Bone Regeneration: Mini-Review and Appealing Perspectives
Abstract
1. Introduction
2. Fundamentals of Bone Healing Materials: Definition, Needs and Expected Properties
2.1. Bone Composition, Structure and Main Properties
2.1.1. Bone Composition
Type I Collagen
Bone Mineral Phase
2.1.2. Bone Structure and Related Mechanical Properties
2.1.3. Bone Cells
2.2. Clinical Challenges in Bone Surgery
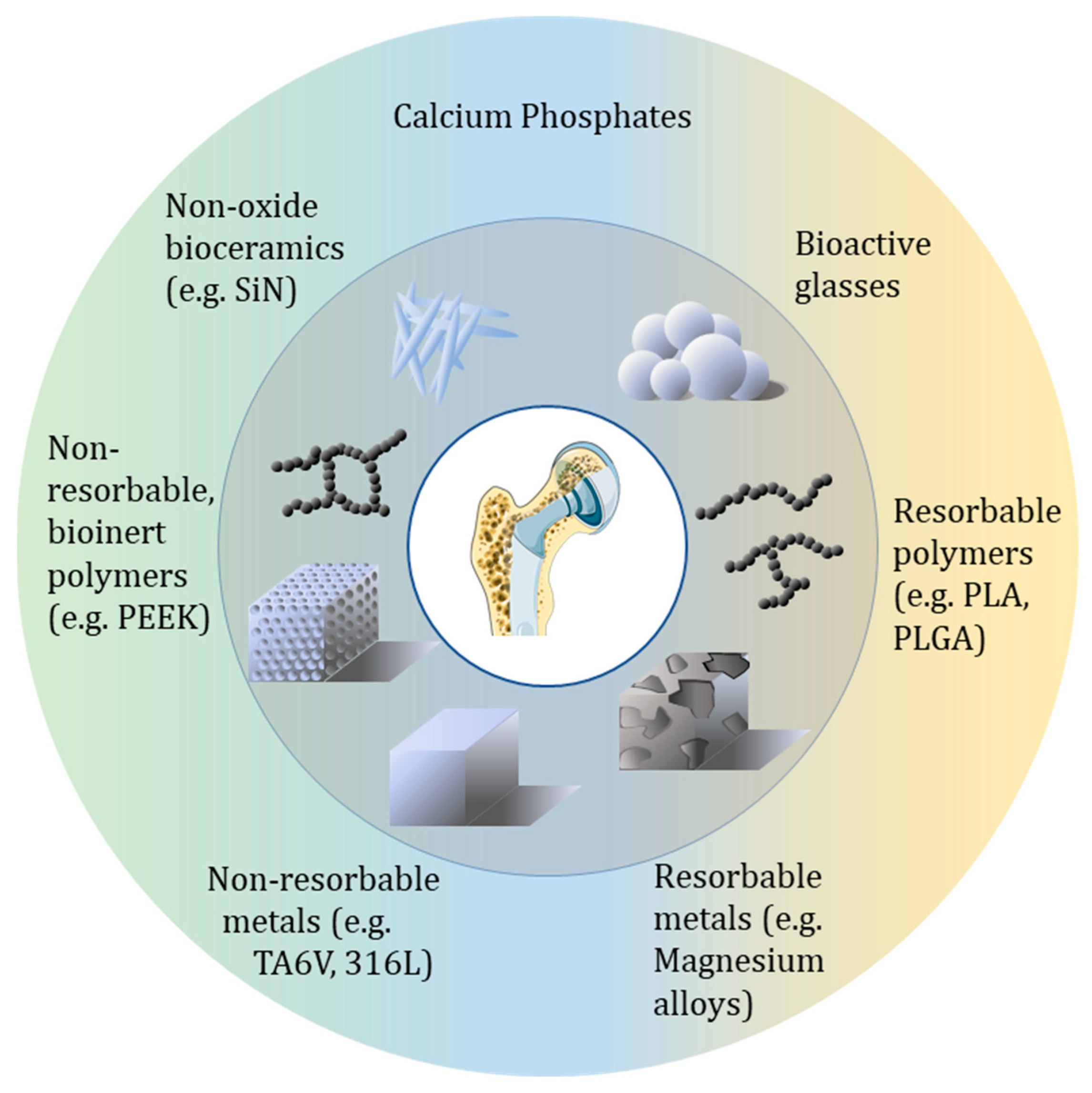
3. Bone Healing Materials Through the Different Families of Materials
3.1. Inorganic-Based Bone Implants
3.1.1. Metal-Based Biomaterials
3.1.2. Ceramics
3.1.3. Bioactive Glasses
3.1.4. Non-Oxide Ceramics
3.2. Organic-Including Materials
3.2.1. Synthetic Polymers
3.2.2. Natural Polymers
3.2.3. Hydrogels
3.3. Composites and Hybrid Biomaterials
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Finch, J. The Ancient Origins of Prosthetic Medicine. Lancet 2011, 377, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Jung, H.; Skirboll, S. Materials Used in Cranioplasty: A History and Analysis. Neurosurg. Focus 2014, 36, E19. [Google Scholar] [CrossRef]
- Hampel, G.A.; Yilmaz, E.; Massrey, C.; Clifton, W.; Iwanaga, J.; Loukas, M.; Tubbs, R.S. History of Bone Grafts in Spine Surgery. Cureus 2022, 14, e24655. [Google Scholar] [CrossRef]
- Kuroda, K.; Okido, M. Hydroxyapatite Coating of Titanium Implants Using Hydroprocessing and Evaluation of Their Osteoconductivity. Bioinorg. Chem. Appl. 2012, 2012, 730693. [Google Scholar] [CrossRef] [PubMed]
- De Groot, K.; Geesink, R.; Klein, C.P.a.T.; Serekian, P. Plasma Sprayed Coatings of Hydroxylapatite. J. Biomed. Mater. Res. 1987, 21, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O. Octacalcium Phosphate (OCP)-Based Bone Substitute Materials. Jpn. Dent. Sci. Rev. 2013, 49, 58–71. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Xu, H.H.K.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial Amorphous Calcium Phosphate Nanocomposites with a Quaternary Ammonium Dimethacrylate and Silver Nanoparticles. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wu, Y.; Guo, Q.; Shi, Q.; Zhang, J.; Meng, Y.; Xia, F.; Wang, J. Preparation and Application of Calcium Phosphate Nanocarriers in Drug Delivery. Mater. Today Bio 2022, 17, 100501. [Google Scholar] [CrossRef] [PubMed]
- Rimondini, L.; Bianchi, C.L.; Verné, E. (Eds.) Calcium Phosphate Surface Tailoring Technologies for Drug Delivering and Tissue Engineering. In Surface Tailoring of Inorganic Materials for Biomedical Applications; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 43–111. ISBN 978-1-60805-462-6. [Google Scholar]
- Verma, A.H.; Kumar, T.S.S.; Madhumathi, K.; Rubaiya, Y.; Ramalingan, M.; Doble, M. Curcumin Releasing Eggshell Derived Carbonated Apatite Nanocarriers for Combined Anti-Cancer, Anti-Inflammatory and Bone Regenerative Therapy. J. Nanosci. Nanotechnol. 2019, 19, 6872–6880. [Google Scholar] [CrossRef]
- Epple, M.; Ganesan, K.; Heumann, R.; Klesing, J.; Kovtun, A.; Neumann, S.; Sokolova, V. Application of Calcium Phosphate Nanoparticles in Biomedicine. J. Mater. Chem. 2010, 20, 18–23. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Zhu, Y.-J.; Chen, F.; Wu, J. Calcium Phosphate Nanocarriers Dual-Loaded with Bovine Serum Albumin and Ibuprofen: Facile Synthesis, Sequential Drug Loading and Sustained Drug Release. Chem.–Asian J. 2012, 7, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.-L.; Zhu, Y.-J.; Wu, J.; Chen, F.; Cao, S.-W. Calcium Phosphate Drug Nanocarriers with Ultrahigh and Adjustable Drug-Loading Capacity: One-Step Synthesis, in Situ Drug Loading and Prolonged Drug Release. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A. Significant Advancements of 4D Printing in the Field of Orthopaedics. J. Clin. Orthop. Trauma 2020, 11, S485–S490. [Google Scholar] [CrossRef]
- Sajjad, R.; Chauhdary, S.T.; Anwar, M.T.; Zahid, A.; Khosa, A.A.; Imran, M.; Sajjad, M.H. A Review of 4D Printing–Technologies, Shape Shifting, Smart Polymer Based Materials, and Biomedical Applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 20–36. [Google Scholar] [CrossRef]
- Farag, M.M. Recent Trends on Biomaterials for Tissue Regeneration Applications: Review. J. Mater. Sci. 2023, 58, 527–558. [Google Scholar] [CrossRef]
- Lee, V.K.; Dias, A.; Ozturk, M.S.; Chen, K.; Tricomi, B.; Corr, D.T.; Intes, X.; Dai, G. 3D Bioprinting and 3D Imaging for Stem Cell Engineering. In Bioprinting in Regenerative Medicine; Springer International Publishing: New York, NY, USA, 2015; pp. 33–66. ISBN 978-3-319-21386-6. [Google Scholar]
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A Review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hayward, R.C. Mimicking Dynamic in Vivo Environments with Stimuli-Responsive Materials for Cell Culture. Trends Biotechnol. 2012, 30, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Buss, D.J.; Kröger, R.; McKee, M.D.; Reznikov, N. Hierarchical Organization of Bone in Three Dimensions: A Twist of Twists. J. Struct. Biol. X 2022, 6, 100057. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone Hierarchical Structure in Three Dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Turner, C.H. Mechanical Signaling for Bone Modeling and Remodeling. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of Bone Development and Repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. The material bone: Structure-Mechanical Function Relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Nair, A.K.; Gautieri, A.; Chang, S.-W.; Buehler, M.J. Molecular Mechanics of Mineralized Collagen Fibrils in Bone. Nat. Commun. 2013, 4, 1724. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Hazan, S.; Sauer, K.; Aladin, V.; Keinan-Adamsky, K.; Corzilius, B.; Shahar, R.; Zaslansky, P.; Goobes, G. Molecular Differences in Collagen Organization and in Organic-Inorganic Interfacial Structure of Bones with and without Osteocytes. Acta Biomater. 2022, 144, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Tzaphlidou, M. Bone Architecture: Collagen Structure and Calcium/Phosphorus Maps. J. Biol. Phys. 2008, 34, 39. [Google Scholar] [CrossRef]
- Coradin, T.; Wang, K.; Law, T.; Trichet, L. Type I Collagen-Fibrin Mixed Hydrogels: Preparation, Properties and Biomedical Applications. Gels 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Azaïs, T.; Robin, M.; Vallée, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.M.; Nassif, N. The Predominant Role of Collagen in the Nucleation, Growth, Structure and Orientation of Bone Apatite. Nat. Mater. 2012, 11, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Von Euw, S.; Wang, Y.; Laurent, G.; Drouet, C.; Babonneau, F.; Nassif, N.; Azaïs, T. Bone Mineral: New Insights into Its Chemical Composition. Sci. Rep. 2019, 9, 8456. [Google Scholar] [CrossRef] [PubMed]
- Edén, M. Structure and Formation of Amorphous Calcium Phosphate and Its Role as Surface Layer of Nanocrystalline Apatite: Implications for Bone Mineralization. Materialia 2021, 17, 101107. [Google Scholar] [CrossRef]
- Roohani, I.; Cheong, S.; Wang, A. How to Build a Bone?-Hydroxyapatite or Posner’s Clusters as Bone Minerals. Open Ceram. 2021, 6, 100092. [Google Scholar] [CrossRef]
- He, K.; Sawczyk, M.; Liu, C.; Yuan, Y.; Song, B.; Deivanayagam, R.; Nie, A.; Hu, X.; Dravid, V.P.; Lu, J.; et al. Revealing Nanoscale Mineralization Pathways of Hydroxyapatite Using in Situ Liquid Cell Transmission Electron Microscopy. Sci. Adv. 2020, 6, eaaz7524. [Google Scholar] [CrossRef] [PubMed]
- Mancardi, G.; Tamargo, C.E.H.; Tommaso, D.D.; Leeuw, N.H. de Detection of Posner’s Clusters during Calcium Phosphate Nucleation: A Molecular Dynamics Study. J. Mater. Chem. B 2017, 5, 7274–7284. [Google Scholar] [CrossRef] [PubMed]
- Mahamid, J.; Aichmayer, B.; Shimoni, E.; Ziblat, R.; Li, C.; Siegel, S.; Paris, O.; Fratzl, P.; Weiner, S.; Addadi, L. Mapping Amorphous Calcium Phosphate Transformation into Crystalline Mineral from the Cell to the Bone in Zebrafish Fin Rays. Proc. Natl. Acad. Sci. USA 2010, 107, 6316–6321. [Google Scholar] [CrossRef]
- Suzuki, O.; Hamai, R.; Sakai, S. The Material Design of Octacalcium Phosphate Bone Substitute: Increased Dissolution and Osteogenecity. Acta Biomater. 2023, 158, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shiwaku, Y.; Hamai, R.; Sato, S.; Sakai, S.; Tsuchiya, K.; Baba, K.; Takahashi, T.; Suzuki, O. Bone Tissue Response to Different Grown Crystal Batches of Octacalcium Phosphate in Rat Long Bone Intramedullary Canal Area. Int. J. Mol. Sci. 2021, 22, 9770. [Google Scholar] [CrossRef] [PubMed]
- Carino, A.; Ludwig, C.; Cervellino, A.; Müller, E.; Testino, A. Formation and Transformation of Calcium Phosphate Phases under Biologically Relevant Conditions: Experiments and Modelling. Acta Biomater. 2018, 74, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Crane, N.J.; Popescu, V.; Morris, M.D.; Steenhuis, P.; Ignelzi, M.A. Raman Spectroscopic Evidence for Octacalcium Phosphate and Other Transient Mineral Species Deposited during Intramembranous Mineralization. Bone 2006, 39, 434–442. [Google Scholar] [CrossRef]
- Drouet, C.; Rey, C. Nanostructured Calcium Phosphates for Hard Tissue Engineering and Nanomedicine. In Nanostructured Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 223–254. ISBN 978-0-08-102594-9. [Google Scholar]
- Hamai, R.; Sakai, S.; Shiwaku, Y.; Anada, T.; Tsuchiya, K.; Ishimoto, T.; Nakano, T.; Suzuki, O. Octacalcium Phosphate Crystals Including a Higher Density Dislocation Improve Its Materials Osteogenecity. Appl. Mater. Today 2022, 26, 101279. [Google Scholar] [CrossRef]
- Nassif, N.; Gobeaux, F.; Seto, J.; Belamie, E.; Davidson, P.; Panine, P.; Mosser, G.; Fratzl, P.; Giraud Guille, M.M. Self-Assembled Collagen-Apatite Matrix with Bone-like Hierarchy. Chem. Mater. 2010, 22, 3307–3309. [Google Scholar] [CrossRef]
- Wagoner Johnson, A.J.; Herschler, B.A. A Review of the Mechanical Behavior of CaP and CaP/Polymer Composites for Applications in Bone Replacement and Repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef]
- Porrelli, D.; Abrami, M.; Pelizzo, P.; Formentin, C.; Ratti, C.; Turco, G.; Grassi, M.; Canton, G.; Grassi, G.; Murena, L. Trabecular Bone Porosity and Pore Size Distribution in Osteoporotic Patients—A Low Field Nuclear Magnetic Resonance and Microcomputed Tomography Investigation. J. Mech. Behav. Biomed. Mater. 2022, 125, 104933. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical Regulation of Bone Remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Webster, D.J.; Schneider, P.; Dallas, S.L.; Müller, R. Studying Osteocytes within Their Environment. Bone 2013, 54, 285–295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 978-0-08-047036-8. [Google Scholar]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. Materials Evolution of Bone Plates for Internal Fixation of Bone Fractures: A Review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Jones, M.D.; Ruth, J.T.; Benjamin, J.B.; Sheppard, J.E.; Hunter, T.B. Fracture Fixation. RadioGraphics 2003, 23, 1569–1590. [Google Scholar] [CrossRef]
- Tian, L.; Tang, N.; Ngai, T.; Wu, C.; Ruan, Y.; Huang, L.; Qin, L. Hybrid Fracture Fixation Systems Developed for Orthopaedic Applications: A General Review. J. Orthop. Transl. 2019, 16, 1–13. [Google Scholar] [CrossRef]
- Arbez, B.; Kün-Darbois, J.-D.; Convert, T.; Guillaume, B.; Mercier, P.; Hubert, L.; Chappard, D. Biomaterial Granules Used for Filling Bone Defects Constitute 3D Scaffolds: Porosity, Microarchitecture and Molecular Composition Analyzed by microCT and Raman Microspectroscopy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 415–423. [Google Scholar] [CrossRef]
- Keppler, A.M.; Saller, M.M.; Alberton, P.; Westphal, I.; Heidenau, F.; Schönitzer, V.; Böcker, W.; Kammerlander, C.; Schieker, M.; Aszodi, A.; et al. Bone Defect Reconstruction with a Novel Biomaterial Containing Calcium Phosphate and Aluminum Oxide Reinforcement. J. Orthop. Surg. Res. 2020, 15, 287. [Google Scholar] [CrossRef] [PubMed]
- Mounika, C.; Tadge, T.; Keerthana, M.; Velyutham, R.; Kapusetti, G. Advancements in Poly(Methyl Methacrylate) Bone Cement for Enhanced Osteoconductivity and Mechanical Properties in Vertebroplasty: A Comprehensive Review. Med. Eng. Phys. 2023, 120, 104049. [Google Scholar] [CrossRef] [PubMed]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for Guided Bone Regeneration: A Road from Bench to Bedside. Adv. Healthc. Mater. 2020, 9, 2000707. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and Current Interpretations of the Bone-Implant Interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abu Alfaraj, T.; Al-Madani, S.; Alqahtani, N.S.; Almohammadi, A.A.; Alqahtani, A.M.; AlQabbani, H.S.; Bajunaid, M.K.; Alharthy, B.A.; Aljalfan, N. Optimizing Osseointegration in Dental Implantology: A Cross-Disciplinary Review of Current and Emerging Strategies. Cureus 2023, 15, e47943. [Google Scholar] [CrossRef] [PubMed]
- Ortali, C.; Julien, I.; Vandenhende, M.; Drouet, C.; Champion, E. Consolidation of Bone-like Apatite Bioceramics by Spark Plasma Sintering of Amorphous Carbonated Calcium Phosphate at Very Low Temperature. J. Eur. Ceram. Soc. 2018, 38, 2098–2109. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, J.H.; Kim, H.W. Novel Bone-Mimetic Nanohydroxyapatite/Collagen Porous Scaffolds Biomimetically Mineralized from Surface Silanized Mesoporous Nanobioglass/Collagen Hybrid Scaffold: Physicochemical, Mechanical and in Vivo Evaluations. Mater. Sci. Eng. C 2020, 110, 110660. [Google Scholar] [CrossRef]
- Galotta, A.; Rubenis, K.; Locs, J.; Sglavo, V.M. Dissolution-Precipitation Synthesis and Cold Sintering of Mussel Shells-Derived Hydroxyapatite and Hydroxyapatite/Chitosan Composites for Bone Tissue Engineering. Open Ceram. 2023, 15, 100418. [Google Scholar] [CrossRef]
- Rubenis, K.; Zemjane, S.; Vecstaudza, J.; Lazdovica, K.; Bitenieks, J. Sintering of Amorphous Calcium Phosphate to Near-Full Density by Uniaxial Compaction at Room Temperature Journal of the European Ceramic Society Sintering of Amorphous Calcium Phosphate to near-Full Density by Uniaxial Compaction at Room Temperature. J. Eur. Ceram. Soc. 2022, 42, 6199–6205. [Google Scholar] [CrossRef]
- Rubenis, K.; Zemjane, S.; Vecstaudza, J.; Bitenieks, J.; Locs, J. Densification of Amorphous Calcium Phosphate Using Principles of the Cold Sintering Process. J. Eur. Ceram. Soc. 2021, 41, 912–919. [Google Scholar] [CrossRef]
- Galotta, A.; Demir, Ö.; Marsan, O.; Sglavo, V.M.; Loca, D.; Combes, C.; Locs, J. Apatite/Chitosan Composites Formed by Cold Sintering for Drug Delivery and Bone Tissue Engineering Applications. Nanomaterials 2024, 14, 441. [Google Scholar] [CrossRef]
- Bian, Y.; Hu, T.; Lv, Z.; Xu, Y.; Wang, Y.; Wang, H.; Zhu, W.; Feng, B.; Liang, R.; Tan, C.; et al. Bone Tissue Engineering for Treating Osteonecrosis of the Femoral Head. Exploration 2023, 3, 20210105. [Google Scholar] [CrossRef]
- Quan, H.; Ren, C.; He, Y.; Wang, F.; Dong, S.; Jiang, H. Application of Biomaterials in Treating Early Osteonecrosis of the Femoral Head: Research Progress and Future Perspectives. Acta Biomater. 2023, 164, 15–73. [Google Scholar] [CrossRef]
- Fitzpatrick, V.; Martín-Moldes, Z.; Deck, A.; Torres-Sanchez, R.; Valat, A.; Cairns, D.; Li, C.; Kaplan, D.L. Functionalized 3D-Printed Silk-Hydroxyapatite Scaffolds for Enhanced Bone Regeneration with Innervation and Vascularization. Biomaterials 2021, 276, 120995. [Google Scholar] [CrossRef] [PubMed]
- Bouakaz, I.; Sadeghian Dehkord, E.; Meille, S.; Schrijnemakers, A.; Boschini, F.; Preux, N.; Hocquet, S.; Geris, L.; Nolens, G.; Grossin, D.; et al. 3D Printed Triply Periodic Minimal Surfaces Calcium Phosphate Bone Substitute: The Effect of Porosity Design on Mechanical Properties. Ceram. Int. 2024, 50, 2623–2636. [Google Scholar] [CrossRef]
- Knychala, J.; Bouropoulos, N.; Catt, C.J.; Katsamenis, O.L.; Please, C.P.; Sengers, B.G. Pore Geometry Regulates Early Stage Human Bone Marrow Cell Tissue Formation and Organisation. Ann. Biomed. Eng. 2013, 41, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Bone Tissue Regeneration: The Role of Scaffold Geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Gibon, E.; Amanatullah, D.F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The Biological Response to Orthopaedic Implants for Joint Replacement: Part I: Metals. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Gibon, E.; Córdova, L.A.; Lu, L.; Lin, T.-H.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The Biological Response to Orthopedic Implants for Joint Replacement. II: Polyethylene, Ceramics, PMMA, and the Foreign Body Reaction. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Taqi, M.; Llewellyn, C.M.; Estefan, M. Fibula Tissue Transfer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Meyer, H.; Khalid, S.I.; Dorafshar, A.H.; Byrne, R.W. The Materials Utilized in Cranial Reconstruction: Past, Current, and Future. Plast Surg. 2021, 29, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.A.; Kuroda, K.; Okido, M. Preparation and Characterization of Hydroxyapatite Coating on AZ31 Mg Alloy for Implant Applications. Bioinorg. Chem. Appl. 2013, 2013, 175756. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wang, Z.; Li, Z.; Gu, L. A Two-Phase and Long-Lasting Multi-Antibacterial Coating Enables Titanium Biomaterials to Prevent Implants-Related Infections. Mater. Today Bio 2022, 15, 100330. [Google Scholar] [CrossRef]
- Mehdizade, M.; Eivani, A.R.; Tabatabaei, F.; Mousavi Anijdan, S.H.; Jafarian, H.R. Enhanced In-Vitro Biodegradation, Bioactivity, and Mechanical Properties of Mg-Based Biocomposite via Addition of Calcium-Silicate-Based Bioceramic through Friction Stir Processing as Resorbable Temporary Bone Implant. J. Mater. Res. Technol. 2023, 26, 4007–4023. [Google Scholar] [CrossRef]
- He, M.; Chen, L.; Yin, M.; Xu, S.; Liang, Z. Review on Magnesium and Magnesium-Based Alloys as Biomaterials for Bone Immobilization. J. Mater. Res. Technol. 2023, 23, 4396–4419. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, A.; Wu, J.; Guo, S.; Sun, Q. Application and Perspectives: Magnesium Materials in Bone Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 3514–3527. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, J.; Geng, W.; Yang, Y.; Li, X.; Xu, K.; Li, K.; Li, Y.; Duan, Q.; Gao, P.; et al. Regulation of Localized Corrosion of 316L Stainless Steel on Osteogenic Differentiation of Bone Morrow Derived Mesenchymal Stem Cells. Biomaterials 2023, 301, 122262. [Google Scholar] [CrossRef]
- Davis, R.; Singh, A.; Jackson, M.J.; Coelho, R.T.; Prakash, D.; Charalambous, C.P.; Ahmed, W.; da Silva, L.R.R.; Lawrence, A.A. A Comprehensive Review on Metallic Implant Biomaterials and Their Subtractive Manufacturing. Int. J. Adv. Manuf. Technol. 2022, 120, 1473–1530. [Google Scholar] [CrossRef] [PubMed]
- Garabano, G.; Rodriguez, J.; Perez Alamino, L.; Pesciallo, C.A.; del Sel, H.; Lopreite, F. Stress Shielding in Total Knee Replacements: Comparative Analysis between Titanium and All-Polyethylene Bases at 10 Years Follow-Up. J. Orthop. 2022, 34, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, C.; Terriault, P.; Jetté, B.; Dumas, M.; Brailovski, V. Development of a Porous Metallic Femoral Stem: Design, Manufacturing, Simulation and Mechanical Testing. Mater. Des. 2017, 114, 546–556. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of Pore Size on Bone Ingrowth into Porous Titanium Implants Fabricated by Additive Manufacturing: An in Vivo Experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, G.; He, W.; Wang, J.; Ye, J.; Sun, C. Design and Performance Prediction of Selective Laser Melted Porous Structure for Femoral Stem. Mater. Today Commun. 2023, 34, 104987. [Google Scholar] [CrossRef]
- Eltit, F.; Noble, J.; Sharma, M.; Benam, N.; Haegert, A.; Bell, R.H.; Simon, F.; Duncan, C.P.; Garbuz, D.S.; Greidanus, N.V.; et al. Cobalt Ions Induce Metabolic Stress in Synovial Fibroblasts and Secretion of Cytokines/Chemokines That May Be Diagnostic Markers for Adverse Local Tissue Reactions to Hip Implants. Acta Biomater. 2021, 131, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Drynda, S.; Drynda, A.; Feuerstein, B.; Kekow, J.; Lohmann, C.H.; Bertrand, J. The Effects of Cobalt and Chromium Ions on Transforming Growth Factor-Beta Patterns and Mineralization in Human Osteoblast-like MG63 and SaOs-2 Cells. J. Biomed. Mater. Res. Part A 2018, 106, 2105–2115. [Google Scholar] [CrossRef]
- Costa, A.I.; Viana, F.; Toptan, F.; Geringer, J. Highly Porous Ti as a Bone Substitute: Triboelectrochemical Characterization of Highly Porous Ti against Ti Alloy under Fretting-Corrosion Conditions. Corros. Sci. 2021, 190, 109696. [Google Scholar] [CrossRef]
- Wazen, R.M.; Currey, J.A.; Guo, H.; Brunski, J.B.; Helms, J.A.; Nanci, A. Micromotion-Induced Strain Fields Influence Early Stages of Repair at Bone-Implant Interfaces. Acta Biomater. 2013, 9, 6663–6674. [Google Scholar] [CrossRef] [PubMed]
- Padhye, N.M.; Calciolari, E.; Zuercher, A.N.; Tagliaferri, S.; Donos, N. Survival and Success of Zirconia Compared with Titanium Implants: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2023, 27, 6279–6290. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.C.; Manaka, M.; Green, D.D.; Williams, P.; Pezzotti, G.; Kim, Y.-H.; Ries, M.; Sugano, N.; Sedel, L.; Delauney, C.; et al. Current Status of Zirconia Used in Total Hip Implants. J. Bone Jt. Surg. Am. 2003, 85 (Suppl. S4), 73–84. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia Surface Modifications for Implant Dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-S.; Salamanca, E.; Lin, J.C.-Y.; Pan, Y.-H.; Wu, Y.-F.; Teng, N.-C.; Watanabe, I.; Sun, Y.-S.; Chang, W.-J. Preparation of Calcium Phosphate Compounds on Zirconia Surfaces for Dental Implant Applications. Int. J. Mol. Sci. 2022, 23, 6675. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Sato, M.; Webster, T.J. Using Hydroxyapatite Nanoparticles and Decreased Crystallinity to Promote Osteoblast Adhesion Similar to Functionalizing with RGD. Biomaterials 2006, 27, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Åhlén, M.; Tai, C.W.; Bajnóczi, É.G.; de Kleijne, F.; Ferraz, N.; Persson, I.; Strømme, M.; Cheung, O. Highly Porous Amorphous Calcium Phosphate for Drug Delivery and Bio-Medical Applications. Nanomaterials 2020, 10, 20. [Google Scholar] [CrossRef]
- Liljensten, E.; Adolfsson, E.; Strid, K.G.; Thomsen, P. Resorbable and Nonresorbable Hydroxyapatite Granules as Bone Graft Substitutes in Rabbit Cortical Defects. Clin. Implant Dent. Relat. Res. 2003, 5, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, W.; Gui, X.; Song, P.; Lei, H.; Li, Z.; Zhou, C.; Fan, Y.; Zhang, X. 3D Printing of Customized Key Biomaterials Genomics for Bone Regeneration. Appl. Mater. Today 2022, 26, 101346. [Google Scholar] [CrossRef]
- Bornert, F.; Clauss, F.; Hua, G.; Idoux-Gillet, Y.; Keller, L.; Fernandez De Grado, G.; Offner, D.; Smaida, R.; Wagner, Q.; Fioretti, F.; et al. Mechanistic Illustration: How Newly-Formed Blood Vessels Stopped by the Mineral Blocks of Bone Substitutes Can Be Avoided by Using Innovative Combined Therapeutics. Biomedicines 2021, 9, 952. [Google Scholar] [CrossRef]
- Kovrlija, I.; Menshikh, K.; Marsan, O.; Rey, C.; Combes, C.; Locs, J.; Loca, D. Exploring the Formation Kinetics of Octacalcium Phosphate from Alpha-Tricalcium Phosphate: Synthesis Scale-Up, Determination of Transient Phases, Their Morphology and Biocompatibility. Biomolecules 2023, 13, 462. [Google Scholar] [CrossRef]
- Wu, C.-J.; Liu, K.-F.; Liu, C.-M.; Lan, W.-C.; Chu, S.-F.; Shen, Y.-K.; Huang, B.-H.; Huang, J.; Cho, Y.-C.; Ou, K.-L.; et al. An Innovative Biomimetic Porous Bioceramic to Facilitate Bone Tissue Regeneration: Microstructural Characteristics, Biocompatibility, and in Vivo Rabbit Model Evaluation. J. Mater. Res. Technol. 2023, 22, 2566–2575. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-Tricalcium Phosphate for Bone Substitution: Synthesis and Properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Baklouti, O.; Marsan, O.; Salles, F.; Bouajila, J.; El-Feki, H.; Drouet, C. Biomimetic Apatites Functionalized with Antioxidant Phytotherapeutics: The Case of Chlorogenic and Sinapic Phenolic Compounds. Materialia 2024, 38, 102271. [Google Scholar] [CrossRef]
- Iafisco, M.; Carella, F.; Degli Esposti, L.; Adamiano, A.; Catalucci, D.; Modica, J.; Bragonzi, A.; Vitali, A.; Torelli, R.; Sanguinetti, M.; et al. Biocompatible Antimicrobial Colistin Loaded Calcium Phosphate Nanoparticles for the Counteraction of Biofilm Formation in Cystic Fibrosis Related Infections. J. Inorg. Biochem. 2022, 230, 111751. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.G.; Mueller, M.; Vandecandelaere, N.; Trick, I.; Burger-Kentischer, A.; Maucher, T.; Drouet, C. Enzyme-Functionalized Biomimetic Apatites: Concept and Perspectives in View of Innovative Medical Approaches. J. Mater. Sci. Mater. Med. 2014, 25, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Kovrlija, I.; Locs, J.; Loca, D. Octacalcium Phosphate: Innovative Vehicle for the Local Biologically Active Substance Delivery in Bone Regeneration. Acta Biomater. 2021, 135, 27–47. [Google Scholar] [CrossRef]
- Petrakova, N.V.; Teterina, A.Y.; Mikheeva, P.V.; Akhmedova, S.A.; Kuvshinova, E.A.; Sviridova, I.K.; Sergeeva, N.S.; Smirnov, I.V.; Fedotov, A.Y.; Kargin, Y.F.; et al. In Vitro Study of Octacalcium Phosphate Behavior in Different Model Solutions. ACS Omega 2021, 6, 7487–7498. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous Calcium Phosphates: Synthesis, Properties and Uses in Biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [PubMed]
- Desbord, M.; Rey, C. Tunable Behavior in Solution of Amorphous Calcium Ortho/Pyrophosphate Materials: An Acellular In Vitro Study. ACS Biomater. Sci. Eng. 2022, 8, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Somrani, S.; Rey, C.; Jemal, M. Thermal Evolution of Amorphous Tricalcium Phosphate. J. Mater. Chem. 2003, 13, 888–892. [Google Scholar] [CrossRef]
- Le Grill, S.; Soulie, J.; Coppel, Y.; Roblin, P.; Lecante, P.; Marsan, O.; Charvillat, C.; Bertrand, G.; Rey, C.; Brouillet, F. Spray-Drying-Derived Amorphous Calcium Phosphate: A Multi-Scale Characterization. J. Mater. Sci. 2021, 56, 1189–1202. [Google Scholar] [CrossRef]
- Le Grill, S.; Drouet, C.; Marsan, O.; Coppel, Y.; Mazel, V.; Barthelemy, M.-C.; Brouillet, F. Consolidation of Spray-Dried Amorphous Calcium Phosphate by Ultrafast Compression: Chemical and Structural Overview. Nanomaterials 2024, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Grossin, D.; Rollin-Martinet, S.; Estournès, C.; Rossignol, F.; Champion, E.; Combes, C.; Rey, C.; Geoffroy, C.; Drouet, C. Biomimetic Apatite Sintered at Very Low Temperature by Spark Plasma Sintering: Physico-Chemistry and Microstructure Aspects. Acta Biomater. 2010, 6, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C.; Largeot, C.; Raimbeaux, G.; Estournès, C. Bioceramics: Spark Plasma Sintering (SPS) of Calcium Phosphates. Adv. Sci. Technol. 2006, 49, 45–50. [Google Scholar]
- Luginina, M.; Orru, R.; Cao, G.; Luginina, M.; Grossin, D.; Brouillet, F.; Chevallier, G.; Thouron, C.; Drouet, C. First Successful Stabilization of Consolidated Amorphous Calcium Phosphate (ACP) by Cold Sintering: Toward Highly-Resorbable Reactive Bioceramics. J. Mater. Chem. B 2020, 8, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Ortali, C.; Julien, I.; Drouet, C.; Champion, E. Influence of Carbonation on the Low-Temperature Consolidation by Spark Plasma Sintering of Carbonated Calcium Phosphate Bioceramics. Ceram. Int. 2020, 46, 5799–5810. [Google Scholar] [CrossRef]
- Vecchio, G.; Darcos, V.; Grill, S.L.; Brouillet, F.; Coppel, Y.; Duttine, M.; Pugliara, A.; Combes, C.; Soulié, J. Spray-Dried Ternary Bioactive Glass Microspheres: Direct and Indirect Structural Effects of Copper-Doping on Acellular Degradation Behavior. Acta Biomater. 2024, 181, 453–468. [Google Scholar] [CrossRef]
- Erol-Taygun, M.; Unalan, I.; Idris, M.I.B.; Mano, J.F.; Boccaccini, A.R. Bioactıve Glass-Polymer Nanocomposites for Bone Tıssue Regeneration Applicatıons: A Revıew. Adv. Eng. Mater. 2019, 21, 1900287. [Google Scholar] [CrossRef]
- Jones, J.R. Acta Biomaterialia Review of Bioactive Glass: From Hench to Hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Vergnaud, F.; Mekonnen, B.; El Abbassi, A.; Vichery, C.; Nedelec, J.-M. Correlating the Effect of Composition and Textural Properties on Bioactivity for Pristine and Copper-Doped Binary Mesoporous Bioactive Glass Nanoparticles. Materials 2023, 16, 6690. [Google Scholar] [CrossRef]
- Schuhladen, K.; Roether, J.A.; Boccaccini, A.R. Bioactive Glasses Meet Phytotherapeutics: The Potential of Natural Herbal Medicines to Extend the Functionality of Bioactive Glasses. Biomaterials 2019, 217, 119288. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, M.; Gardini, D.; Traverso, P.L.; Faga, M.G.; Bellosi, A. On the Possibility of Silicon Nitride as a Ceramic for Structural Orthopaedic Implants. Part II: Chemical Stability and Wear Resistance in Body Environment. J. Mater. Sci. Mater. Med. 2008, 19, 2889–2901. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, N.; Awad, K.R.; Brotto, M.; Aswath, P.B.; Varanasi, V. A Comparative Study on Silicon Nitride, Titanium and Polyether Ether Ketone on Mouse Pre-Osteoblast Cells. Med. Devices Sens. 2021, 4, e10139. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Laganenka, L.; Du, X.; Hardt, W.-D.; Ferguson, S.J. Silicon Nitride, a Bioceramic for Bone Tissue Engineering: A Reinforced Cryogel System With Antibiofilm and Osteogenic Effects. Front. Bioeng. Biotechnol. 2021, 9, 794586. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G. Silicon Nitride as a Biomaterial. J. Ceram. Soc. Jpn. 2023, 131, 398–428. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic Magnesium Incorporated into PLGA/TCP Porous Scaffold by 3D Printing for Repairing Challenging Bone Defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef]
- Smaida, R.; Pijnenburg, L.; Irusta, S.; Himawan, E.; Mendoza, G.; Harmouch, E.; Idoux-Gillet, Y.; Kuchler-Bopp, S.; Benkirane-Jessel, N.; Hua, G. Potential Implantable Nanofibrous Biomaterials Combined with Stem Cells for Subchondral Bone Regeneration. Materials 2020, 13, 3087. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK Biomaterials in Trauma, Orthopedic, and Spinal Implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Z.; Jiao, Z.; Wu, Z.; Guo, M.; Wang, Y.; Liu, J.; Zhang, P. Enhancing Antibacterial Capability and Osseointegration of Polyetheretherketone (PEEK) Implants by Dual-Functional Surface Modification. Mater. Des. 2021, 205, 109733. [Google Scholar] [CrossRef]
- Usuda, Y.; Okihara, T.; Moriyama, S.; Uemura, T.; Kamanaka, T.; Omi, A.W.; Saito, N.; Takahashi, J.; Aoki, K.; Nishimura, N. Development of Phosphate-Treated PEEK Implants with High Osseointegration. Mater. Today Commun. 2024, 38, 107717. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, H.; Ru, J.; Wang, F.; Xu, M.; Zhou, Q.; Stanikzai, H.; Yerlan, I.; Xu, Z.; Niu, Y.; et al. Creating Micro-Submicro Structure and Grafting Hydroxyl Group on PEEK by Femtosecond Laser and Hydroxylation to Synergistically Activate Cellular Response. Mater. Des. 2021, 199, 109413. [Google Scholar] [CrossRef]
- Durham, J.W.; Montelongo, S.A.; Ong, J.L.; Guda, T.; Allen, M.J.; Rabiei, A. Hydroxyapatite Coating on PEEK Implants: Biomechanical and Histological Study in a Rabbit Model. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 723–731. [Google Scholar] [CrossRef]
- Hu, G.; Zhu, Y.; Xu, F.; Ye, J.; Guan, J.; Jiang, Y.; Di, M.; Li, Z.; Guan, H.; Yao, X. Comparison of Surface Properties, Cell Behaviors, Bone Regeneration and Osseointegration between Nano Tantalum/PEEK Composite and Nano Silicon Nitride/PEEK Composite. J. Biomater. Sci. Polym. Ed. 2022, 33, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, Z.; Sun, A.; Huang, J.; Wu, W.; Chen, M.; Hao, X.; Huang, Z.; Lin, X.; Weng, S. Rapid Construction of Polyetheretherketone (PEEK) Biological Implants Incorporated with Brushite (CaHPO4·2H2O) and Antibiotics for Anti-Infection and Enhanced Osseointegration. Mater. Sci. Eng. C 2020, 111, 110782. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Galotta, A.; Agostinacchio, F.; Motta, A.; Dirè, S.; Sglavo, V.M. Mechanochemical Synthesis and Cold Sintering of Mussel Shell-Derived Hydroxyapatite Nano-Powders for Bone Tissue Regeneration. J. Eur. Ceram. Soc. 2023, 43, 639–647. [Google Scholar] [CrossRef]
- Visan, A.I.; Ristoscu, C.; Popescu-Pelin, G.; Sopronyi, M.; Matei, C.E.; Socol, G.; Chifiriuc, M.C.; Bleotu, C.; Grossin, D.; Brouillet, F.; et al. Composite Drug Delivery System Based on Amorphous Calcium Phosphate–Chitosan: An Efficient Antimicrobial Platform for Extended Release of Tetracycline. Pharmaceutics 2021, 13, 1659. [Google Scholar] [CrossRef]
- Kyyak, S.; Blatt, S.; Wiesmann, N.; Smeets, R.; Kaemmerer, P.W. Hyaluronic Acid with Bone Substitutes Enhance Angiogenesis In Vivo. Materials 2022, 15, 3839. [Google Scholar] [CrossRef] [PubMed]
- Hsia, T.-L.; Lin, Z.; Xia, Y.; Shu, R.; Xie, Y. A Photoresponsive Recombinant Human Amelogenin-Loaded Hyaluronic Acid Hydrogel Promotes Bone Regeneration. J. Periodontal Res. 2024, 59, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Pijnenburg, L.; Idoux-Gillet, Y.; Bornert, F.; Benameur, L.; Tabrizian, M.; Auvray, P.; Rosset, P.; María Gonzalo-Daganzo, R.; Gómez Barrena, E.; et al. Preclinical Safety Study of a Combined Therapeutic Bone Wound Dressing for Osteoarticular Regeneration. Nat. Commun. 2019, 10, 2156. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, M.; Zhang, B.; Gui, X.; Han, Y.; Wang, L.; Zhou, W.; Guo, L.; Zhang, Z.; Li, Z.; et al. DLP Fabricating of Precision GelMA/HAp Porous Composite Scaffold for Bone Tissue Engineering Application. Compos. Part B Eng. 2022, 244, 110163. [Google Scholar] [CrossRef]
- Zheng, A.; Wang, X.; Xin, X.; Peng, L.; Su, T.; Cao, L.; Jiang, X. Promoting Lacunar Bone Regeneration with an Injectable Hydrogel Adaptive to the Microenvironment. Bioact. Mater. 2023, 21, 403–421. [Google Scholar] [CrossRef]
- Wu, S.; Gai, T.; Chen, J.; Chen, X.; Chen, W. Smart Responsive in Situ Hydrogel Systems Applied in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2024, 12, 1389733. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, Z.; Sun, X.; Li, K. Bone Morphogenetic Protein 7-Loaded Gelatin Methacrylate/Oxidized Sodium Alginate/Nano-Hydroxyapatite Composite Hydrogel for Bone Tissue Engineering. Int. J. Nanomed. 2024, 19, 6359–6376. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wu, Y.; Ding, M.; Chen, X.; Zhou, T.; Huang, C.; Wang, X.; Zong, C.; Liu, Y.; Tian, L.; et al. Strong and Tough β-TCP/PCL Composite Scaffolds with Gradient Structure for Bone Tissue Engineering: Development and Evaluation. Ceram. Int. 2024, 50, 31905–31917. [Google Scholar] [CrossRef]
- Pagani, S.; Salerno, M.; Filardo, G.; Locs, J.; van Osch, G.J.V.M.; Vecstaudza, J.; Dolcini, L.; Borsari, V.; Fini, M.; Giavaresi, G.; et al. Human Osteoblasts’ Response to Biomaterials for Subchondral Bone Regeneration in Standard and Aggressive Environments. Int. J. Mol. Sci. 2023, 24, 14764. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qi, X.; Bao, M.; Zhou, T.; Shi, J.; Xu, Z.; Zhou, M.; Boccaccini, A.R.; Zheng, K.; Jiang, X. Biomineralization Inspired 3D Printed Bioactive Glass Nanocomposite Scaffolds Orchestrate Diabetic Bone Regeneration by Remodeling Micromilieu. Bioact. Mater. 2023, 25, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Avnet, S.; Pompo, G.D.; Borciani, G.; Fischetti, T.; Graziani, G.; Baldini, N. Advantages and Limitations of Using Cell Viability Assays for 3D Bioprinted Constructs. Biomed. Mater. 2024, 19, 025033. [Google Scholar] [CrossRef] [PubMed]
- Ravoor, J.; Karuppan, D.; Renold Elsen, S. Binder Optimization for Extrusion Based 3D Printing of Hydroxyapatite Materials Using Different Polymeric Binders. Mater. Today Proc. 2023, in press. [CrossRef]
- Zauchner, D.; Müller, M.Z.; Horrer, M.; Bissig, L.; Zhao, F.; Fisch, P.; Lee, S.S.; Zenobi-Wong, M.; Müller, R.; Qin, X.-H. Synthetic Biodegradable Microporous Hydrogels for in Vitro 3D Culture of Functional Human Bone Cell Networks. Nat. Commun. 2024, 15, 5027. [Google Scholar] [CrossRef]
- Wu, M.; Liu, H.; Li, D.; Zhu, Y.; Wu, P.; Chen, Z.; Chen, F.; Chen, Y.; Deng, Z.; Cai, L. Smart-Responsive Multifunctional Therapeutic System for Improved Regenerative Microenvironment and Accelerated Bone Regeneration via Mild Photothermal Therapy. Adv. Sci. 2024, 11, 2304641. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Wang, S.; Zhang, Y.; Yang, A.; Cheng, Y.; Chen, X. Ultra-Durable Cell-Free Bioactive Hydrogel with Fast Shape Memory and on-Demand Drug Release for Cartilage Regeneration. Nat. Commun. 2023, 14, 7771. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Wang, D.; Mai, H.; Chen, R.; Xu, Y.; Lei, M.; Xie, J.; Tang, Z.; Fu, J.; Chen, Y.; et al. Injectable Thermo-Responsive Poloxamer Hydrogel/Methacrylate Gelatin Microgels Stimulates Bone Regeneration through Biomimetic Programmed Release of SDF-1a and IGF-1. Int. J. Biol. Macromol. 2024, 271, 132742. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Grill, S.; Brouillet, F.; Drouet, C. Bone Regeneration: Mini-Review and Appealing Perspectives. Bioengineering 2025, 12, 38. https://doi.org/10.3390/bioengineering12010038
Le Grill S, Brouillet F, Drouet C. Bone Regeneration: Mini-Review and Appealing Perspectives. Bioengineering. 2025; 12(1):38. https://doi.org/10.3390/bioengineering12010038
Chicago/Turabian StyleLe Grill, Sylvain, Fabien Brouillet, and Christophe Drouet. 2025. "Bone Regeneration: Mini-Review and Appealing Perspectives" Bioengineering 12, no. 1: 38. https://doi.org/10.3390/bioengineering12010038
APA StyleLe Grill, S., Brouillet, F., & Drouet, C. (2025). Bone Regeneration: Mini-Review and Appealing Perspectives. Bioengineering, 12(1), 38. https://doi.org/10.3390/bioengineering12010038








