestion of Heat-Treated Quinoa Protein Isolates Obtained at Various Extraction PH. Food Biophys. 2016, 11, 184–197. [Google Scholar] [CrossRef]
- Toosi, S.; Naderi-Meshkin, H.; Kalalinia, F.; Peivandi, M.T.; HosseinKhani, H.; Bahrami, A.R.; Heirani-Tabasi, A.; Mirahmadi, M.; Behravan, J. PGA-incorporated Collagen: Toward a Biodegradable Composite Scaffold for Bone-tissue Engineering. J. Biomed. Mater. Res. Part A 2023, 104, 2020–2028. [Google Scholar] [CrossRef]
- Takamoto, T.; Hiraoka, Y.; Tabata, Y. Enhanced Proliferation and Osteogenic Differentiation of Rat Mesenchymal Stem Cells in Collagen Sponge Reinforced with Different Poly(Ethylene Terephthalate) Fibers. J. Biomater. Sci. Polym. Ed. 2007, 18, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Goldberg-Bockhorn, E.; Wenzel, U.; Theodoraki, M.; Döscher, J.; Riepl, R.; Wigand, M.C.; Brunner, C.; Heßling, M.; Hoffmann, T.K.; Kern, J.; et al. Enhanced Cellular Migration and Prolonged Chondrogenic Differentiation in Decellularized Cartilage Scaffolds under Dynamic Culture Conditions. J. Tissue Eng. Regen. Med. 2022, 16, 36–50. [Google Scholar] [CrossRef]
- Sinlapabodin, S.; Amornsudthiwat, P.; Damrongsakkul, S.; Kanokpanont, S. An Axial Distribution of Seeding, Proliferation, and Osteogenic Differentiation of MC3T3-E1 Cells and Rat Bone Marrow-Derived Mesenchymal Stem Cells across a 3D Thai Silk Fibroin/Gelatin/Hydroxyapatite Scaffold in a Perfusion Bioreactor. Mater. Sci. Eng. C 2016, 58, 960–970. [Google Scholar] [CrossRef]
- Rivera, F.J.C.; Chen, J. Computational Fluid Dynamics Modeling of Cell Cultures in Bioreactors and Its Potential for Cultivated Meat Production—A Mini-Review. Future Foods 2022, 6, 100195. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bektas, C.; Lee, K.; Jackson, A.; Bhatia, M.; Mao, Y. Bovine Placentome-Derived Extracellular Matrix: A Sustainable 3D Scaffold for Cultivated Meat. Bioengineering 2024, 11, 854. https://doi.org/10.3390/bioengineering11080854
Bektas C, Lee K, Jackson A, Bhatia M, Mao Y. Bovine Placentome-Derived Extracellular Matrix: A Sustainable 3D Scaffold for Cultivated Meat. Bioengineering. 2024; 11(8):854. https://doi.org/10.3390/bioengineering11080854
Chicago/Turabian StyleBektas, Cemile, Kathleen Lee, Anisha Jackson, Mohit Bhatia, and Yong Mao. 2024. "Bovine Placentome-Derived Extracellular Matrix: A Sustainable 3D Scaffold for Cultivated Meat" Bioengineering 11, no. 8: 854. https://doi.org/10.3390/bioengineering11080854
APA StyleBektas, C., Lee, K., Jackson, A., Bhatia, M., & Mao, Y. (2024). Bovine Placentome-Derived Extracellular Matrix: A Sustainable 3D Scaffold for Cultivated Meat. Bioengineering, 11(8), 854. https://doi.org/10.3390/bioengineering11080854









