Pretreatment of Vine Shoot Biomass by Choline Chloride-Based Deep Eutectic Solvents to Promote Biomass Fractionation and Enhance Sugar Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass
2.2. Materials and Chemicals
2.3. DESs Preparation
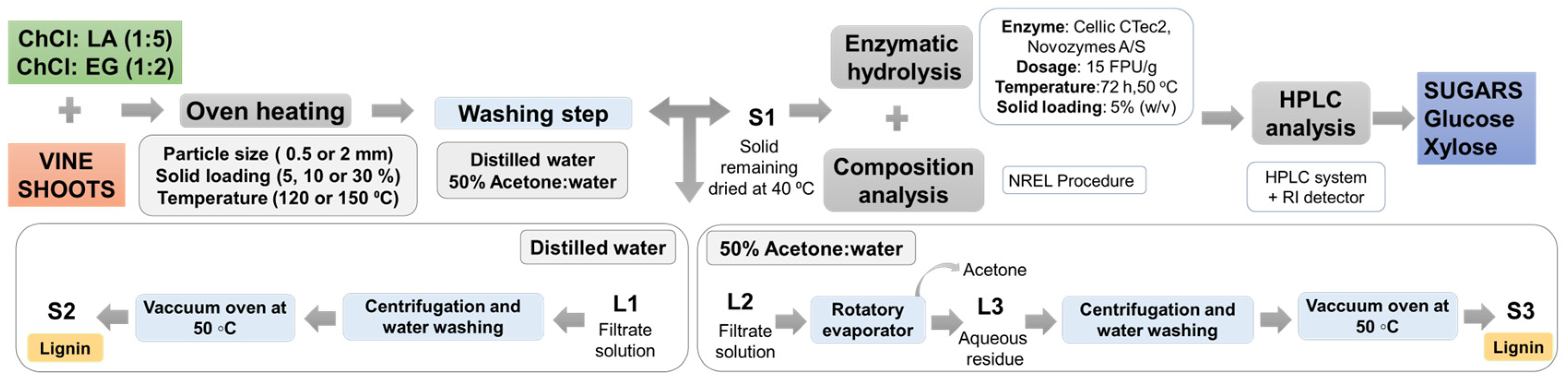
2.4. Solvent Pretreatment
Testing of an Alternative Washing Methodology
2.5. Compositional Analysis of Samples
2.6. Enzymatic Hydrolysis
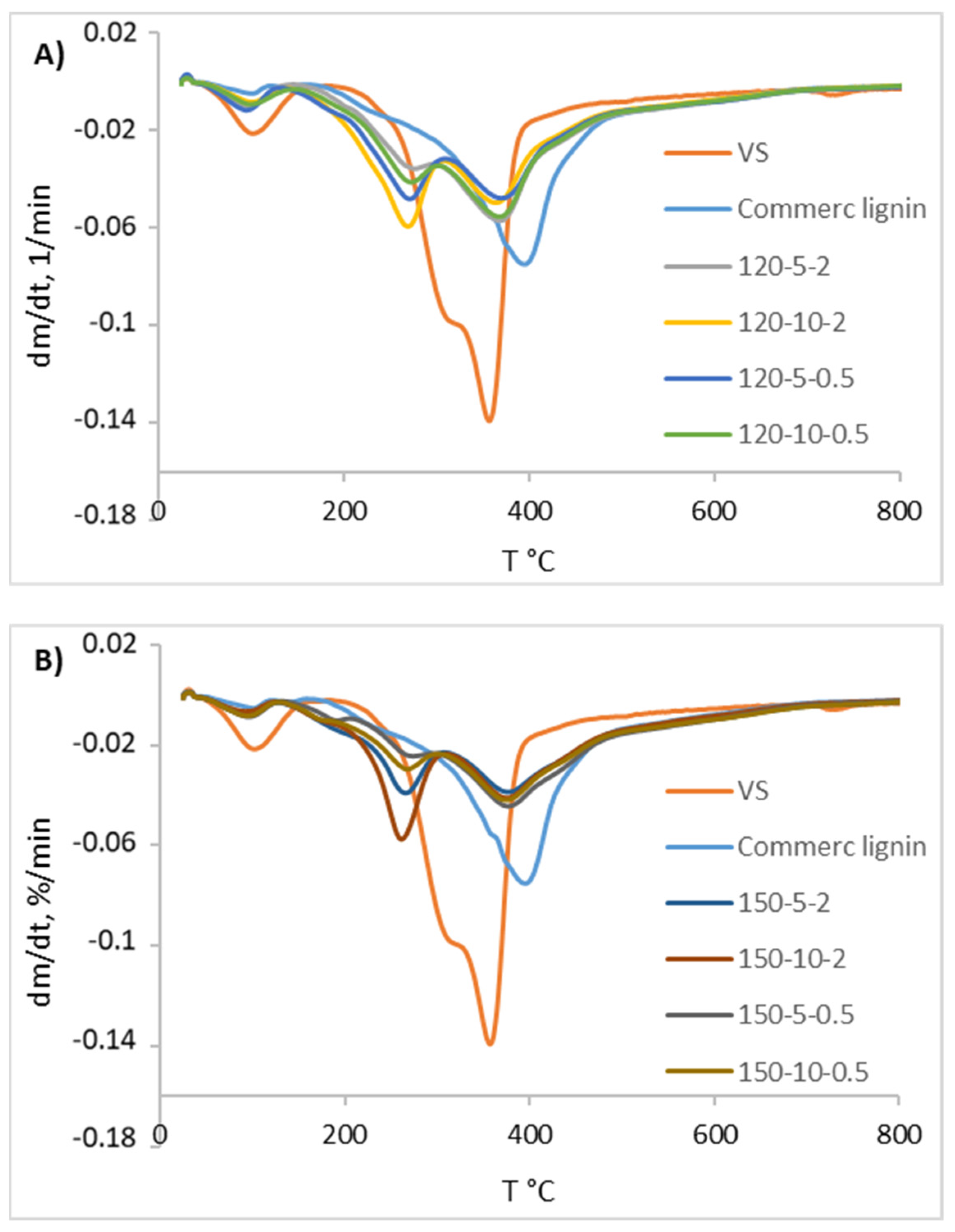
2.7. Thermogravimetric Analysis
3. Results and Discussion
3.1. Chemical Composition of VS Biomass
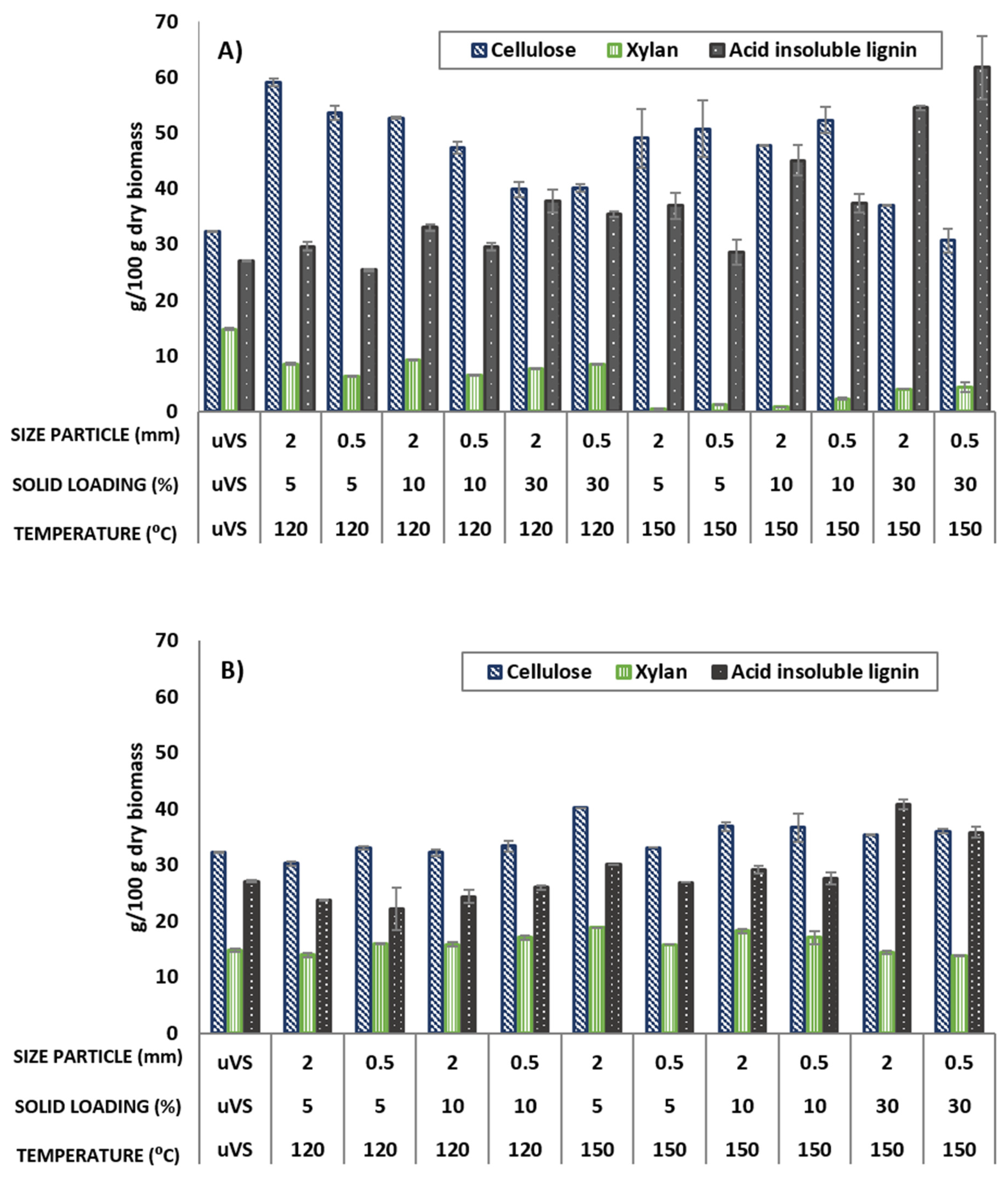
3.2. Effect of DES Pretreatment Conditions on Chemical Composition of VS Biomass
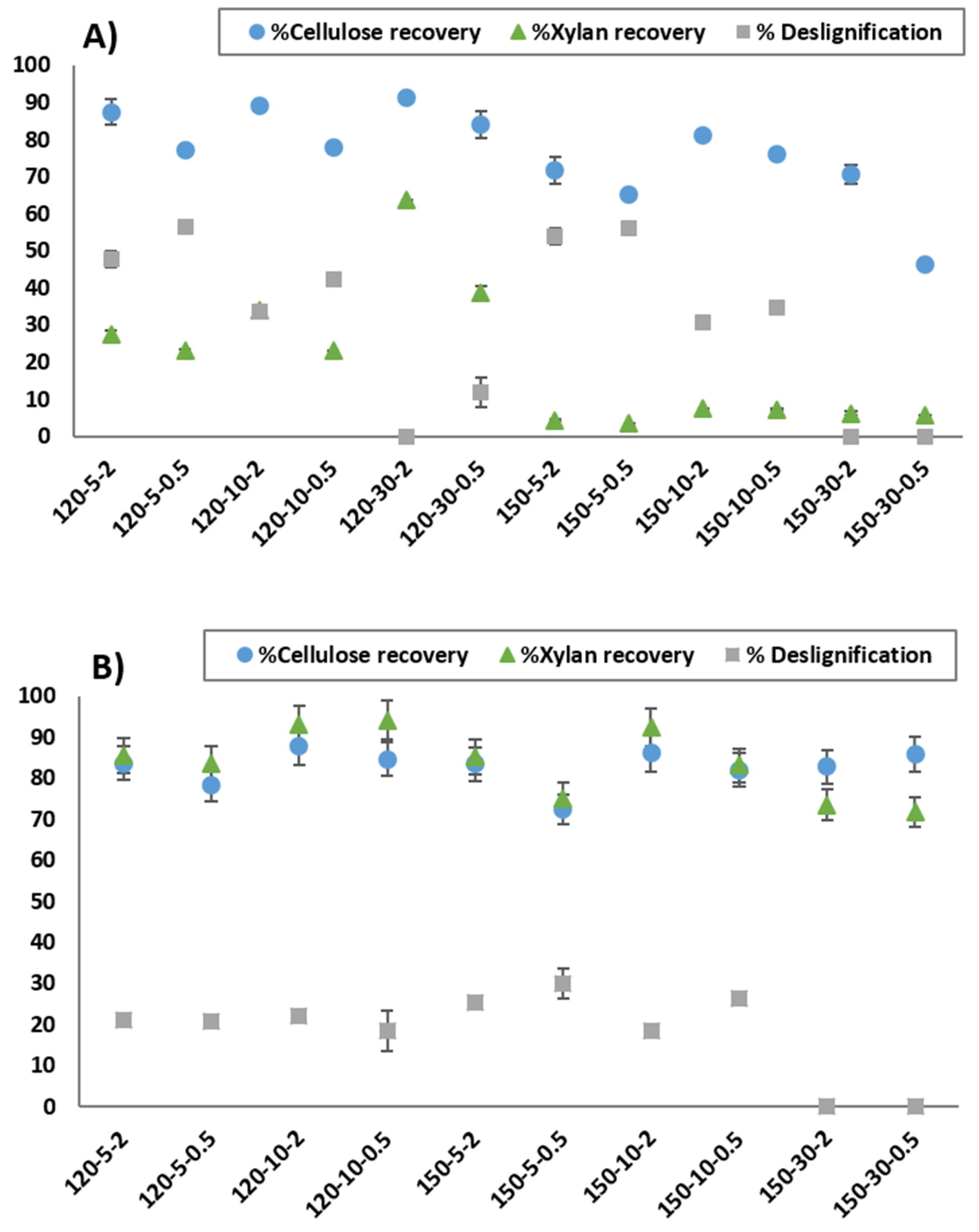
3.3. Effect of DES Pretreatment Conditions on Biomass Fractionation
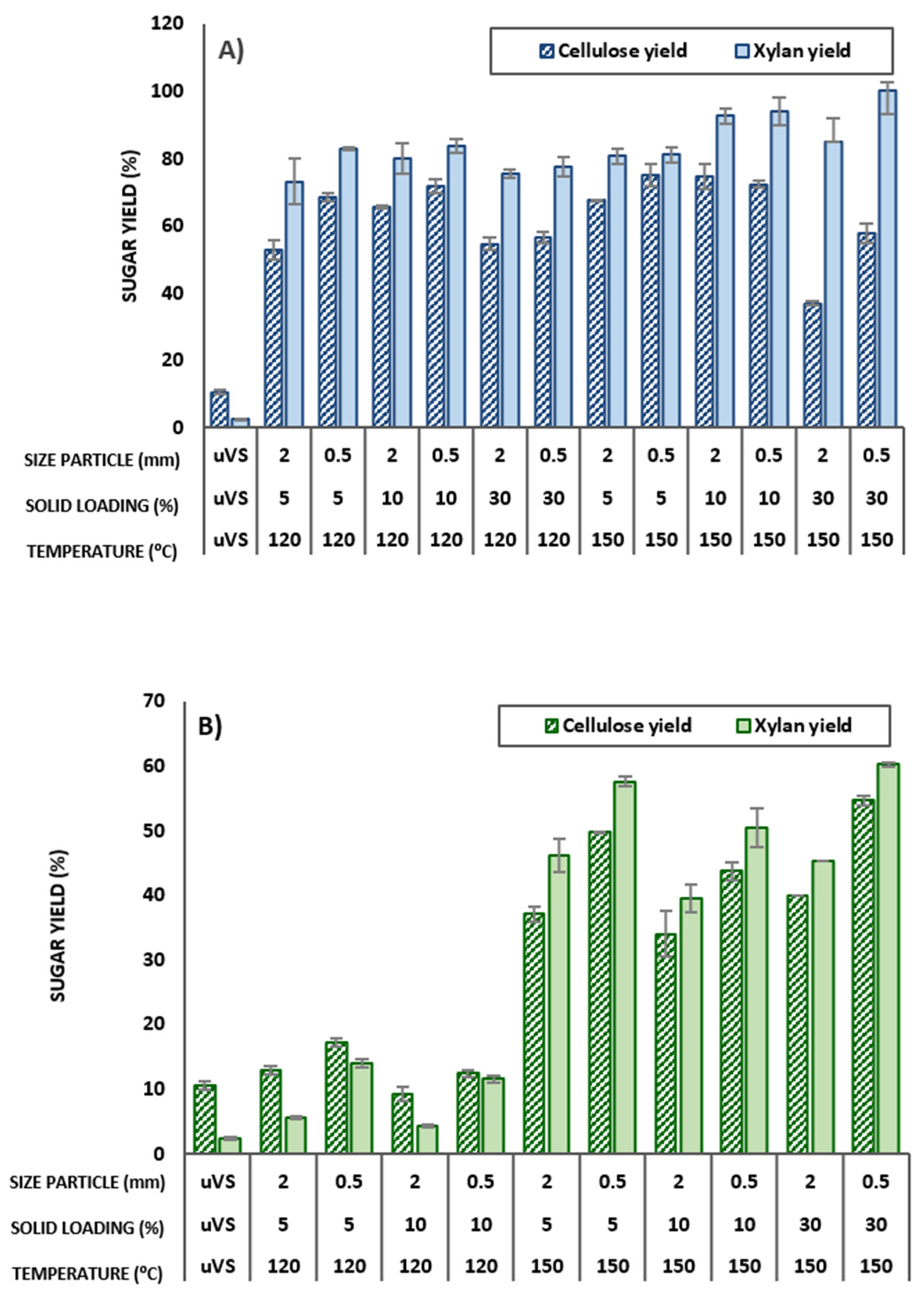
3.4. Sugar Production by Enzymatic Hydrolysis of VS after DES Pretreatment
3.5. Effect of the Washing Agent
3.6. Characterization of Recovered Lignin-Rich Solid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hingsamer, M.; Jungmeier, G. The Role of Bioenergy in the Emerging Bioeconomy. In Biorefineries; Lago, C., Caldés, N., Lechón, Y., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 179–222. [Google Scholar]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An Overview of Biorefinery-Derived Platform Chemicals from a Cellulose and Hemicellulose Biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.D.; Patel, A.K.; Puri, M. Global Status of Lignocellulosic Biorefinery: Challenges and Perspectives. Bioresour. Technol. 2022, 344, 126415. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Eibes, G.; Dávila, I.; Vila, C.; Labidi, J.; Gullón, P. Valorization of Vine Shoots Based on the Autohydrolysis Fractionation Optimized by a Kinetic Approach. Ind. Eng. Chem. Res. 2017, 56, 14164–14171. [Google Scholar] [CrossRef]
- Senila, L.; Tenu, I.; Carlescu, P.; Corduneanu, O.R.; Dumitrachi, E.P.; Kovacs, E.; Scurtu, D.A.; Cadar, O.; Becze, A.; Senila, M.; et al. Sustainable Biomass Pellets Production Using Vineyard Wastes. Agriculture 2020, 10, 501. [Google Scholar] [CrossRef]
- García-Galindo, D.; Dyjakon, A.; Villa-Ceballos, F.C. Building Variable Productivity Ratios for Improving Large Scale Spatially Explicit Pruning Biomass Assessments. Energies 2019, 12, 957. [Google Scholar] [CrossRef]
- Yiin, C.L.; Yap, K.L.; Ku, A.Z.E.; Chin, B.L.F.; Lock, S.S.M.; Cheah, K.W.; Loy, A.C.M.; Chan, Y.H. Recent Advances in Green Solvents for Lignocellulosic Biomass Pretreatment: Potential of Choline Chloride (ChCl) Based Solvents. Bioresour. Technol. 2021, 333, 125195. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; Van Den Bruinhorst, A.; Kroon, M.C. New Natural and Renewable Low Transition Temperature Mixtures (LTTMs): Screening as Solvents for Lignocellulosic Biomass Processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Dong, Y.Y.; Wang, D.; Deng, J.; Shi, Z.; Yang, J.; Yang, H. Pretreatment of Bamboo with Choline Chloride-Lactic Acid Integrated with Calcium Chloride Hydrates Deep Eutectic Solvent to Boost Bioconversion for Ethanol Production. Ind. Crops Prod. 2023, 200, 116879. [Google Scholar] [CrossRef]
- Shen, X.J.; Wen, J.L.; Mei, Q.Q.; Chen, X.; Sun, D.; Yuan, T.Q.; Sun, R.C. Facile Fractionation of Lignocelluloses by Biomass-Derived Deep Eutectic Solvent (DES) Pretreatment for Cellulose Enzymatic Hydrolysis and Lignin Valorization. Green Chem. 2019, 21, 275–283. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Z.; Tian, D.; Zhao, J.; Zhang, J.; Deng, P.; Zou, H.; Lu, C. Acidic Deep Eutectic Solvent Assisted Mechanochemical Delignification of Lignocellulosic Biomass at Room Temperature. Int. J. Biol. Macromol. 2023, 234, 123593. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, T.; Fu, Q.J.; Bai, Y.Y.; Peng, F.; Yao, C.L. Choline Chloride-Lactic Acid Deep Eutectic Solvent for Delignification and Nanocellulose Production of Moso Bamboo. Cellulose 2019, 26, 9447–9462. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, X.; You, T.T.; Xu, F. Deep Eutectic Solvents (DESs) for Cellulose Dissolution: A Mini-Review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Yin, X.; Wei, L.; Pan, X.; Liu, C.; Jiang, J.; Wang, K. The Pretreatment of Lignocelluloses With Green Solvent as Biorefinery Preprocess: A Minor Review. Front. Plant Sci. 2021, 12, 670061. [Google Scholar] [CrossRef] [PubMed]
- Loow, Y.L.; New, E.K.; Yang, G.H.; Ang, L.Y.; Foo, L.Y.W.; Wu, T.Y. Potential Use of Deep Eutectic Solvents to Facilitate Lignocellulosic Biomass Utilization and Conversion. Cellulose 2017, 24, 3591–3618. [Google Scholar] [CrossRef]
- Li, C.; Huang, C.; Zhao, Y.; Zheng, C.; Su, H.; Zhang, L.; Luo, W.; Zhao, H.; Wang, S.; Huang, L.J. Effect of Choline-Based Deep Eutectic Solvent Pretreatment on the Structure of Cellulose and Lignin in Bagasse. Processes 2021, 9, 384. [Google Scholar] [CrossRef]
- Oh, Y.; Park, S.; Jung, D.; Oh, K.K.; Lee, S.H. Effect of Hydrogen Bond Donor on the Choline Chloride-Based Deep Eutectic Solvent-Mediated Extraction of Lignin from Pine Wood. Int. J. Biol. Macromol. 2020, 165, 187–197. [Google Scholar] [CrossRef]
- Smink, D.; Kersten, S.R.A.; Schuur, B. Recovery of Lignin from Deep Eutectic Solvents by Liquid-Liquid Extraction. Sep. Purif. Technol. 2020, 235, 116127. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep Eutectic Solvents for Pretreatment, Extraction, and Catalysis of Biomass and Food Waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef]
- Lynam, J.G.; Kumar, N.; Wong, M.J. Deep Eutectic Solvents’ Ability to Solubilize Lignin, Cellulose, and Hemicellulose; Thermal Stability; and Density. Bioresour. Technol. 2017, 238, 684–689. [Google Scholar] [CrossRef]
- Zhang, C.W.; Xia, S.Q.; Ma, P.S. Facile Pretreatment of Lignocellulosic Biomass Using Deep Eutectic Solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Y.; Peng, J.; Song, X.; Liu, Y.; Su, Z.; Li, B.; Gao, C.; Tian, W. Comprehensive Analysis of Important Parameters of Choline Chloride-Based Deep Eutectic Solvent Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2021, 319, 124209. [Google Scholar] [CrossRef] [PubMed]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural Deep Eutectic Solvents for Lignocellulosic Biomass Pretreatment: Recent Developments, Challenges and Novel Opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Rahaman, M.S.; Yelle, D.; Shang, H.; Sun, Z.; Renneckar, S.; Dong, J.; Tulaphol, S.; Sathitsuksanoh, N. Effects of Polyol-Based Deep Eutectic Solvents on the Efficiency of Rice Straw Enzymatic Hydrolysis. Ind. Crops Prod. 2021, 167, 113480. [Google Scholar] [CrossRef]
- Mamilla, J.L.K.; Novak, U.; Grilc, M.; Likozar, B. Natural Deep Eutectic Solvents (DES) for Fractionation of Waste Lignocellulosic Biomass and Its Cascade Conversion to Value-Added Bio-Based Chemicals. Biomass Bioenergy 2019, 120, 417–425. [Google Scholar] [CrossRef]
- del Mar Contreras-Gámez, M.; Galán-Martín, Á.; Seixas, N.; da Costa Lopes, A.M.; Silvestre, A.; Castro, E. Deep Eutectic Solvents for Improved Biomass Pretreatment: Current Status and Future Prospective towards Sustainable Processes. Bioresour. Technol. 2023, 369, 128396. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, D.; Zhang, T.; Lei, T.; Zhang, H.; Yang, J.; Shui, X.; Li, F.; Zhang, Y.; Zhang, Q. Comparison of Three Ionic Liquids Pretreatment of Arundo Donax L. For Enhanced Photo-Fermentative Hydrogen Production. Bioresour. Technol. 2022, 343, 126088. [Google Scholar] [CrossRef]
- NREL. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) (Revised August 2012): Issue Date: 4/25/2008. 2012; 18p. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 11 September 2024).
- Moreno, A.D.; Duque, A.; González, A.; Ballesteros, I.; Negro, M.J. Valorization of Greenhouse Horticulture Waste from a Biorefinery Perspective. Foods 2021, 10, 814. [Google Scholar] [CrossRef]
- Zhai, Q.; Long, F.; Jiang, X.; Hse, C.Y.; Jiang, J.; Xu, J. Facile and Rapid Fractionation of Bamboo Wood with a P-Toluenesulfonic Acid-Based Three-Constituent Deep Eutectic Solvent. Ind. Crops Prod. 2020, 158, 113018. [Google Scholar] [CrossRef]
- Chang, L.; Sun, Y.; Gan, L. Insights into Cellulose Deconstruction and Pseudo-Lignin Formation during Deep Eutectic Solvent Treatment. Cellulose 2023, 30, 141–152. [Google Scholar] [CrossRef]
- Cui, P.; Ye, Z.; Chai, M.; Yuan, J.; Xiong, Y.; Yang, H.; Yao, L. Effective Fractionation of Lignocellulose Components and Lignin Valorization by Combination of Deep Eutectic Solvent with Ethanol. Front. Bioeng. Biotechnol. 2023, 10, 1–11. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J. Lignocellulosic Biomass Pretreatment by Deep Eutectic Solvents on Lignin Extraction and Saccharification Enhancement: A Review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, D.; Contreras, M.d.M.; Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martín, J.M.; Romero, I.; Castro, E. Sustainable Vine Shoots-to-Ethanol Valorisation by a Sequential Acid/Organosolv Pretreatment. Process Saf. Environ. Prot. 2024, 183, 1059–1070. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, P.; Andrés, M.A.; Labidi, J. Coproduction of Lignin and Glucose from Vine Shoots by Eco-Friendly Strategies: Toward the Development of an Integrated Biorefinery. Bioresour. Technol. 2017, 244, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Quintero, L.P.; de Souza, N.P.Q.; Milagres, A.M.F. The Effect of Xylan Removal on the High-Solid Enzymatic Hydrolysis of Sugarcane Bagasse. BioEnergy Res. 2022, 15, 1096–1106. [Google Scholar] [CrossRef]
- Paulsen Thoresen, P.; Lange, H.; Rova, U.; Christakopoulos, P.; Matsakas, L. Role and Importance of Solvents for the Fractionation of Lignocellulosic Biomass. Bioresour. Technol. 2023, 369, 128447. [Google Scholar] [CrossRef]
- Li, D.; Qi, L.; Yang, M.; Gu, Y.; Xue, Y.; Chen, J.; He, M.; Yang, G. Switchable Deep Eutectic Solvents for Lignin Dissolution and Regeneration. Polymers 2023, 15, 4233. [Google Scholar] [CrossRef]
- Kroon., M.C.; Casal., M.F.; Van Den Bruinhorst, A. Pretreatment of Lignocellulosc Biomass and Recovery of Substituents Using Natural Deep Eutectic Solvents/Compound Mixtures with Low Transtion Temperatures. Patent No. WO2013153203, 17 October 2013. [Google Scholar]
- Domínguez-Robles, J.; Tamminen, T.; Liitiä, T.; Peresin, M.S.; Rodríguez, A.; Jääskeläinen, A.S. Aqueous Acetone Fractionation of Kraft, Organosolv and Soda Lignins. Int. J. Biol. Macromol. 2018, 106, 979–987. [Google Scholar] [CrossRef]
- Moradi, H.; Farzi, N. Experimental and Computational Assessment of the Physicochemical Properties of Choline Chloride/Ethylene Glycol Deep Eutectic Solvent in 1:2 and 1:3 Mole Fractions and 298.15–398.15 K. J. Mol. Liq. 2021, 339, 116669. [Google Scholar] [CrossRef]
- Jin, W.; Shen, D.; Liu, Q.; Xiao, R. Evaluation of the Co-Pyrolysis of Lignin with Plastic Polymers by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2016, 133, 65–74. [Google Scholar] [CrossRef]
- Xiang, A.; Ebdon, J.R.; Horrocks, A.R.; Kandola, B.K. On the Utility of Thermogravimetric Analysis for Exploring the Kinetics of Thermal Degradation of Lignins. Bioresour. Technol. Reports 2022, 20, 101214. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.; Bustam, M.A.; Mutalib, M.I.A.; Rafiq, S. Investigations of Novel Nitrile-Based Ionic Liquids as Pre-Treatment Solvent for Extraction of Lignin from Bamboo Biomass. J. Ind. Eng. Chem. 2013, 19, 207–214. [Google Scholar] [CrossRef]
- Li, W.; Amos, K.; Li, M.; Pu, Y.; DeBolt, S.; Ragauskas, A.J.; Shi, J. Fractionation and Characterization of Lignin Streams from Unique High-Lignin Content Endocarp Feedstocks. Biotechnol. Biofuels 2018, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhu, Y.; Qi, B.; Li, S.; Luo, J.; Wan, Y. Structure-Property-Performance Relationships of Lactic Acid-Based Deep Eutectic Solvents with Different Hydrogen Bond Acceptors for Corn Stover Pretreatment. Bioresour. Technol. 2021, 336, 125312. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, V.R.; Pandey, A.; Pant, K.K.; Henry, R.J. Improving Enzymatic Digestibility of Sugarcane Bagasse from Different Varieties of Sugarcane Using Deep Eutectic Solvent Pretreatment. Bioresour. Technol. 2021, 337, 125480. [Google Scholar] [CrossRef] [PubMed]
- Alavijeh, R.S.; Shahvandi, A.; Okoro, O.V.; Denayer, J.F.M.; Karimi, K. Biorefining of Corn Stover for Efficient Production of Bioethanol, Biodiesel, Biomethane, and Value-Added Byproducts. Energy Convers. Manag. 2023, 283, 116877. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of Physicochemical Properties and Analytical Characterization of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, J.; Lan, P.; Yang, H.; Lu, J.; Wang, Z. Study on the Dissolution Mechanism of Cellulose by ChCl-Based Deep Eutectic Solvents. Materials 2020, 13, 278. [Google Scholar] [CrossRef]
- Kwon, G.J.; Yang, B.S.; Park, C.W.; Bandi, R.; Lee, E.A.; Park, J.S.; Han, S.Y.; Kim, N.H.; Lee, S.H. Treatment Effects of Choline Chloride-Based Deep Eutectic Solvent on the Chemical Composition of Red Pine (Pinus Densiflora). BioResources 2020, 15, 6457–6470. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural Deep Eutectic Solvent Mediated Pretreatment of Rice Straw: Bioanalytical Characterization of Lignin Extract and Enzymatic Hydrolysis of Pretreated Biomass Residue. Environ. Sci. Pollut. Res. 2016, 23, 9265–9275. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z.; Liu, T.; Wang, Y. Production of High-Concentration Fermentable Sugars from Lignocellulosic Biomass by Using High Solids Fed-Batch Enzymatic Hydrolysis. Biochem. Eng. J. 2021, 176, 108186. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Zhao, J.; Wang, D. Effects of Particle Size on Biomass Pretreatment and Hydrolysis Performances in Bioethanol Conversion. Biomass Convers. Biorefinery 2023, 13, 13023–13036. [Google Scholar] [CrossRef]
- Yan, M.; Tian, C.; Wu, T.; Huang, X.; Zhong, Y.; Yang, P.; Zhang, L.; Ma, J.; Lu, H.; Zhou, X. Insights into Structure and Properties of Cellulose Nanofibrils (CNFs) Prepared by Screw Extrusion and Deep Eutectic Solvent Permeation. Int. J. Biol. Macromol. 2021, 191, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Gajardo-Parra, N.F.; Cotroneo-Figueroa, V.P.; Aravena, P.; Vesovic, V.; Canales, R.I. Viscosity of Choline Chloride-Based Deep Eutectic Solvents: Experiments and Modeling. J. Chem. Eng. Data 2020, 65, 5581–5592. [Google Scholar] [CrossRef]
- Schmatz, A.A.; Salazar-Bryam, A.M.; Contiero, J.; Sant’Anna, C.; Brienzo, M. Pseudo-Lignin Content Decreased with Hemicellulose and Lignin Removal, Improving Cellulose Accessibility, and Enzymatic Digestibility. BioEnergy Res. 2021, 14, 106–121. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Jiang, J.; Zhang, Y.; Bi, S.; Wang, H.-M. Revealing Structural and Functional Specificity of Lignin from Tobacco Stalk during Deep Eutectic Solvents Deconstruction Aiming to Targeted Valorization. Ind. Crops Prod. 2022, 180, 114696. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Wu, P.; Yagoub, A.E.-G.A.; Chen, L.; Abdullateef Taiye, M.; Zhou, C. Pretreatment of Sugarcane Bagasse with Deep Eutectic Solvents Affect the Structure and Morphology of Lignin. Ind. Crops Prod. 2021, 173, 114108. [Google Scholar] [CrossRef]

| DES Solvent Pretreatment with ChCl:LA (1:5) and ChCl:EG (1:2) | |||
|---|---|---|---|
| Assay Conditions | Temperature (°C) | Solid Loading (%) | Particle Size (mm) |
| 120-5-2 | 120 | 5 | 2 |
| 120-5-0.5 | 0.5 | ||
| 120-10-2 | 10 | 2 | |
| 120-10-0.5 | 0.5 | ||
| 120-30-2 * | 30 | 2 | |
| 120-30-0.5 * | 0.5 | ||
| 150-5-2 | 150 | 5 | 2 |
| 150-5-0.5 | 0.5 | ||
| 150-10-2 | 10 | 2 | |
| 150-10-0.5 | 0.5 | ||
| 150-30-2 | 30 | 2 | |
| 150-30-0.5 | 0.5 | ||
| Component | Composition (g/100 g of Dry Biomass, (%)) |
|---|---|
| Cellulose | 32.3 ± 0.5 |
| Hemicellulose | 18.4 ± 0.3 |
| Xylan | 14.8 ± 0.3 |
| Galactan | 1.9 ± 0.06 |
| Arabinan | 1.0 ± 0.04 |
| Mannan | 0.7 ± 0.01 |
| Acetyl groups | 5.3 ± 0.05 |
| Acid-insoluble lignin | 26.0 ± 0.1 |
| Acid-soluble lignin | 1.6 ± 0.05 |
| Ash | 3.6 ± 0.3 |
| Aqueous extractives | 6.9 ± 0.1 |
| Ethanol extractives | 1.4 ± 0.2 |
| Assay Conditions | ChCl:LA (1:5) | ChCl:EG (1:2) |
|---|---|---|
| Solid Recovery (%) | Solid Recovery (%) | |
| 120-5-2 | 69.2 ± 1.5 | 91.7 ± 0.2 |
| 120-5-0.5 | 60.2 ± 0.2 | 81.9 ± 0.0 |
| 120-10-2 | 65.0 ± 4.3 | 89.2 ± 0.0 |
| 120-10-0.5 | 66.0 ± 2.8 | 85.6 ± 5.1 |
| 120-30-2 | 66.6 ± 1.9 | - |
| 120-30-0.5 | 67.4 ± 3.0 | - |
| 150-5-2 | 73.6 ± 5.6 | 77.1 ± 2.2 |
| 150-5-0.5 | 69.4 ± 5.7 | 78.4 ± 0.2 |
| 150-10-2 | 62.9 ± 1.4 | 86.8 ± 0.0 |
| 150-10-0.5 | 65.6 ± 1.2 | 78.4 ± 0.0 |
| 150-30-2 | 58.4 ± 5.4 | 80.4 ± 1.7 |
| 150-30-0.5 | 70.4 ± 0.7 | 82.1 ± 0.4 |
| Assay Conditions | ChCl:LA (1:5) | ChCl:EG (1:2) | |||
|---|---|---|---|---|---|
| 120 °C-30%-0.5 mm | 150 °C-30%-0.5 mm | ||||
| Washing agent | H2O | 50% Acetone: H2O | H2O | 50% Acetone: H2O | |
| Composition analysis (g/100 g dry biomass) | Cellulose | 40.2 ± 0.7 | 45.0 ± 1.8 | 36.0 ± 0.2 | 40.0 ± 0.6 |
| Xylan | 8.5 ± 0.1 | 9.3 ± 0.3 | 13.8 ± 0.1 | 15.6 ± 0.0 | |
| Acid-insoluble lignin | 35.4 ± 0.5 | 29.6 ±1.0 | 35.9 ± 0.2 | 29.7 ± 1.4 | |
| Component recovery yield (%) | Cellulose | 84.0 ± 3.8 | 82.0 ± 0.8 | 85.9 ± 0.4 | 84.3 ± 5.1 |
| Xylan | 38.8 ± 1.8 | 42.0 ± 0.4 | 71.8 ± 0.3 | 71.9 ± 4.3 | |
| Delignification | 11.7 ± 3.9 | 31.5 ± 0.7 | 0.0 ± 0.5 | 23.5 ± 0.0 | |
| Solid recovery | 67.4 ± 3.0 | 71.5 ± 0.1 | 82.1 ± 0.4 | 88.5 ± 0.0 | |
| EH conversion yield (%) | Cellulose | 56.4 ± 1.7 | 49.4 ± 2.1 | 54.6 ± 0.8 | 61.7 ± 3.2 |
| Xylan | 77.6 ± 2.9 | 69.4 ± 4.2 | 60.3 ± 0.3 | 66.9 ± 2.5 | |
| Assay | Mass Loss (g/100 g Dry Biomass) | Range T 260–280 °C | Range T 355–395 °C | |||
|---|---|---|---|---|---|---|
| Tmax (°C) | Mass Loss (%/min) | Tmax (°C) | Mass Loss (g/100 g Dry Biomass/min) | |||
| raw VS | 80.8 | 357 | 13.9 | |||
| Commercial lignin | 61.8 | 395 | 7.5 | |||
| ChCl:LA H2O | 120-5-2 | 62.9 | 278 | 3.6 | 368 | 5.7 |
| 120-5-0.5 | 64.6 | 271 | 4.8 | 371 | 4.8 | |
| 120-10-2 | 65.7 | 269 | 6.0 | 363 | 5.0 | |
| 120-10-0.5 | 63.9 | 273 | 4.2 | 369 | 5.6 | |
| 150-5-2 | 58.6 | 266 | 3.9 | 378 | 3.9 | |
| 150-5-0.5 | 56.7 | 273 | 2.4 | 376 | 4.4 | |
| 150-10-2 | 60.9 | 261 | 5.8 | 375 | 4.1 | |
| 150-10-0.5 | 57.7 | 269 | 3.0 | 375 | 4.2 | |
| ChCl:LA 50% acetone:H2O | 120-30-0.5 | 64.6 | 274 | 3.8 | 365 | 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañadas, R.; Duque, A.; Bahíllo, A.; Iglesias, R.; Manzanares, P. Pretreatment of Vine Shoot Biomass by Choline Chloride-Based Deep Eutectic Solvents to Promote Biomass Fractionation and Enhance Sugar Production. Bioengineering 2024, 11, 935. https://doi.org/10.3390/bioengineering11090935
Cañadas R, Duque A, Bahíllo A, Iglesias R, Manzanares P. Pretreatment of Vine Shoot Biomass by Choline Chloride-Based Deep Eutectic Solvents to Promote Biomass Fractionation and Enhance Sugar Production. Bioengineering. 2024; 11(9):935. https://doi.org/10.3390/bioengineering11090935
Chicago/Turabian StyleCañadas, Raquel, Aleta Duque, Alberto Bahíllo, Raquel Iglesias, and Paloma Manzanares. 2024. "Pretreatment of Vine Shoot Biomass by Choline Chloride-Based Deep Eutectic Solvents to Promote Biomass Fractionation and Enhance Sugar Production" Bioengineering 11, no. 9: 935. https://doi.org/10.3390/bioengineering11090935
APA StyleCañadas, R., Duque, A., Bahíllo, A., Iglesias, R., & Manzanares, P. (2024). Pretreatment of Vine Shoot Biomass by Choline Chloride-Based Deep Eutectic Solvents to Promote Biomass Fractionation and Enhance Sugar Production. Bioengineering, 11(9), 935. https://doi.org/10.3390/bioengineering11090935









