Toward Digital Periodontal Health: Recent Advances and Future Perspectives
Abstract
1. Introduction
2. Periodontal Disease and Systemic Disease
2.1. Cardiovascular Disease (CVD)
2.2. Atherosclerosis Cardiovascular Disease (ACVD)
2.3. Diabetes
2.4. Adverse Pregnancy Outcome
2.5. WHIM Syndrome
2.6. Chediak–Higashi Syndrome
2.7. Leukocyte Adhesion Deficiency-I (LAD-I)
2.8. Osteoporosis
2.9. Other Diseases
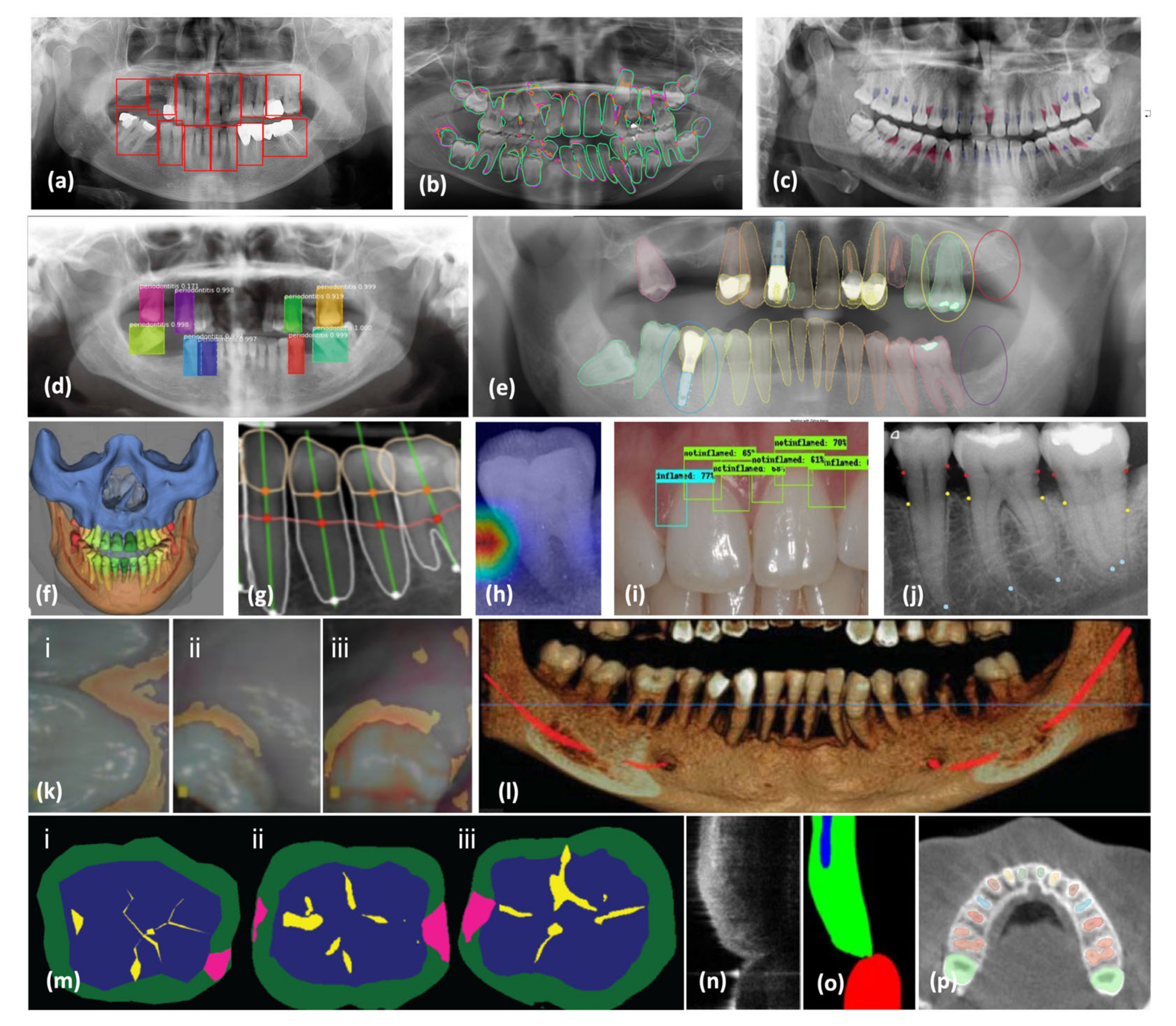
3. AI-Assisted Periodontal Diagnosis in Radiographs
3.1. Radiograph Modalities
3.2. AI Periodontal Diagnosis
3.3. Evaluation Metrics in AI Models
3.4. Advances in AI-Assisted Periodontal Diagnosis
| Work | Remarks | Modality | Size Of Dataset | Preprocessing | Deep Learning Model | CV Strategy | Performance |
|---|---|---|---|---|---|---|---|
| PBL Detection | |||||||
| [87] | Periodontal Inspection | OCT | 18 | ROI Crop | Authors Specific CNN | 1-Fold | IoU = 97.8 |
| [122] | Panoramic | 85 | - | Authors Specific CNN | 10-Times Repeated Group Shuffling | ACC = 81 Sens = 81 Spec = 81 | |
| [123] | Panoramic | 12,179 | ROI SEG | Dentnet | 1-Fold | F1 Score: 75.00 | |
| [118] | Detection of PBL | Panoramic | 1432 | - | AlexNet + SVM | 10-fold | ACC = 81.4 Sens = 84.5 Spec = 79.1 |
| [76] | Alveolar Bone Loss | Panoramic | 1121 | - | U-Net | 1-Fold | ACC = 99.4 F1-Score = 99.7 |
| Horizontal Bone Loss | 1120 | ACC = 89.2 F1-Score = 94.3 | |||||
| Vertical Bone Loss | 828 | ACC = 50.6 F1-Score = 67.3 | |||||
| Furcation Defect | 890 | ROI Crop | ACC = 83.7 F1-Score = 91.2 | ||||
| [74] | Edentulous VS Healthy VS Periodontitis | Panoramic | 4083 | AUG | Faster R-CNN + Region Proposal Network | 5-Fold | AUC = 91 F1-Score = 90 Precision = 90 Recall = 90 |
| [19] | SEG Of PBL, CEJL, and Teeth Structures for Periodontitis Staging | Panoramic | 330 | - | Mask R-CNN + Resnet101 | 1-Fold | Pixel ACC = 92.0 Dice = 93.0 |
| [124] | Staging of PBL | Panoramic | 640 | AUG + ROI SEG (U Net) | Cspdarknet + Spatial Pyramid Pooling Module + Path Aggregation Network + Yolov4 | 1-Fold | ACC = 77 Sens = 77 Spec = 88 |
| [125] | Staging of PBL | panoramic | 1747 | ROI DET (Modified CNN) + AUG | PDCNN | 1-Fold | ACC = 76.2 |
| [126] | Assess Periodontal Bone Level | Periapical | 1724 | VGG-16 | 1-Fold | ||
| [120] | Classification of PBL | Periapical | 21,819 | - | ViT-base | 1-Fold | ACC = 85.2 Sens = 89.8 Spec = 74.5 |
| [127] | Detection of PBL | Periapical | 21,819 | AUG | ConvNeXT-base | 1-Fold | ACC = 84.8 Sens = 90.7 Spec = 71.2 |
| [128] | Assessment of PBL | Periapical | 30 | ROI Crop + AUG + super-resolution algorithm | Inception | 1-Fold | ACC = 95.2 Sens = 90.4 Spec = 48.1 |
| [129] | Detection and classification of PBL | Periapical | 340 | AUG + Landmark LOC (KNEEL) + ROI Crop | - | 3-Fold | ACC = 58 |
| [83] | Estimation of Alveolar Bone Loss | Periapical | 446 | AUG + ROI Crop | Modified CNN | 1-Fold | ACC = 80 Sens = 96 Spec = 41 |
| [81] | Periodontists | Periapical | 1525 | ROI Crop (Yolov7) + Adaptive Histogram Equalization + AUG | Efficientnet-B0 | 10-Fold | ACC = 95.4 Sens = 93.2 Spec = 96.8 |
| [130] | Periodontists | Periapical | 4129 | ROI Crop | Modified Resnet18 | Single-Fold | Sens = 82 Spec = 84 F1-Score = 82.8 |
| [131] | Periodontists | Bitewing | 384 | Tooth Position Identification (Yolov4) + AUG | Alexnet | 5-Fold | ACC = 88.8 Precision = 88.8 Recall = 89.0 |
| [119] | PBL Grading | CBCT | 219 | ROI Crop (U-Net) | Densenet | 1-Fold | Sens = 93.2 Spec = 97.4 (Mild) Sens = 91.1 Spec = 98.6 (Moderate) Sens = 92.8 Spec = 99.6 (Severe) |
| PBL VS Normal | Sens = 94.8 Spec = 96.6 | ||||||
| [132] | Periodontal Disease Segmentation | Periapical | 2000 | RGB To Gray + Semantic SEG | Inception Resnet V2 | Single-Fold | ACC = 93.3 |
| [133] | Normal VS Calculus/Inflammation | Intraoral | 220 | ROI Crop (Yolov5) | Parallel 1D-CNN Blocks | 10-Fold | ACC = 74.5 |
| [134] | Normal VS Caries VS Periodontitis VS Periapical Cysts | Periapical | 188 | AUG | Densenet121 | 1-Fold | ACC: 99.5 Sens = 100 Spec = 99.3 |
| [80] | Periodontal Bone Level Segmentation | Periapical | 8000 | AUG | Detectron2 | 1-Fold | ACC = 92.6 |
| [135] | Periodontitis Detection | Periapical | 2900 | Faster R-CNN | 5-Fold | IoU = 68.0 | |
| [136] | Radiographic Bone Loss Segmentation | Periapical | 693 | - | U Net + Resnet34 | 1-Fold | ACC: Stage 1 = 91 Stage 2 = 88 Stage 3 = 99 No Loss = 99 |
| PCT Detection | |||||||
| [137] | Determine t Severity of PCT for Premolars and Molar | Periapical | 1740 | ROI Crop + AUG | VGG 19 | 1-Fold | ACC = 82.2 (Premolar) ACC = 73.4 (Molar) |
| [77] | Panoramic | 100 | ROI Crop | Faster R-CNN | 5-Fold | Sens = 84 Spec = 88 F1-Score = 81 | |
| Dental Diseases | |||||||
| [90] | Gingival Inflammation | Intraoral | 134 | - | Faster R-CNN | 1-Fold | ACC = 77.1 Precision = 88.0 Recall = 41.7 |
| [138] | Gingival Diseases Segmentation (Healthy, Diseased, or Questionable) | Intraoral | 567 | - | Deeplabv3 | 1-Fold | Sens = 92 Spec = 94 |
| [17] | Gingivitis VS Calculus VS Soft Deposit | Intraoral | 3932 | - | CNN With Multi-Task Learning | 1-Fold | AUC = 87.11 Sens = 60.1 Spec = 83.9 (Gingivitis) AUC = 80.1 Sens = 54.2 Spec = 83.6 (Calculus) AUC = 78.5 Sens = 56.5 Spec = 80.0 (Soft Deposit) |
| [139] | Dental Caries, Dental Fluorosis, Periodontal Disease, Cracked Tooth, Dental Calculus, Dental Plaque, And Tooth Loss | Intraoral | 12,600 | Retinex Algorithm | MASK R-CNN | ACC Caries = 90.1 Fluorosis = 95 Periodontists = 94.3 Cracked Tooth = 94.1 Calculus = 98.1 Plaque = 100 Tooth Loss = 98.4 | |
| [140] | Early-Stage Caries VS Dental Plaque | Intraoral | 7200 | - | Authors Specific CNN | 1-Fold | ACC = 95.9 |
| [121] | Plaque Segmentation | Intraoral | 886 | ROI Crop | Deeplabv3+ | 1-Fold | MIoU: 72.60 |
3.5. AI-Assisted Periodontal Risk Assessment (PRA)
3.6. The Role of AI in Enhancing Periodontal Staging and Grading
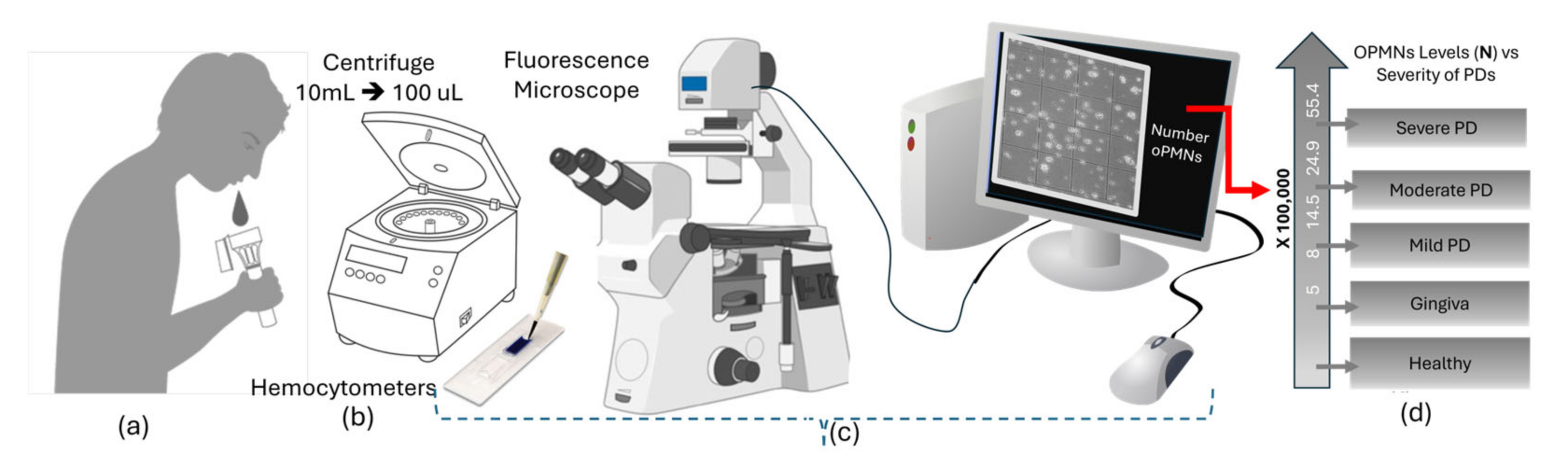
4. Screening Oral Neutrophil Level for Periodontal Diseases Diagnosis and Treatment
4.1. Physiology of Saliva
4.2. Physiology of oPMNs
4.3. Oral Cavity
4.4. oPMNs as a Biomarker of Periodontal Diseases
4.5. oPMNs as a Biomarker of Blood Cancer’s Treatment
5. Toward AI-Assisted oPMN Qualifications
5.1. Traditional oPMN Assessment Methods
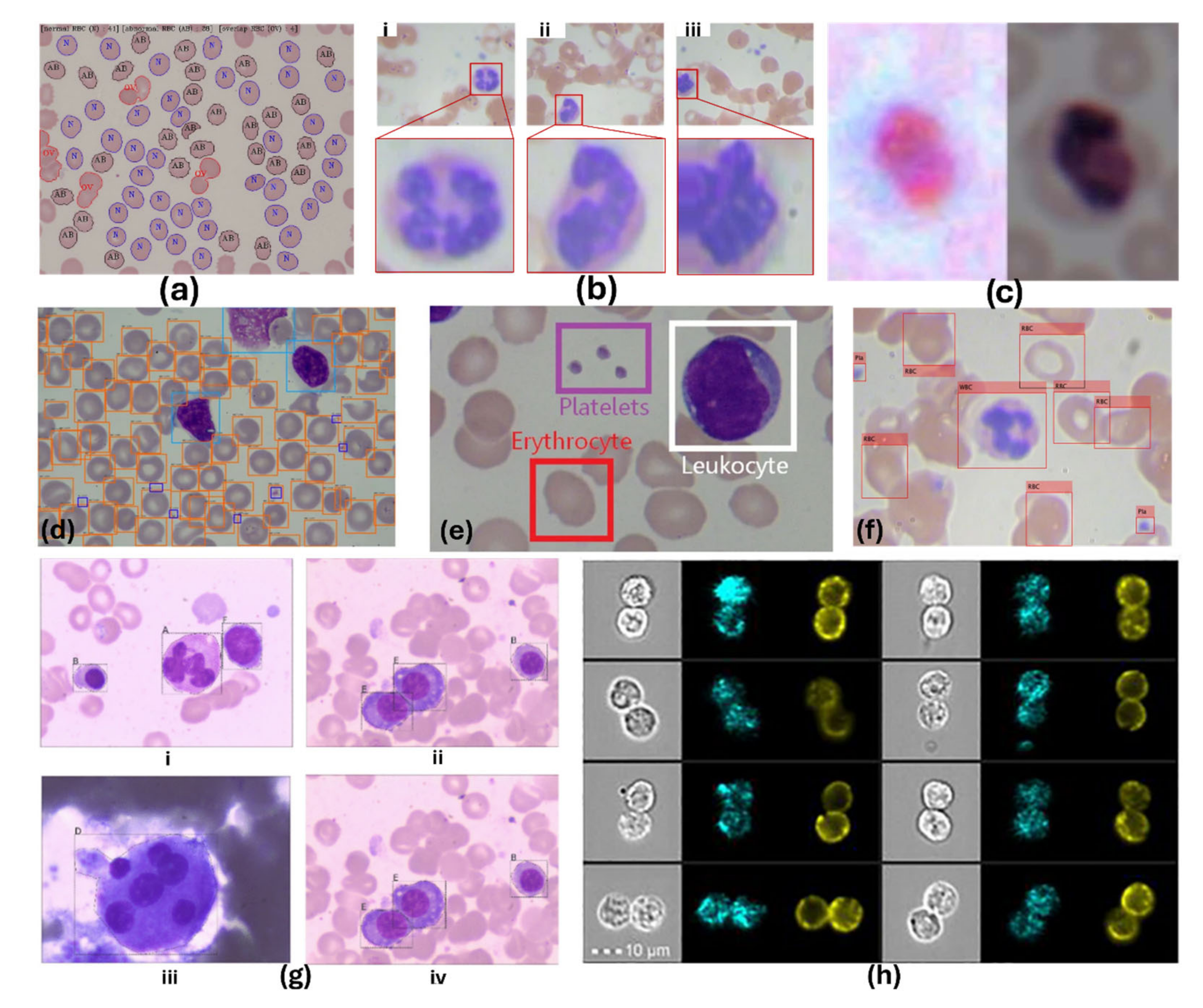
5.2. AI-Assisted Cell Detection and Counting
5.2.1. Conventional Machine Learning Approaches
5.2.2. Limitations of Conventional Machine Learning
5.2.3. Advances in Deep Learning for Cellular Monitoring
5.2.4. Segmentation Approaches
5.2.5. Advances in AI-Assisted Cellular Analysis
| Work | Images Number (Mag–FOV) | Cell | APP | AI Technique | Evaluation |
|---|---|---|---|---|---|
| Prinyakupt et al. [211] | 555/477 (100×–C) | WBC | SEG CL | Thresholding, mathematical morphology, distance modeling/feature extraction/linear and naïve Bayes (NB) classification | Ave. nucleus SEG Dice: 92.9% |
| Nassar et al. [212] | 98 (NA–C) | WBC | CL | Morphological feature extraction/AdaBoost, Gradient Boosting (best), (k-NN), random forest (RF), and SVM classification | Ave. cell SEG Dice: 94.7% |
| López et al. [214] | 1315 (NA–C) | WBC | CL | Keypoint detection/SIFT feature extraction/SVM classification | Ave. CL acc: 98.7% |
| Chen et al. [215] | 60 (400×–W) | Breast Cancer Cells | SEG COUNT | HSV color feature extraction/SVM-based pixel classification/Mathematical morphology-based refinement | Ave. WBC CL F1-Score: 97% |
| Abdeldaim et al. [216] | 260 (300–500×–C) | WBC | CL | Shape, color, texture features extraction/k-NN (best), NB, SVM, and Decision Trees classification | Lymphocyte CL F1-Score: 78% |
| Kumar et al. [238] | 70 (1000×–C) | WBC | SEG CL | k-means, mathematical morphology/GLCM, geometrical, color features extraction/multiclass SVM classification | Max. mean acc: 79% |
| Hegde et al. [218] | 1418 (NA–C) | WBC | CL | Shape, color, texture features extraction/NN, Autoencoders, CNN classification | Peak acc: 85% |
| Alam et al. [39] | 360/100 (100×–W) | WBC/RBC/Platelet | CL DET | YOLO cell detection | Mean (SD) label index error: −0.53% (2.26%) |
| Zhang et al. [38] | 314 (63×–R) | RBC | SEG CL | dU-Net | CL acc: 90% |
| Fan et al. [230] | 300/100/268/257 (NA–C) | WBC | LOC SEG | LeukocyteMask (Modified Mask-RCNN) | Ave. CL acc (NN + handcrafted features): 99.8% |
| Li et al. [227] | 108 (300–500×–C) | WBC | SEG | Enhanced U-Net | Ave. CL acc (CNN): 99% |
| Long et al. [228] | 599 (NA–C) | Various Cell Types | SEG | Enhanced U-Net | Ave. DET acc: RBC: 96.1%, WBC: 86.9%, Platelet: 96.4% |
| Sahlol et al. [223] | 260/10,661 (300–500×–C) | WBC | CL | VGGNet + SESSA feature filtering + SVM classification | Ave. DET acc: Lymphocyte: 99.5%, Monocyte: 98.4%, Basophil: 98.5%, Eosinophil: 96.2%, Neutrophil: 95% |
| Patil et al. [30] | 12,442 (NA–C) | WBC | CL | CNN: VGG16, InceptionV3, ResNet50, Xception (best) + RNN: (LSTM) | Dice: 96.5 |
| Yang et al. [225] | 1819 (100×–C) | WBC/Parasites | CL | Thresholding, IGMS, modified VGG-19 | Multiclass SEG Dice: 0.74 (0.016) |
| Kassim et al. [37] | 965 (NA–W) | RBC | SEG DET | Dual deep learning architecture: U-Net + Faster R-CNN | Binary SEG Ave. Dice: 0.97–0.98 |
| He et al. [31] | 410 (100×–C) | WBC/RBC/Platelet | DET | Improved CycleGAN for fully labeled data generation. Tested with YOLO and Faster R-CNN (best) | (four different datasets) |
| Chen et al. [36] | 31,058 | WBC | CL | Deep Feature Fusion Neural Network | ACC = 80.3 |
| Kutlu et al. [34] | 6259 | WBC | DET | R-CNN | ACC: Lymphocyte = 99.52 Monocyte = 98.40 Basophil = 98.48 Eosinophil 96.16 |
| Leng et al. [35] | 10,323 | WBC | SEG | DETR | Precision = 96.1 |
| Cheuque et al. [40] | 365 | WBC | DET, CL | Faster R-CNN + MobileNet | ACC = 98.4 |
| Wu et al. [239] | 268 | WBC | SEG | ResNet50 + Attentional Mechanisms | Dice = 98.13 |
| 248 | Dice = 95.31 | ||||
| Elhassan et al. [32] | 18,365 | WBC | LOC | CMYK-moment + modified CNN + RF | ACC = 97.57 |
| 17,092 | ACC = 95.47 | ||||
| Revanda et al. [33] | 31 | WBC | CL | Mask R-CNN | ACC= 83.72 |
| Zhong et al. [240] | 6038 | TBS | SEG, DET | AlexNet | ACC = 96.22 |
| 111 | |||||
| Olayah et al. [241] | 12,507 | WBC | CL | Deep Fusion Model based on VGG-19, MobileNet and ResNet-101 | ACC = 99.80 |
| Wang et al. [242] | 12,515 | WBC | CL | WBC-AMNet | ACC = 89.22 |
| 4358 | ACC = 98.39 | ||||
| Prasad et al. [243] | 12,500 | WBC | CL | DCRNet | ACC = 97.39 |
| 400 | ACC = 94.39 | ||||
| Prasad et al. [244] | 300 | WBC | SEG, Size determination | Deep U_ClusterNet | ACC = 98.8 |
| 100 | ACC = 97.8 | ||||
| Batool et al. [245] | 15,114 | WBC | CL | EfficientNetB3 | ACC = 99.31 |
| Katar et al. [246] | 16,633 | WBC | CL, LOC | ViT | ACC = 99.70 |
| Khan et al. [247] | 182,711 | WBC | CL | DCGAN + MobileNet + ATT Module | ACC = 99.83 |
| 5000 | ACC = 99.35 | ||||
| 21,740 | ACC = 99.60 | ||||
| Bairaboina et al. [248] | 12,444 | WBC | CL | Ghost-ResNeXt | ACC = 99.24 |
| 242 | ACC = 99.16 | ||||
| 3517 | ACC = 98.61 | ||||
| Lu et al. [249] | 300 | WBC | SEG | ResNet + UNet++ | Dice = 98.92 |
| 100 | Dice = 99.28 | ||||
| 242 | Dice = 92.24 | ||||
| 231 | Dice = 97.60 | ||||
| Haider et al. [250] | 60 | WBC | SEG | Deep aggregation segmentation network | Dice = 98.97 |
| 20 | |||||
| 48 | Dice = 99.00 | ||||
| 46 | Dice = 96.05 | ||||
| Dice = 88.62 |
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. Study Selection
Appendix A.2. Databases
Appendix A.3. Search Period
Appendix A.4. Selection Criteria
Appendix A.5. Synthesis and Analysis
References
- Tichenor, M.; Sridhar, D. Metric partnerships: Global burden of disease estimates within the World Bank, the World Health Organisation and the Institute for Health Metrics and Evaluation. Wellcome Open Res. 2019, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Highfield, J. Diagnosis and classification of periodontal disease. Aust. Dent. J. 2009, 54, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar]
- Mealey, B.L.; Moritz, A.J. Hormonal influences: Effects of diabetes mellitus and endogenous female sex steroid hormones on the periodontium. Periodontol. 2000 2003, 32, 59–81. [Google Scholar] [CrossRef]
- Chen, F.; Song, Y.; Li, W.; Xu, H.; Dan, H.; Chen, Q. Association between periodontitis and mortality of patients with cardiovascular diseases: A cohort study based on NHANES. J. Periodontol. 2024, 95, 175–184. [Google Scholar] [CrossRef]
- Dababneh, R.; Al-wahadneh, A.M.; Hamadneh, S.; Khouri, A.; Bissada, N.F. Periodontal manifestation of leukocyte adhesion deficiency type I. J. Periodontol. 2008, 79, 764–768. [Google Scholar] [CrossRef]
- Thinkhamrop, J.; Hofmeyr, G.J.; Adetoro, O.; Lumbiganon, P.; Ota, E. Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity. Cochrane Database Syst. Rev. 2015, 1, CD002250. [Google Scholar]
- Takeuchi, N.; Ekuni, D.; Irie, K.; Furuta, M.; Tomofuji, T.; Morita, M.; Watanabe, T. Relationship between periodontal inflammation and fetal growth in pregnant women: A cross-sectional study. Arch. Gynecol. Obstet. 2013, 287, 951–957. [Google Scholar] [CrossRef]
- Gorlin, R.J.; Gelb, B.; Diaz, G.A.; Lofsness, K.G.; Pittelkow, M.R.; Fenyk, J.R., Jr. WHIM syndrome, an autosomal dominant disorder: Clinical, hematological, and molecular studies. Am. J. Med. Genet. 2000, 91, 368–376. [Google Scholar] [CrossRef]
- Hajishengallis, E.; Hajishengallis, G. Neutrophil homeostasis and periodontal health in children and adults. J. Dent. Res. 2014, 93, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 1 September 2024).
- Wu, C.-Z.; Yuan, Y.-H.; Liu, H.-H.; Li, S.-S.; Zhang, B.-W.; Chen, W.; An, Z.-J.; Chen, S.-Y.; Wu, Y.-Z.; Han, B. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health 2020, 20, 204. [Google Scholar] [CrossRef] [PubMed]
- Andrade, K.M.; Silva, B.P.M.; de Oliveira, L.R.; Cury, P.R. Automatic dental biofilm detection based on deep learning. J. Clin. Periodontol. 2023, 50, 571–581. [Google Scholar] [CrossRef]
- Shen, K.L.; Huang, C.L.; Lin, Y.C.; Du, J.K.; Chen, F.L.; Kabasawa, Y.; Chen, C.C.; Huang, H.L. Effects of artificial intelligence-assisted dental monitoring intervention in patients with periodontitis: A randomized controlled trial. J. Clin. Periodontol. 2022, 49, 988–998. [Google Scholar] [CrossRef]
- Li, W.; Liang, Y.; Zhang, X.; Liu, C.; He, L.; Miao, L.; Sun, W. A deep learning approach to automatic gingivitis screening based on classification and localization in RGB photos. Sci. Rep. 2021, 11, 16831. [Google Scholar] [CrossRef]
- Mupparapu, M.; Nadeau, C. Oral and maxillofacial imaging. Dent. Clin. 2016, 60, 1–37. [Google Scholar] [CrossRef]
- Chang, H.-J.; Lee, S.-J.; Yong, T.-H.; Shin, N.-Y.; Jang, B.-G.; Kim, J.-E.; Huh, K.-H.; Lee, S.-S.; Heo, M.-S.; Choi, S.-C. Deep learning hybrid method to automatically diagnose periodontal bone loss and stage periodontitis. Sci. Rep. 2020, 10, 7531. [Google Scholar] [CrossRef]
- Morimoto, Y.; Tanaka, T.; Yamamoto, N.; Kodama, M.; Seta, Y.; Habu, M.; Oda, M.; Kito, S.; Wakasugi-Sato, N.; Matsumoto-Takeda, S. New trends and advances in oral and maxillofacial imaging. Curr. Med. Imaging 2009, 5, 226–237. [Google Scholar] [CrossRef]
- Scott, D.A.; Krauss, J. Neutrophils in periodontal inflammation. Periodontal Dis. 2012, 15, 56–83. [Google Scholar]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil diversity in health and disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Mühlberg, S.; Jäger, J.; Krohn-Grimberghe, B.; Patschan, S.; Mausberg, R.F.; Schmalz, G.; Haak, R.; Ziebolz, D. Oral health-related quality of life depending on oral health in patients with rheumatoid arthritis. Clin. Oral Investig. 2017, 21, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Păunică, I.; Giurgiu, M.; Dumitriu, A.S.; Păunică, S.; Pantea Stoian, A.M.; Martu, M.-A.; Serafinceanu, C. The bidirectional relationship between periodontal disease and diabetes mellitus—A review. Diagnostics 2023, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Francisconi, C.F.; Caldas, R.J.; Oliveira Martins, L.J.; Fischer Rubira, C.M.; da Silva Santos, P.S. Leukemic oral manifestations and their management. Asian Pac. J. Cancer Prev. 2016, 17, 911–915. [Google Scholar] [CrossRef]
- Bennett, J.S. Structure and function of the platelet integrin α IIb β 3. J. Clin. Investig. 2005, 115, 3363–3369. [Google Scholar] [CrossRef]
- Bramantoro, T.; Zulfiana, A.A.; Amir, M.S.; Irmalia, W.R.; Nor, N.A.M.; Nugraha, A.P.; Krismariono, A. The contradictory effects of coffee intake on periodontal health: A systematic review of experimental and observational studies. F1000Research 2022, 11, 924. [Google Scholar] [CrossRef]
- Landzberg, M.; Doering, H.; Aboodi, G.; Tenenbaum, H.; Glogauer, M. Quantifying oral inflammatory load: Oral neutrophil counts in periodontal health and disease. J. Periodontal Res. 2015, 50, 330–336. [Google Scholar] [CrossRef]
- Lakschevitz, F.S.; Aboodi, G.M.; Glogauer, M. Oral neutrophil transcriptome changes result in a pro-survival phenotype in periodontal diseases. PLoS ONE 2013, 8, e68983. [Google Scholar] [CrossRef]
- Patil, A.; Patil, M.; Birajdar, G. White blood cells image classification using deep learning with canonical correlation analysis. Irbm 2021, 42, 378–389. [Google Scholar] [CrossRef]
- He, J.; Wang, C.; Jiang, D.; Li, Z.; Liu, Y.; Zhang, T. CycleGAN with an improved loss function for cell detection using partly labeled images. IEEE J. Biomed. Health Inform. 2020, 24, 2473–2480. [Google Scholar] [CrossRef]
- Elhassan, T.A.M.; Rahim, M.S.M.; Swee, T.T.; Hashim, S.Z.M.; Aljurf, M. Feature extraction of white blood cells using CMYK-moment localization and deep learning in acute myeloid leukemia blood smear microscopic images. IEEE Access 2022, 10, 16577–16591. [Google Scholar] [CrossRef]
- Revanda, A.R.; Fatichah, C.; Suciati, N. Classification of Acute Lymphoblastic Leukemia on White Blood Cell Microscopy Images Based on Instance Segmentation Using Mask R-CNN. Int. J. Intell. Eng. Syst. 2022, 15, 625–637. [Google Scholar]
- Kutlu, H.; Avci, E.; Özyurt, F. White blood cells detection and classification based on regional convolutional neural networks. Med. Hypotheses 2020, 135, 109472. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Wang, C.; Leng, M.; Ge, M.; Dong, W. Deep learning detection network for peripheral blood leukocytes based on improved detection transformer. Biomed. Signal Process. Control 2023, 82, 104518. [Google Scholar] [CrossRef]
- Chen, J.; Fu, L.; Wei, M.; Zheng, S.; Zheng, J.; Lyu, Z.; Huang, X.; Sun, L. Label-free White Blood Cells Classification Using A Deep Feature Fusion Neural Network. Heliyon 2024, 10, e31496. [Google Scholar] [CrossRef]
- Kassim, Y.M.; Palaniappan, K.; Yang, F.; Poostchi, M.; Palaniappan, N.; Maude, R.J.; Antani, S.; Jaeger, S. Clustering-based dual deep learning architecture for detecting red blood cells in malaria diagnostic smears. IEEE J. Biomed. Health Inform. 2020, 25, 1735–1746. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Xu, M.; Li, Q. Automated semantic segmentation of red blood cells for sickle cell disease. IEEE J. Biomed. Health Inform. 2020, 24, 3095–3102. [Google Scholar] [CrossRef]
- Alam, M.M.; Islam, M.T. Machine learning approach of automatic identification and counting of blood cells. Healthc. Technol. Lett. 2019, 6, 103–108. [Google Scholar] [CrossRef]
- Cheuque, C.; Querales, M.; León, R.; Salas, R.; Torres, R. An efficient multi-level convolutional neural network approach for white blood cells classification. Diagnostics 2022, 12, 248. [Google Scholar] [CrossRef]
- Zia, E.; Melander, O.; Björkbacka, H.; Hedblad, B.; Engström, G. Total and differential leucocyte counts in relation to incidence of stroke subtypes and mortality: A prospective cohort study. J. Intern. Med. 2012, 272, 298–304. [Google Scholar] [CrossRef]
- Pfister, R.; Sharp, S.J.; Luben, R.; Wareham, N.J.; Khaw, K.-T. Differential white blood cell count and incident heart failure in men and women in the EPIC-Norfolk study. Eur. Heart J. 2012, 33, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Satish, B.; Srikala, P.; Maharudrappa, B.; Awanti, S.M.; Kumar, P.; Hugar, D. Saliva: A tool in assessing glucose levels in Diabetes Mellitus. J. Int. Oral Health: JIOH 2014, 6, 114. [Google Scholar]
- Zhang, C.-Z.; Cheng, X.-Q.; Li, J.-Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.-D. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 2016, 8, 133–137. [Google Scholar] [CrossRef]
- Genco, R.; Grossi, S. Is estrogen deficiency a risk factor for periodontal disease? Compend. Contin. Educ. Dentistry. Suppl. 1998, 22, S23–S29. [Google Scholar]
- Aitken, J.P.; Ortiz, C.; Morales-Bozo, I.; Rojas-Alcayaga, G.; Baeza, M.; Beltran, C.; Escobar, A. α-2-macroglobulin in saliva is associated with glycemic control in patients with type 2 diabetes mellitus. Dis. Markers 2015, 2015, 128653. [Google Scholar] [CrossRef]
- Collins, J.; Smith, M.; Arnold, R.; Offenbacher, S. Effects of Escherichia coli and Porphyromonas gingivalis lipopolysaccharide on pregnancy outcome in the golden hamster. Infect. Immun. 1994, 62, 4652–4655. [Google Scholar] [CrossRef]
- Offenbacher, S.; Katz, V.; Fertik, G.; Collins, J.; Boyd, D.; Maynor, G.; McKaig, R.; Beck, J. Periodontal infection as a possible risk factor for preterm low birth weight. J. Periodontol. 1996, 67, 1103–1113. [Google Scholar] [CrossRef]
- Mahapatra, A.; Nayak, R.; Satpathy, A.; Pati, B.K.; Mohanty, R.; Mohanty, G.; Beura, R. Maternal periodontal status, oral inflammatory load, and systemic inflammation are associated with low infant birth weight. J. Periodontol. 2021, 92, 1107–1116. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Gorlin, R.J.; Lukens, J.N.; Taniuchi, S.; Bohinjec, J.; Francois, F.; Klotman, M.E.; Diaz, G.A. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat. Genet. 2003, 34, 70–74. [Google Scholar] [CrossRef]
- Nagle, D.L.; Karim, M.A.; Woolf, E.A.; Holmgren, L.; Bork, P.; Misumi, D.J.; McGrail, S.H.; Dussault Jr, B.J.; Perou, C.M.; Boissy, R.E. Identification and mutation analysis of the complete gene for Chediak–Higashi syndrome. Nat. Genet. 1996, 14, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Blume, R.S.; Bennett, J.M.; Yankee, R.A.; Wolff, S.M. Defective granulocyte regulation in the Chediak–Higashi syndrome. New Engl. J. Med. 1968, 279, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Thumbigere Math, V.; Rebouças, P.; Giovani, P.; Puppin-Rontani, R.; Casarin, R.; Martins, L.; Wang, L.; Krzewski, K.; Introne, W.; Somerman, M. Periodontitis in Chédiak-Higashi syndrome: An altered immunoinflammatory response. JDR Clin. Transl. Res. 2018, 3, 35–46. [Google Scholar] [CrossRef]
- Hanna, S.; Etzioni, A. Leukocyte adhesion deficiencies. Ann. N. Y. Acad. Sci. 2012, 1250, 50–55. [Google Scholar] [CrossRef]
- Meyle, J. Leukocyte adhesion deficiency and prepubertal periodontitis. Periodontology 2000, 6, 26–36. [Google Scholar] [CrossRef]
- Roberts, M.W.; Atkinson, J. Oral manifestations associated with leukocyte adhesion deficiency: A five-year case study. Pediatr Dent 1990, 12, 107–111. [Google Scholar]
- Wactawski-Wende, J.; Grossi, S.G.; Trevisan, M.; Genco, R.J.; Tezal, M.; Dunford, R.G.; Ho, A.W.; Hausmann, E.; Hreshchyshyn, M.M. The role of osteopenia in oral bone loss and periodontal disease. J. Periodontol. 1996, 67, 1076–1084. [Google Scholar] [CrossRef]
- Garnero, P. Markers of bone turnover for the prediction of fracture risk. Osteoporos. Int. 2000, 11, S55. [Google Scholar] [CrossRef]
- Jeffcoat, M.K.; Chesnut, C.H. Systemic osteoporosis and oral bone loss: Evidence shows increased risk factors. J. Am. Dent. Assoc. 1993, 124, 49–56. [Google Scholar] [CrossRef]
- Moutsopoulos, N.M.; Konkel, J.; Sarmadi, M.; Eskan, M.A.; Wild, T.; Dutzan, N.; Abusleme, L.; Zenobia, C.; Hosur, K.B.; Abe, T. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17–driven inflammatory bone loss. Sci. Transl. Med. 2014, 6, 229ra40. [Google Scholar] [CrossRef]
- Sharma, P.; Dietrich, T.; Sidhu, A.; Vithlani, V.; Rahman, M.; Stringer, S.; Jesky, M.; Kaur, O.; Ferro, C.; Cockwell, P. The periodontal health component of the Renal Impairment In Secondary Care (RIISC) cohort study: A description of the rationale, methodology and initial baseline results. J. Clin. Periodontol. 2014, 41, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.; Wilson, N. Manifesto for a paradigm shift: Periodontal health for a better life. Br. Dent. J. 2014, 216, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Arigbede, A.O.; Babatope, B.O.; Bamidele, M.K. Periodontitis and systemic diseases: A literature review. J. Indian Soc. Periodontol. 2012, 16, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, N.R.; Smith, T.O.; Kaul, A.; Sofat, N. Hand to mouth: A systematic review and meta-analysis of the association between rheumatoid arthritis and periodontitis. Front. Immunol. 2016, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-T.; Tu, M.-L.; Liu, D.-Y.; Zheng, D.; Zhang, J.; Leng, W. Periodontal disease and risk of chronic obstructive pulmonary disease: A meta-analysis of observational studies. PLoS ONE 2012, 7, e46508. [Google Scholar] [CrossRef]
- Caglayan, F.; Bayrakdar, I.S. The intraoral ultrasonography in dentistry. Niger. J. Clin. Pract. 2018, 21, 125–133. [Google Scholar]
- Wenzel, A. Radiographic display of carious lesions and cavitation in approximal surfaces: Advantages and drawbacks of conventional and advanced modalities. Acta Odontol. Scand. 2014, 72, 251–264. [Google Scholar] [CrossRef]
- Sansare, K.; Raghav, M.; Sontakke, S.; Karjodkar, F.; Wenzel, A. Clinical cavitation and radiographic lesion depth in proximal surfaces in an Indian population. Acta Odontol. Scand. 2014, 72, 1084–1088. [Google Scholar] [CrossRef]
- Acar, B.; Kamburoğlu, K. Use of cone beam computed tomography in periodontology. World J. Radiol. 2014, 6, 139. [Google Scholar] [CrossRef]
- Nasseh, I.; Al-Rawi, W. Cone beam computed tomography. Dent. Clin. 2018, 62, 361–391. [Google Scholar] [CrossRef]
- Evans, C.A.; Scarfe, W.C.; Ahmad, M.; Cevidanes, L.H.; Ludlow, J.B.; Palomo, J.M.; Simmons, K.E.; White, S.C. Clinical recommendations regarding use of cone beam computed tomography in orthodontics. Position statement by the American Academy of Oral and Maxillofacial Radiology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 238–257. [Google Scholar]
- Singh, N.K.; Raza, K. Progress in deep learning-based dental and maxillofacial image analysis: A systematic review. Expert Syst. Appl. 2022, 199, 116968. [Google Scholar] [CrossRef]
- Ryu, J.; Lee, D.-M.; Jung, Y.-H.; Kwon, O.; Park, S.; Hwang, J.; Lee, J.-Y. Automated detection of periodontal bone loss using deep learning and panoramic radiographs: A convolutional neural network approach. Appl. Sci. 2023, 13, 5261. [Google Scholar] [CrossRef]
- Koch, T.L.; Perslev, M.; Igel, C.; Brandt, S.S. Accurate segmentation of dental panoramic radiographs with U-Nets. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venezia, Italy, 8–11 April 2019; pp. 15–19. [Google Scholar]
- Kurt-Bayrakdar, S.; Bayrakdar, İ.Ş.; Yavuz, M.B.; Sali, N.; Çelik, Ö.; Köse, O.; Uzun Saylan, B.C.; Kuleli, B.; Jagtap, R.; Orhan, K. Detection of periodontal bone loss patterns and furcation defects from panoramic radiographs using deep learning algorithm: A retrospective study. BMC Oral Health 2024, 24, 155. [Google Scholar] [CrossRef]
- Thanathornwong, B.; Suebnukarn, S. Automatic detection of periodontal compromised teeth in digital panoramic radiographs using faster regional convolutional neural networks. Imaging Sci. Dent. 2020, 50, 169. [Google Scholar] [CrossRef]
- De Angelis, F.; Pranno, N.; Franchina, A.; Di Carlo, S.; Brauner, E.; Ferri, A.; Pellegrino, G.; Grecchi, E.; Goker, F.; Stefanelli, L.V. Artificial intelligence: A new diagnostic software in dentistry: A preliminary performance diagnostic study. Int. J. Environ. Res. Public Health 2022, 19, 1728. [Google Scholar] [CrossRef]
- Hung, K.F.; Ai, Q.Y.H.; Wong, L.M.; Yeung, A.W.K.; Li, D.T.S.; Leung, Y.Y. Current applications of deep learning and radiomics on CT and CBCT for maxillofacial diseases. Diagnostics 2022, 13, 110. [Google Scholar] [CrossRef]
- Chen, C.-C.; Wu, Y.-F.; Aung, L.M.; Lin, J.C.-Y.; Ngo, S.T.; Su, J.-N.; Lin, Y.-M.; Chang, W.-J. Automatic recognition of teeth and periodontal bone loss measurement in digital radiographs using deep-learning artificial intelligence. J. Dent. Sci. 2023, 18, 1301–1309. [Google Scholar] [CrossRef]
- Chen, I.D.S.; Yang, C.-M.; Chen, M.-J.; Chen, M.-C.; Weng, R.-M.; Yeh, C.-H. Deep learning-based recognition of periodontitis and dental caries in dental x-ray images. Bioengineering 2023, 10, 911. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, K.; Lyu, P.; Li, H.; Zhang, L.; Wu, J.; Lee, C.-H. A deep learning approach to automatic teeth detection and numbering based on object detection in dental periapical films. Sci. Rep. 2019, 9, 3840. [Google Scholar] [CrossRef]
- Tsoromokos, N.; Parinussa, S.; Claessen, F.; Moin, D.A.; Loos, B.G. Estimation of alveolar bone loss in periodontitis using machine learning. Int. Dent. J. 2022, 72, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, H.; Li, Z. A weakly supervised learning-based segmentation network for dental diseases. Math. Biosci. Eng. 2023, 20, 2039–2060. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Mark, R.; Sing, I.; Jain, A. Diagnostic accuracy of CBCT for aggressive periodontitis. J. Clin. Imaging Sci. 2014, 4, 2. [Google Scholar] [CrossRef]
- Casalegno, F.; Newton, T.; Daher, R.; Abdelaziz, M.; Lodi-Rizzini, A.; Schürmann, F.; Krejci, I.; Markram, H. Caries detection with near-infrared transillumination using deep learning. J. Dent. Res. 2019, 98, 1227–1233. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Chiu, C.-H.; Cai, Z.-Q.; Lin, J.-Y.; Yao, C.-Y.; Lyu, D.-Y.; Lee, S.-Y.; Chen, K.-W.; Chen, I.-Y. OCT-based periodontal inspection framework. Sensors 2019, 19, 5496. [Google Scholar] [CrossRef]
- Cui, Z.; Li, C.; Wang, W. ToothNet: Automatic tooth instance segmentation and identification from cone beam CT images. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, New Orleans, LA, USA, 18–24 June 2022; pp. 6368–6377. [Google Scholar]
- Musleh, D.; Almossaeed, H.; Balhareth, F.; Alqahtani, G.; Alobaidan, N.; Altalag, J.; Aldossary, M.I. Advancing Dental Diagnostics: A Review of Artificial Intelligence Applications and Challenges in Dentistry. Big Data Cogn. Comput. 2024, 8, 66. [Google Scholar] [CrossRef]
- Collins, C.; Dennehy, D.; Conboy, K.; Mikalef, P. Artificial intelligence in information systems research: A systematic literature review and research agenda. Int. J. Inf. Manag. 2021, 60, 102383. [Google Scholar] [CrossRef]
- Ding, H.; Wu, J.; Zhao, W.; Matinlinna, J.P.; Burrow, M.F.; Tsoi, J.K. Artificial intelligence in dentistry—A review. Front. Dent. Med. 2023, 4, 1085251. [Google Scholar] [CrossRef]
- Khanagar, S.B.; Al-Ehaideb, A.; Maganur, P.C.; Vishwanathaiah, S.; Patil, S.; Baeshen, H.A.; Sarode, S.C.; Bhandi, S. Developments, application, and performance of artificial intelligence in dentistry–A systematic review. J. Dent. Sci. 2021, 16, 508–522. [Google Scholar] [CrossRef]
- Zhang, B.; Dai, N.; Tian, S.; Yuan, F.; Yu, Q. The extraction method of tooth preparation margin line based on S-Octree CNN. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3241. [Google Scholar] [CrossRef]
- Mehl, A.; Blanz, V. New procedure for fully automatic occlusal surface reconstruction by means of a biogeneric tooth model. Int. J. Comput. Dent. 2005, 8, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.H.; Sporring, J. Reconstructing teeth with bite information. In Proceedings of the Image Analysis: 15th Scandinavian Conference, SCIA 2007, Aalborg, Denmark, 10–14 June 2007; pp. 102–111. [Google Scholar]
- Minnema, J.; Ernst, A.; van Eijnatten, M.; Pauwels, R.; Forouzanfar, T.; Batenburg, K.J.; Wolff, J. A review on the application of deep learning for CT reconstruction, bone segmentation and surgical planning in oral and maxillofacial surgery. Dentomaxillofacial Radiol. 2022, 51, 20210437. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Gómez-Polo, M.; Vyas, S.; Barmak, B.A.; Galluci, G.O.; Att, W.; Krishnamurthy, V.R. Artificial intelligence applications in implant dentistry: A systematic review. J. Prosthet. Dent. 2023, 129, 293–300. [Google Scholar] [CrossRef]
- Revilla-León, M.; Gómez-Polo, M.; Barmak, A.B.; Inam, W.; Kan, J.Y.; Kois, J.C.; Akal, O. Artificial intelligence models for diagnosing gingivitis and periodontal disease: A systematic review. J. Prosthet. Dent. 2023, 130, 816–824. [Google Scholar] [CrossRef]
- Carter, K.; Landini, G.; Walmsley, A.D. Automated quantification of dental plaque accumulation using digital imaging. J. Dent. 2004, 32, 623–628. [Google Scholar] [CrossRef]
- Joseph, B.; Prasanth, C.S.; Jayanthi, J.L.; Presanthila, J.; Subhash, N. Detection and quantification of dental plaque based on laser-induced autofluorescence intensity ratio values. J. Biomed. Opt. 2015, 20, 048001. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Pazinatto, J.; Zanatta, F.B. Are oral hygiene instructions with aid of plaque-disclosing methods effective in improving self-performed dental plaque control? A systematic review of randomized controlled trials. Int. J. Dent. Hyg. 2021, 19, 239–254. [Google Scholar] [CrossRef]
- Mensi, M.; Scotti, E.; Sordillo, A.; Agosti, R.; Calza, S. Plaque disclosing agent as a guide for professional biofilm removal: A randomized controlled clinical trial. Int. J. Dent. Hyg. 2020, 18, 285–294. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, R.; Qu, W.; Wu, W.; Chen, J.; Fang, J.; Chen, Y.; Farella, M.; Mei, L. Effect of visual method vs plaque disclosure in enhancing oral hygiene in adolescents and young adults: A single-blind randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 280–286. [Google Scholar] [CrossRef]
- Van der Veen, M.; Thomas, R.; Huysmans, M.; De Soet, J. Red autofluorescence of dental plaque bacteria. Caries Res. 2006, 40, 542–545. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Lee, E.-S.; Kwon, H.-K.; Kim, B.-I. Monitoring the maturation process of a dental microcosm biofilm using the Quantitative Light-induced Fluorescence-Digital (QLF-D). J. Dent. 2014, 42, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, M.H.; Volgenant, C.M.; Keijser, B.; Ten Cate, J.B.M.; Crielaard, W. Dynamics of red fluorescent dental plaque during experimental gingivitis—A cohort study. J. Dent. 2016, 48, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Choi, D.-H.; Mah, Y.-J.; Pang, E.-K. Validity assessment of quantitative light-induced fluorescence-digital (QLF-D) for the dental plaque scoring system: A cross-sectional study. BMC Oral Health 2018, 18, 187. [Google Scholar] [CrossRef]
- Marjorie, K.; Jeffcoat, I.; Wang, C.; Reddy, M.S. Radiographic diagnosis in periodontics. Periodontol. 2000 1995, 7, 54–68. [Google Scholar]
- Tugnait, A.; Clerehugh, V.; Hirschmann, P. The usefulness of radiographs in diagnosis and management of periodontal diseases: A review. J. Dent. 2000, 28, 219–226. [Google Scholar] [CrossRef]
- Mol, A. Imaging methods in periodontology. Periodontol. 2000 2004, 34, 34–48. [Google Scholar] [CrossRef]
- Brägger, U. Radiographic parameters: Biological significance and clinical use. Periodontol. 2000 2005, 39, 73–90. [Google Scholar] [CrossRef]
- Corbet, E.; Ho, D.; Lai, S. Radiographs in periodontal disease diagnosis and management. Aust. Dent. J. 2009, 54, S27–S43. [Google Scholar] [CrossRef]
- Hausmann, E.; Allen, K.; Christersson, L.; Genco, R. Effect of x-ray beam vertical angulation on radiographic alveolar crest level measurement. J. Periodontal Res. 1989, 24, 8–19. [Google Scholar] [CrossRef]
- Wouters, F.R.; Lavstedt, S.; Frithiof, L.; Söder, P.-Ö.; Hellden, L.; Salonen, L. A computerized system to measure interproximal alveolar bone levels in epidemiologic, radiographic investigations: II. Intra-and inter-examiner variation study. Acta Odontol. Scand. 1988, 46, 33–39. [Google Scholar] [CrossRef]
- Khanagar, S.B.; Alfouzan, K.; Alkadi, L.; Albalawi, F.; Iyer, K.; Awawdeh, M. Performance of Artificial Intelligence (AI) models designed for application in pediatric dentistry—A systematic review. Appl. Sci. 2022, 12, 9819. [Google Scholar] [CrossRef]
- Ghaffari, M.; Zhu, Y.; Shrestha, A. A Review of Advancements of Artificial Intelligence in Dentistry. Dent. Rev. 2024, 4, 100081. [Google Scholar] [CrossRef]
- Sunnetci, K.M.; Ulukaya, S.; Alkan, A. Periodontal bone loss detection based on hybrid deep learning and machine learning models with a user-friendly application. Biomed. Signal Process. Control 2022, 77, 103844. [Google Scholar]
- Ezhov, M.; Gusarev, M.; Golitsyna, M.; Yates, J.M.; Kushnerev, E.; Tamimi, D.; Aksoy, S.; Shumilov, E.; Sanders, A.; Orhan, K. Clinically applicable artificial intelligence system for dental diagnosis with CBCT. Sci. Rep. 2021, 11, 15006. [Google Scholar] [CrossRef]
- Dujic, H.; Meyer, O.; Hoss, P.; Wölfle, U.C.; Wülk, A.; Meusburger, T.; Meier, L.; Gruhn, V.; Hesenius, M.; Hickel, R. Automatized Detection of Periodontal Bone Loss on Periapical Radiographs by Vision Transformer Networks. Diagnostics 2023, 13, 3562. [Google Scholar] [CrossRef]
- You, W.; Hao, A.; Li, S.; Wang, Y.; Xia, B. Deep learning-based dental plaque detection on primary teeth: A comparison with clinical assessments. BMC Oral Health 2020, 20, 141. [Google Scholar] [CrossRef]
- Krois, J.; Ekert, T.; Meinhold, L.; Golla, T.; Kharbot, B.; Wittemeier, A.; Dörfer, C.; Schwendicke, F. Deep learning for the radiographic detection of periodontal bone loss. Sci. Rep. 2019, 9, 8495. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.-S.; Song, I.-S.; Jung, K.-H. DeNTNet: Deep Neural Transfer Network for the detection of periodontal bone loss using panoramic dental radiographs. Sci. Rep. 2019, 9, 17615. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, D.; Cao, Z.; Wu, F.; Zhu, H.; Zhu, F. A two-stage deep learning architecture for radiographic staging of periodontal bone loss. BMC Oral Health 2022, 22, 106. [Google Scholar] [CrossRef]
- Kong, Z.; Ouyang, H.; Cao, Y.; Huang, T.; Ahn, E.; Zhang, M.; Liu, H. Automated periodontitis bone loss diagnosis in panoramic radiographs using a bespoke two-stage detector. Comput. Biol. Med. 2023, 152, 106374. [Google Scholar] [CrossRef]
- Alotaibi, G.; Awawdeh, M.; Farook, F.F.; Aljohani, M.; Aldhafiri, R.M.; Aldhoayan, M. Artificial intelligence (AI) diagnostic tools: Utilizing a convolutional neural network (CNN) to assess periodontal bone level radiographically—A retrospective study. BMC Oral Health 2022, 22, 399. [Google Scholar] [CrossRef] [PubMed]
- Hoss, P.; Meyer, O.; Wölfle, U.C.; Wülk, A.; Meusburger, T.; Meier, L.; Hickel, R.; Gruhn, V.; Hesenius, M.; Kühnisch, J. Detection of Periodontal Bone Loss on Periapical Radiographs—A Diagnostic Study Using Different Convolutional Neural Networks. J. Clin. Med. 2023, 12, 7189. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.; Faria, M.; Giraldi, G.; Bastos, L.; Conci, A. Do radiographic assessments of periodontal bone loss improve with deep learning methods for enhanced image resolution? Sensors 2021, 21, 2013. [Google Scholar] [CrossRef] [PubMed]
- Danks, R.P.; Bano, S.; Orishko, A.; Tan, H.J.; Moreno Sancho, F.; D’Aiuto, F.; Stoyanov, D. Automating periodontal bone loss measurement via dental landmark localisation. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 1189–1199. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhou, Z.; Zhou, Z.; Wu, X.; Li, Y.; Wang, S.; Liao, W.; Ying, S.; Zhao, Z. Artificial intelligence for caries and periapical periodontitis detection. J. Dent. 2022, 122, 104107. [Google Scholar] [CrossRef]
- Li, K.-C.; Mao, Y.-C.; Lin, M.-F.; Li, Y.-Q.; Chen, C.-A.; Chen, T.-Y.; Abu, P.A.R. Detection of tooth position by YOLOv4 and various dental problems based on CNN with bitewing radiograph (July 2023). IEEE Access 2024, 12, 11822–11835. [Google Scholar] [CrossRef]
- Rajee, M.; Mythili, C. Dental image segmentation and classification using inception Resnetv2. IETE J. Res. 2023, 69, 4972–4988. [Google Scholar] [CrossRef]
- Park, S.; Erkinov, H.; Hasan, M.A.M.; Nam, S.-H.; Kim, Y.-R.; Shin, J.; Chang, W.-D. Periodontal disease classification with color teeth images using convolutional neural networks. Electronics 2023, 12, 1518. [Google Scholar] [CrossRef]
- Liu, F.; Gao, L.; Wan, J.; Lyu, Z.-L.; Huang, Y.-Y.; Liu, C.; Han, M. Recognition of digital dental X-ray images using a convolutional neural network. J. Digit. Imaging 2023, 36, 73–79. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Zhao, Y.; Zhao, J.; Wang, Y. Dental disease detection on periapical radiographs based on deep convolutional neural networks. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 649–661. [Google Scholar] [CrossRef]
- Lee, C.T.; Kabir, T.; Nelson, J.; Sheng, S.; Meng, H.W.; Van Dyke, T.E.; Walji, M.F.; Jiang, X.; Shams, S. Use of the deep learning approach to measure alveolar bone level. J. Clin. Periodontol. 2022, 49, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, D.-h.; Jeong, S.-N.; Choi, S.-H. Diagnosis and prediction of periodontally compromised teeth using a deep learning-based convolutional neural network algorithm. J. Periodontal Implant Sci. 2018, 48, 114. [Google Scholar] [CrossRef] [PubMed]
- Alalharith, D.M.; Alharthi, H.M.; Alghamdi, W.M.; Alsenbel, Y.M.; Aslam, N.; Khan, I.U.; Shahin, S.Y.; Dianišková, S.; Alhareky, M.S.; Barouch, K.K. A deep learning-based approach for the detection of early signs of gingivitis in orthodontic patients using faster region-based convolutional neural networks. Int. J. Environ. Res. Public Health 2020, 17, 8447. [Google Scholar] [CrossRef] [PubMed]
- Chau, R.C.W.; Li, G.-H.; Tew, I.M.; Thu, K.M.; McGrath, C.; Lo, W.-L.; Ling, W.-K.; Hsung, R.T.-C.; Lam, W.Y.H. Accuracy of artificial intelligence-based photographic detection of gingivitis. Int. Dent. J. 2023, 73, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, J.; Huan, Y.; Zou, Z.; Yeh, S.-C.; Zheng, L.-R. A smart dental health-IoT platform based on intelligent hardware, deep learning, and mobile terminal. IEEE J. Biomed. Health Inform. 2019, 24, 898–906. [Google Scholar] [CrossRef]
- Wang, C.; Qin, H.; Lai, G.; Zheng, G.; Xiang, H.; Wang, J.; Zhang, D. Automated classification of dual channel dental imaging of auto-fluorescence and white lightby convolutional neural networks. J. Innov. Opt. Health Sci. 2020, 13, 2050014. [Google Scholar] [CrossRef]
- Saydzai, S.; Buontempo, Z.; Patel, P.; Hasan, F.; Sun, C.; Akcalı, A.; Lin, G.H.; Donos, N.; Nibali, L. Comparison of the efficacy of periodontal prognostic systems in predicting tooth loss. J. Clin. Periodontol. 2022, 49, 740–748. [Google Scholar] [CrossRef]
- Garnick, J.J.; Silverstein, L. Periodontal probing: Probe tip diameter. J. Periodontol. 2000, 71, 96–103. [Google Scholar] [CrossRef]
- Leroy, R.; Eaton, K.A.; Savage, A. Methodological issues in epidemiological studies of periodontitis-how can it be improved? BMC Oral Health 2010, 10, 8. [Google Scholar] [CrossRef]
- Meusburger, T.; Wülk, A.; Kessler, A.; Heck, K.; Hickel, R.; Dujic, H.; Kühnisch, J. The Detection of Dental Pathologies on Periapical Radiographs—Results from a Reliability Study. J. Clin. Med. 2023, 12, 2224. [Google Scholar] [CrossRef]
- Chang, J.; Chang, M.-F.; Angelov, N.; Hsu, C.-Y.; Meng, H.-W.; Sheng, S.; Glick, A.; Chang, K.; He, Y.-R.; Lin, Y.-B. Application of deep machine learning for the radiographic diagnosis of periodontitis. Clin. Oral Investig. 2022, 26, 6629–6637. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Huang, P.; Huang, P. Automatic methods for alveolar bone loss degree measurement in periodontitis periapical radiographs. Comput. Methods Programs Biomed. 2017, 148, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Dai, F.; Zhu, H.; Yang, H.; Huang, Y.; Jiang, L.; Tang, X.; Deng, L.; Song, L. Deep learning for the early identification of periodontitis: A retrospective, multicentre study. Clin. Radiol. 2023, 78, e985–e992. [Google Scholar] [CrossRef]
- Widyaningrum, R.; Candradewi, I.; Aji, N.R.A.S.; Aulianisa, R. Comparison of Multi-Label U-Net and Mask R-CNN for panoramic radiograph segmentation to detect periodontitis. Imaging Sci. Dent. 2022, 52, 383. [Google Scholar] [CrossRef]
- Koshi, E.; Rajesh, S.; Koshi, P.; Arunima, P. Risk assessment for periodontal disease. J. Indian Soc. Periodontol. 2012, 16, 324–328. [Google Scholar] [CrossRef]
- Moosa, Y.; Bacha, S.H.; Raza, S.A.; Zia, M.H.; Fatima, A.; Shaikh, A.A. Role of Artificial Intelligence in Periodontology. Pak. J. Med. Health Sci. 2023, 17, 363. [Google Scholar] [CrossRef]
- Patel, J.S.; Patel, K.; Vo, H.; Jiannan, L.; Tellez, M.M.; Albandar, J.; Wu, H. Enhancing an AI-Empowered Periodontal CDSS and Comparing with Traditional Perio-risk Assessment Tools. AMIA Annu. Symp. Proc. 2023, 2022, 846–855. [Google Scholar]
- Yauney, G.; Rana, A.; Wong, L.C.; Javia, P.; Muftu, A.; Shah, P. Automated process incorporating machine learning segmentation and correlation of oral diseases with systemic health. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3387–3393. [Google Scholar]
- Shirmohammadi, A.; Oskouei, S.G. The growing footprint of artificial intelligence in periodontology & implant dentistry. J. Adv. Periodontol. Implant Dent. 2023, 15, 1–2. [Google Scholar]
- Miller, A.; Huang, C.; Brody, E.; Siqueira, R. Artificial intelligence applications for the radiographic detection o f periodontal disease: A scoping review. J. Califor. Dent. Asso. 2023, 51, 1. [Google Scholar]
- Li, X.; Zhao, D.; Xie, J.; Wen, H.; Liu, C.; Li, Y.; Li, W.; Wang, S. Deep learning for classifying the stages of periodontitis on dental images: A systematic review and meta-analysis. BMC Oral Health 2023, 23, 1017. [Google Scholar] [CrossRef]
- Khan, A.; Khan, K.J.; Ghaza, M.A.; Dave, T.; Shahnoor, S.; Khan, A.M.; Oduoye, M.O.; Nafula, W.P.; Ubechu, S.C. Celebrating breakthrough in dental diagnostics: FDA approval of an AI model for diagnosis of periodontal diseases: A correspondence. Health Sci. Rep. 2023, 6, e1573. [Google Scholar] [CrossRef] [PubMed]
- Abdul, N.S.; AlGhannam, S.M.; Almughaiseeb, A.A.; Bindawoad, F.A.; Shenoy, M. A review on salivary constituents and their role in diagnostics. Bioinformation 2022, 18, 1021. [Google Scholar]
- Sumbayak, I.A.; Masulili, S.L.C.; Tadjoedin, F.M.; Sulijaya, B.; Mutiara, A.; Khoirowati, D.; Soeroso, Y.; Bachtiar, B.M. Changes in interleukin-1β, tumor necrosis factor-α, and interleukin-10 cytokines in older people with periodontitis. Geriatrics 2023, 8, 79. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Jayaprakash, D.; Aghanashini, S.; Vijayendra, R.R.; Chatterjee, A.; Rosh, R.M.; Bharwani, A. Effect of periodontal therapy on C-reactive protein levels in gingival crevicular fluid of patients with gingivitis and chronic periodontitis: A clinical and biochemical study. J. Indian Soc. Periodontol. 2014, 18, 456–460. [Google Scholar] [CrossRef]
- Barros, S.P.; Williams, R.; Offenbacher, S.; Morelli, T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol. 2000 2016, 70, 53–64. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Taba, M.; Kinney, J.; Kim, A.S.; Giannobile, W.V. Diagnostic biomarkers for oral and periodontal diseases. Dent. Clin. 2005, 49, 551–571. [Google Scholar] [CrossRef]
- Tanwar, H.; Gnanasekaran, J.M.; Allison, D.; Chuang, L.-s.; He, X.; Aimetti, M.; Baima, G.; Costalonga, M.; Cross, R.K.; Sears, C. Unraveling the link between periodontitis and inflammatory bowel disease: Challenges and outlook. arXiv 2023, arXiv:2308.10907v1. [Google Scholar]
- Şenel, S. An overview of physical, microbiological and immune barriers of oral mucosa. Int. J. Mol. Sci. 2021, 22, 7821. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, D.W.; Donowitz, G.R.; Mandell, G.L. Polymorphonuclear neutrophils: An effective antimicrobial force. Rev. Infect. Dis. 1989, 11, S1532–S1544. [Google Scholar] [CrossRef] [PubMed]
- Cruchley, A.T.; Bergmeier, L.A. Structure and functions of the oral mucosa. In Oral Mucosa in Health and Disease: A Concise Handbook; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–18. [Google Scholar]
- Min, B.-M. Oral Mucosa and Gingiva. In Oral Biochemistry; Springer: Berlin/Heidelberg, Germany, 2023; pp. 87–97. [Google Scholar]
- Lehman, H.K.; Segal, B.H. The role of neutrophils in host defense and disease. J. Allergy Clin. Immunol. 2020, 145, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018, 9, 324475. [Google Scholar] [CrossRef]
- Silva, L.M.; Doyle, A.D.; Greenwell-Wild, T.; Dutzan, N.; Tran, C.L.; Abusleme, L.; Juang, L.J.; Leung, J.; Chun, E.M.; Lum, A.G. Fibrin is a critical regulator of neutrophil effector function at the oral mucosal barrier. Science 2021, 374, eabl5450. [Google Scholar] [CrossRef]
- Rijkschroeff, P.; Loos, B.G.; Nicu, E.A. Oral polymorphonuclear neutrophil contributes to oral health. Curr. Oral Health Rep. 2018, 5, 211–220. [Google Scholar] [CrossRef]
- Nicu, E.A.; Rijkschroeff, P.; Wartewig, E.; Nazmi, K.; Loos, B.G. Characterization of oral polymorphonuclear neutrophils in periodontitis patients: A case-control study. BMC Oral Health 2018, 18, 149. [Google Scholar] [CrossRef]
- Nanci, A.; Bosshardt, D.D. Structure of periodontal tissues in health and disease. Periodontol. 2000 2006, 40, 11. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernandez, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.; Wei, L.; Thornton-Evans, G.; Genco, R. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Polymicrobial communities in periodontal disease: Their quasi-organismal nature and dialogue with the host. Periodontol. 2000 2021, 86, 210–230. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C.; Kornman, K.S. The pathogenesis of human periodontitis: An introduction. Periodontol. 2000 1997, 14, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, J. Neutrophil subsets in periodontal health and disease: A mini review. Front. Immunol. 2020, 10, 423235. [Google Scholar] [CrossRef]
- Marsh, P.D.; Devine, D.A. How is the development of dental biofilms influenced by the host? J. Clin. Periodontol. 2011, 38, 28–35. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Nishihara, T.; Koseki, T. Microbial etiology of periodontitis. Periodontol. 2000 2004, 36, 14–26. [Google Scholar] [CrossRef]
- Khoury, W.; Glogauer, J.; Tenenbaum, H.C.; Glogauer, M. Oral inflammatory load: Neutrophils as oral health biomarkers. J. Periodontal Res. 2020, 55, 594–601. [Google Scholar] [CrossRef]
- Elebyary, O.; Sun, C.; Batistella, E.A.; Van Dyke, T.E.; Low, S.B.; Singhal, S.; Tenenbaum, H.; Glogauer, M. Utilizing Oral Neutrophil Counts as an Indicator of Oral Inflammation Associated With Periodontal Disease: A Blinded Multicentre Study. J. Clin. Periodontol. 2024. [Google Scholar] [CrossRef]
- Cilloni, D.; Petiti, J.; Campia, V.; Podestà, M.; Squillario, M.; Montserrat, N.; Bertaina, A.; Sabatini, F.; Carturan, S.; Berger, M. Transplantation induces profound changes in the transcriptional asset of hematopoietic stem cells: Identification of specific signatures using machine learning techniques. J. Clin. Med. 2020, 9, 1670. [Google Scholar] [CrossRef]
- Solves, P.; Sanz, J.; Gómez, I.; de la Puerta, R.; Arnao, M.; Montoro, J.; Piñana, J.L.; Carretero, C.; Balaguer, A.; Guerreiro, M. Comparison of transfusion requirements in adult patients undergoing Haploidentical or single-unit umbilical cord blood stem cell transplantation. Eur. J. Haematol. 2019, 103, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Manabe, N.; Kawaguchi, H.; Chikuda, H.; Miyaura, C.; Inada, M.; Nagai, R.; Nabeshima, Y.-i.; Nakamura, K.; Sinclair, A.M.; Scheuermann, R.H. Connection between B lymphocyte and osteoclast differentiation pathways. J. Immunol. 2001, 167, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Ghyselen, J.; Koninckx, P.; van Steenberghe, D. Long-term bone mass evaluation of mandible and lumbar spine in a group of women receiving hormone replacement therapy. Eur. J. Oral Sci. 1996, 104, 10–16. [Google Scholar] [CrossRef]
- Tezal, M.; Wactawski-Wende, J.; Grossi, S.G.; Ho, A.W.; Dunford, R.; Genco, R.J. The relationship between bone mineral density and periodontitis in postmenopausal women. J. Periodontol. 2000, 71, 1492–1498. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okubo, M.; Furuta, Y.; Tokida, M.; Ichikawa, K.; Ohsaka, A. Impact of CD34+ pre-counting and plerixafor on autologous peripheral blood stem cell collection in Japanese university hospitals in eight years. Transfus. Apher. Sci. 2019, 58, 102664. [Google Scholar] [CrossRef]
- Yanamandra, U.; Deo, P.; Sahu, K.K.; Nampoothiri, R.V.; Gupta, N.; Prabhakaran, A.; Dhibhar, D.P.; Khadwal, A.; Prakash, G.; Sachdeva, M.U.S. Clinicopathological profile of myelomatous pleural effusion: Single-center real-world experience and review of literature. Clin. Lymphoma Myeloma Leuk. 2019, 19, 183–189.e1. [Google Scholar] [CrossRef]
- Mawardi, H.; Hashmi, S.K.; Elad, S.; Aljurf, M.; Treister, N. Chronic graft-versus-host disease: Current management paradigm and future perspectives. Oral Dis. 2019, 25, 931–948. [Google Scholar] [CrossRef]
- Inagaki, K.; Kurosu, Y.; Yoshinari, N.; Noguchi, T.; Krall, E.; Garcia, R. Efficacy of periodontal disease and tooth loss to screen for low bone mineral density in Japanese women. Calcif. Tissue Int. 2005, 77, 9–14. [Google Scholar] [CrossRef]
- Alfraih, F.; Alawwami, M.; Aljurf, M.; Alhumaidan, H.; Alsaedi, H.; El Fakih, R.; Alotaibi, B.; Rasheed, W.; Bernas, S.N.; Massalski, C. High-resolution HLA allele and haplotype frequencies of the Saudi Arabian population based on 45,457 individuals and corresponding stem cell donor matching probabilities. Hum. Immunol. 2021, 82, 97–102. [Google Scholar] [CrossRef]
- Chanprapaph, K.; Leerunyakul, K.; Niparuck, P.; Rutnin, S. A clinical and histological comparison between acute cutaneous graft-versus-host disease and other maculopapular eruptions following hematopoietic stem cell transplantation: A retrospective cohort. Int. J. Dermatol. 2021, 60, 60–69. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Sakellari, I.; Anyfanti, P.; Batsis, I.; Vardi, A.; Bousiou, Z.; Lazaridis, A.; Nikolaidou, B.; Zarifis, I.; Masmanidou, M. Assessment of endothelial injury and pro-coagulant activity using circulating microvesicles in survivors of allogeneic hematopoietic cell transplantation. Int. J. Mol. Sci. 2020, 21, 9768. [Google Scholar] [CrossRef] [PubMed]
- Cheretakis, C.; Dror, Y.; Glogauer, M. A noninvasive oral rinse assay to monitor engraftment, neutrophil tissue delivery and susceptibility to infection following HSCT in pediatric patients. Bone Marrow Transpl. 2005, 36, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.-J.; Zhao, X.-Y.; Yu, X.-X.; Lv, M.; Han, T.-T.; Han, W.; Huang, X.-J. Quantity and quality reconstitution of NKG2A+ natural killer cells are associated with graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transpl. 2019, 25, 1–11. [Google Scholar] [CrossRef]
- George, J.; Rapsomaniki, E.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; Herrett, E.; Smeeth, L.; Timmis, A.; Hemingway, H. How does cardiovascular disease first present in women and men? Incidence of 12 cardiovascular diseases in a contemporary cohort of 1,937,360 people. Circulation 2015, 132, 1320–1328. [Google Scholar] [CrossRef]
- Rindom Schiött, C.; Löe, H. The origin and variation in number of leukocytes in the human saliva. J. Periodontal Res. 1970, 5, 36–41. [Google Scholar] [CrossRef]
- Raaeste, A.-M.; Tapanila, T.; Tupakka, R. Leukocyte migration into the healthy dentulous mouth. J. Periodontal Res. 1977, 12, 444–449. [Google Scholar] [CrossRef]
- Raeste, A.M.; Aura, A. Rate of migration of oral leukocytes in patients with periodontitis. Eur. J. Oral Sci. 1978, 86, 43–51. [Google Scholar] [CrossRef]
- Bender, J.; Thang, H.; Glogauer, M. Novel rinse assay for the quantification of oral neutrophils and the monitoring of chronic periodontal disease. J. Periodontal Res. 2006, 41, 214–220. [Google Scholar] [CrossRef]
- Hong, K.-Y.; Ghafari, A.; Mei, Y.; Williams, J.S.; Attia, D.; Forsyth, J.; Wang, K.; Wyeld, T.; Sun, C.; Glogauer, M. Oral inflammatory load predicts vascular function in a young adult population: A pilot study. Front. Oral Health 2023, 4, 1233881. [Google Scholar] [CrossRef]
- Theda, C.; Hwang, S.H.; Czajko, A.; Loke, Y.J.; Leong, P.; Craig, J.M. Quantitation of the cellular content of saliva and buccal swab samples. Sci. Rep. 2018, 8, 6944. [Google Scholar] [CrossRef]
- Domnich, M.; Riedesel, J.; Pylaeva, E.; Kürten, C.H.; Buer, J.; Lang, S.; Jablonska, J. Oral neutrophils: Underestimated players in oral cancer. Front. Immunol. 2020, 11, 565683. [Google Scholar] [CrossRef] [PubMed]
- Marini, O.; Costa, S.; Bevilacqua, D.; Calzetti, F.; Tamassia, N.; Spina, C.; De Sabata, D.; Tinazzi, E.; Lunardi, C.; Scupoli, M.T. Mature CD10+ and immature CD10− neutrophils present in G-CSF–treated donors display opposite effects on T cells. Blood J. Am. Soc. Hematol. 2017, 129, 1343–1356. [Google Scholar] [CrossRef]
- Sack Jr, G.H. Serum amyloid A–a review. Mol. Med. 2018, 24, 46. [Google Scholar] [CrossRef]
- Prinyakupt, J.; Pluempitiwiriyawej, C. Segmentation of white blood cells and comparison of cell morphology by linear and naïve Bayes classifiers. Biomed. Eng. Online 2015, 14, 63. [Google Scholar] [CrossRef]
- Nassar, M.; Doan, M.; Filby, A.; Wolkenhauer, O.; Fogg, D.K.; Piasecka, J.; Thornton, C.A.; Carpenter, A.E.; Summers, H.D.; Rees, P. Label-free identification of white blood cells using machine learning. Cytom. Part A 2019, 95, 836–842. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef]
- Lopez-Puigdollers, D.; Traver, V.J.; Pla, F. Recognizing white blood cells with local image descriptors. Expert Syst. Appl. 2019, 115, 695–708. [Google Scholar] [CrossRef]
- Chen, L.; Bao, J.; Huang, Q.; Sun, H. A robust and automated cell counting method in quantification of digital breast cancer immunohistochemistry images. Pol. J. Pathol. 2019, 70, 162–173. [Google Scholar] [CrossRef]
- Abdeldaim, A.M.; Sahlol, A.T.; Elhoseny, M.; Hassanien, A.E. Computer-aided acute lymphoblastic leukemia diagnosis system based on image analysis. Adv. Soft Comput. Mach. Learn. Image Process. 2018, 131–147. [Google Scholar]
- Mirmohammadi, P.; Rasooli, A.; Ashtiyani, M.; Amin, M.M.; Deevband, M.R. Automatic recognition of acute lymphoblastic leukemia using multi-SVM classifier. Curr. Sci. 2018, 115, 1512–1518. [Google Scholar] [CrossRef]
- Hegde, R.B.; Prasad, K.; Hebbar, H.; Singh, B.M.K. Comparison of traditional image processing and deep learning approaches for classification of white blood cells in peripheral blood smear images. Biocybern. Biomed. Eng. 2019, 39, 382–392. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Adv. Neural Inf. Process. Syst. 2012, 60, 25. [Google Scholar] [CrossRef]
- Redmon, J.; Divvala, S.; Girshick, R.; Farhadi, A. You only look once: Unified, real-time object detection. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 779–788. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the inception architecture for computer vision. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 2818–2826. [Google Scholar]
- Sahlol, A.T.; Kollmannsberger, P.; Ewees, A.A. Efficient classification of white blood cell leukemia with improved swarm optimization of deep features. Sci. Rep. 2020, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Yang, F.; Poostchi, M.; Yu, H.; Zhou, Z.; Silamut, K.; Yu, J.; Maude, R.J.; Jaeger, S.; Antani, S. Deep learning for smartphone-based malaria parasite detection in thick blood smears. IEEE J. Biomed. Health Inform. 2019, 24, 1427–1438. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015: 18th International Conference, Munich, Germany, 5–9 October 2015; part III 18. pp. 234–241. [Google Scholar]
- Li, H.; Zhao, X.; Su, A.; Zhang, H.; Liu, J.; Gu, G. Color space transformation and multi-class weighted loss for adhesive white blood cell segmentation. IEEE Access 2020, 8, 24808–24818. [Google Scholar] [CrossRef]
- Long, F. Microscopy cell nuclei segmentation with enhanced U-Net. BMC Bioinform. 2020, 21, 8. [Google Scholar] [CrossRef]
- Zhou, Z.; Siddiquee, M.M.R.; Tajbakhsh, N.; Liang, J. Unet++: Redesigning skip connections to exploit multiscale features in image segmentation. IEEE Trans. Med. Imaging 2019, 39, 1856–1867. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, F.; Xi, L.; Li, Z.; Liu, G.; Xu, Y. LeukocyteMask: An automated localization and segmentation method for leukocyte in blood smear images using deep neural networks. J. Biophotonics 2019, 12, e201800488. [Google Scholar] [CrossRef]
- Tomari, R.; Zakaria, W.N.W.; Jamil, M.M.A.; Nor, F.M.; Fuad, N.F.N. Computer aided system for red blood cell classification in blood smear image. Procedia Comput. Sci. 2014, 42, 206–213. [Google Scholar] [CrossRef]
- Drałus, G.; Mazur, D.; Czmil, A. Automatic detection and counting of blood cells in smear images using retinanet. Entropy 2021, 23, 1522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Tsai, J.-T.; Ho, W.-H. Automatic identifying and counting blood cells in smear images by using single shot detector and Taguchi method. BMC Bioinform. 2021, 22, 635. [Google Scholar] [CrossRef]
- Anand, V.; Gupta, S.; Koundal, D.; Alghamdi, W.Y.; Alsharbi, B.M. Deep learning-based image annotation for leukocyte segmentation and classification of blood cell morphology. BMC Med. Imaging 2024, 24, 83. [Google Scholar] [CrossRef]
- Di Ruberto, C.; Loddo, A.; Puglisi, G. Blob detection and deep learning for leukemic blood image analysis. Appl. Sci. 2020, 10, 1176. [Google Scholar] [CrossRef]
- Wang, D.; Hwang, M.; Jiang, W.-C.; Ding, K.; Chang, H.C.; Hwang, K.-S. A deep learning method for counting white blood cells in bone marrow images. BMC Bioinform. 2021, 22, 94. [Google Scholar] [CrossRef]
- Rivenson, Y.; Ceylan Koydemir, H.; Wang, H.; Wei, Z.; Ren, Z.; Gunaydın, H.; Zhang, Y.; Gorocs, Z.; Liang, K.; Tseng, D. Deep learning enhanced mobile-phone microscopy. Acs Photonics 2018, 5, 2354–2364. [Google Scholar] [CrossRef]
- Kumar, P.; Vasuki, S. Automated diagnosis of acute lymphocytic leukemia and acute myeloid leukemia using multi-SV. J. Biomed. Imaging Bioeng. 2017, 1, 20–24. [Google Scholar]
- Wu, J.; Zheng, X.; Liu, D.; Ai, L.; Tang, P.; Wang, B.; Wang, Y. WBC image segmentation based on residual networks and attentional mechanisms. Comput. Intell. Neurosci. 2022, 2022, 1610658. [Google Scholar] [CrossRef]
- Zhong, Y.; Dan, Y.; Cai, Y.; Lin, J.; Huang, X.; Mahmoud, O.; Hald, E.S.; Kumar, A.; Fang, Q.; Mahmoud, S.S. Efficient Malaria Parasite Detection From Diverse Images of Thick Blood Smears for Cross-Regional Model Accuracy. IEEE Open J. Eng. Med. Biol. 2023, 4, 226–233. [Google Scholar] [CrossRef]
- Olayah, F.; Senan, E.M.; Ahmed, I.A.; Awaji, B. Blood slide image analysis to classify WBC types for prediction haematology based on a hybrid model of CNN and handcrafted features. Diagnostics 2023, 13, 1899. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, J.; Li, J.; Li, H.; Wang, L. WBC-AMNet: Automatic classification of WBC images using deep feature fusion network based on focalized attention mechanism. PLoS ONE 2022, 17, e0261848. [Google Scholar] [CrossRef] [PubMed]
- Krishna Prasad, P.; Reddy, E.S.; Chandra Sekharaiah, K. An intelligent white blood cell detection and multi-class classification using fine optimal DCRNet. Multimed. Tools Appl. 2024, 83, 75825–75853. [Google Scholar] [CrossRef]
- Prasad, P.K.; Reddy, E.S.; Sekharaiah, K.C. Deep U_ClusterNet: Automatic deep clustering based segmentation and robust cell size determination in white blood cell. Multimed. Tools Appl. 2024, 83, 25923–25949. [Google Scholar] [CrossRef]
- Batool, A.; Byun, Y.-C. Lightweight EfficientNetB3 model based on depthwise separable convolutions for enhancing classification of leukemia white blood cell images. IEEE Access 2023, 11, 37203–37215. [Google Scholar] [CrossRef]
- Katar, O.; Yildirim, O. An explainable vision transformer model based white blood cells classification and localization. Diagnostics 2023, 13, 2459. [Google Scholar] [CrossRef]
- Khan, S.; Sajjad, M.; Abbas, N.; Escorcia-Gutierrez, J.; Gamarra, M.; Muhammad, K. Efficient leukocytes detection and classification in microscopic blood images using convolutional neural network coupled with a dual attention network. Comput. Biol. Med. 2024, 174, 108146. [Google Scholar] [CrossRef]
- Bairaboina, S.S.R.; Battula, S.R. Ghost-resNeXt: An effective deep learning based on mature and immature WBC classification. Appl. Sci. 2023, 13, 4054. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, X.; Fan, H.; Lai, T.; Li, Z. WBC-Net: A white blood cell segmentation network based on UNet++ and ResNet. Appl. Soft Comput. 2021, 101, 107006. [Google Scholar] [CrossRef]
- Haider, A.; Arsalan, M.; Lee, Y.W.; Park, K.R. Deep features aggregation-based joint segmentation of cytoplasm and nuclei in white blood cells. IEEE J. Biomed. Health Inform. 2022, 26, 3685–3696. [Google Scholar] [CrossRef]
- Surdilovic, D.; Ille, T.; D’Souza, J. Artificial intelligence and dental practice management. Eur. J. Artif. Intell. Mach. Learn. 2022, 1, 11–14. [Google Scholar] [CrossRef]


| Classification | Mild Periodontitis | Moderate Periodontitis | Severe Periodontitis | Chronic Periodontitis | Aggressive Periodontitis |
|---|---|---|---|---|---|
| Clinical Attachment Loss (CAL) | 1–2 mm | 3–4 mm | ≥5 mm | Varies (based on mild, moderate, or severe criteria) | Rapid attachment loss and bone destruction |
| Probing Depths (PD) | 3–4 mm | 5–6 mm | ≥7 mm | Varies | Probing depths often deep (≥6 mm) |
| Radiographic Bone Loss (RBL) | <15% bone loss (coronal third) | 15–33% bone loss | >33% bone loss | Bone loss correlating to clinical stage | Vertical bone loss often seen, especially in younger individuals |
| Bleeding on Probing | Present | Present | Present | Present, but may vary | Usually present, can be more pronounced |
| Tooth Mobility | Minimal or none | Possible slight mobility | Moderate to severe mobility | May be present in later stages | Frequent due to rapid bone loss |
| Furcation Involvement | None or minimal | May involve early furcation | Significant furcation involvement | May or may not be present, depending on severity | Frequent in advanced cases |
| Tooth Loss due to Periodontitis | None | Rare or few | Potential for tooth loss | Tooth loss can occur in severe stages | Early tooth loss may occur |
| Classification | Stage I | Stage II | Stage III | Stage IV | Grade A | Grade B | Grade C |
|---|---|---|---|---|---|---|---|
| Stage/Grade Focus | Initial Periodontitis | Moderate Periodontitis | Severe Periodontitis (with potential tooth loss) | Severe Periodontitis (with complex rehabilitation needed) | Slow rate of progression | Moderate rate of progression | Rapid rate of progression |
| CAL | 1–2 mm | 3–4 mm | ≥5 mm | ≥5 mm | - | - | - |
| PD | ≤4 mm | ≤5 mm | ≥6 mm | ≥6 mm | - | - | - |
| Tooth Loss due to Periodontitis | No tooth loss | No tooth loss | ≤4 teeth | ≥5 teeth | - | - | - |
| RBL | Coronal third (<15%) | Coronal third (15–33%) | Extending to mid-third of root and beyond | Extending to mid-third of root and beyond | - | - | - |
| Bone Destruction Pattern | Horizontal | Horizontal | Vertical > 3 mm | Vertical > 3 mm | - | - | - |
| Furcation Involvement | None | None | Possible | Likely | - | - | - |
| Rate of Bone Loss | - | - | - | - | No additional bone loss over 5 years | <2 mm bone loss over 5 years | ≥2 mm bone loss over 5 years |
| Metric | Description | Formulation |
| Accuracy (Acc) | Measures the overall correctness of the model’s predictions | (TP + TN)/(TP + TN + FP + FN) |
| Precision | Proportion of true positives among positive predictions | TP/(TP + FP) |
| Sensitivity (Sens) | Proportion of true negatives correctly identified | TP/(TP + FN) |
| Specificity (Spec) | Proportion of true negatives correctly identified | TN/(TN + FP) |
| F1 Score | Harmonic mean of precision and recall | 2 * (Precision * Recall)/(Precision + Recall) |
| Area Under ROC Curve (AUC-ROC) | Measures the model’s ability to rank predicted probabilities | ROC curve represents the TPR plotted against the FPR |
| Intersection over Union (IoU) | Measure the accuracy of an object detector on a particular dataset. | Area of overlap/Area of union |
| Mean Absolute Error (MAE) | Average absolute difference between predicted and actual | (1/N) * Σ |
| Mean Squared Error (MSE) | Average squared difference between predicted and actual | (1/N) * Σ (y − ˆy) ^2 |
| Root Mean Squared Error (RMSE) | Square root of the MSE | √ (1/N) * Σ (y − ˆy) ^2 |
| R-squared | Proportion of the variance in the dependent variable | 1 − (SSE/SST) |
| Confusion Matrix | Summarizes the performance of a classification algorithm | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soheili, F.; Delfan, N.; Masoudifar, N.; Ebrahimni, S.; Moshiri, B.; Glogauer, M.; Ghafar-Zadeh, E. Toward Digital Periodontal Health: Recent Advances and Future Perspectives. Bioengineering 2024, 11, 937. https://doi.org/10.3390/bioengineering11090937
Soheili F, Delfan N, Masoudifar N, Ebrahimni S, Moshiri B, Glogauer M, Ghafar-Zadeh E. Toward Digital Periodontal Health: Recent Advances and Future Perspectives. Bioengineering. 2024; 11(9):937. https://doi.org/10.3390/bioengineering11090937
Chicago/Turabian StyleSoheili, Fatemeh, Niloufar Delfan, Negin Masoudifar, Shahin Ebrahimni, Behzad Moshiri, Michael Glogauer, and Ebrahim Ghafar-Zadeh. 2024. "Toward Digital Periodontal Health: Recent Advances and Future Perspectives" Bioengineering 11, no. 9: 937. https://doi.org/10.3390/bioengineering11090937
APA StyleSoheili, F., Delfan, N., Masoudifar, N., Ebrahimni, S., Moshiri, B., Glogauer, M., & Ghafar-Zadeh, E. (2024). Toward Digital Periodontal Health: Recent Advances and Future Perspectives. Bioengineering, 11(9), 937. https://doi.org/10.3390/bioengineering11090937










