Medical 3D Printing Using Material Jetting: Technology Overview, Medical Applications, and Challenges
Abstract
1. Introduction
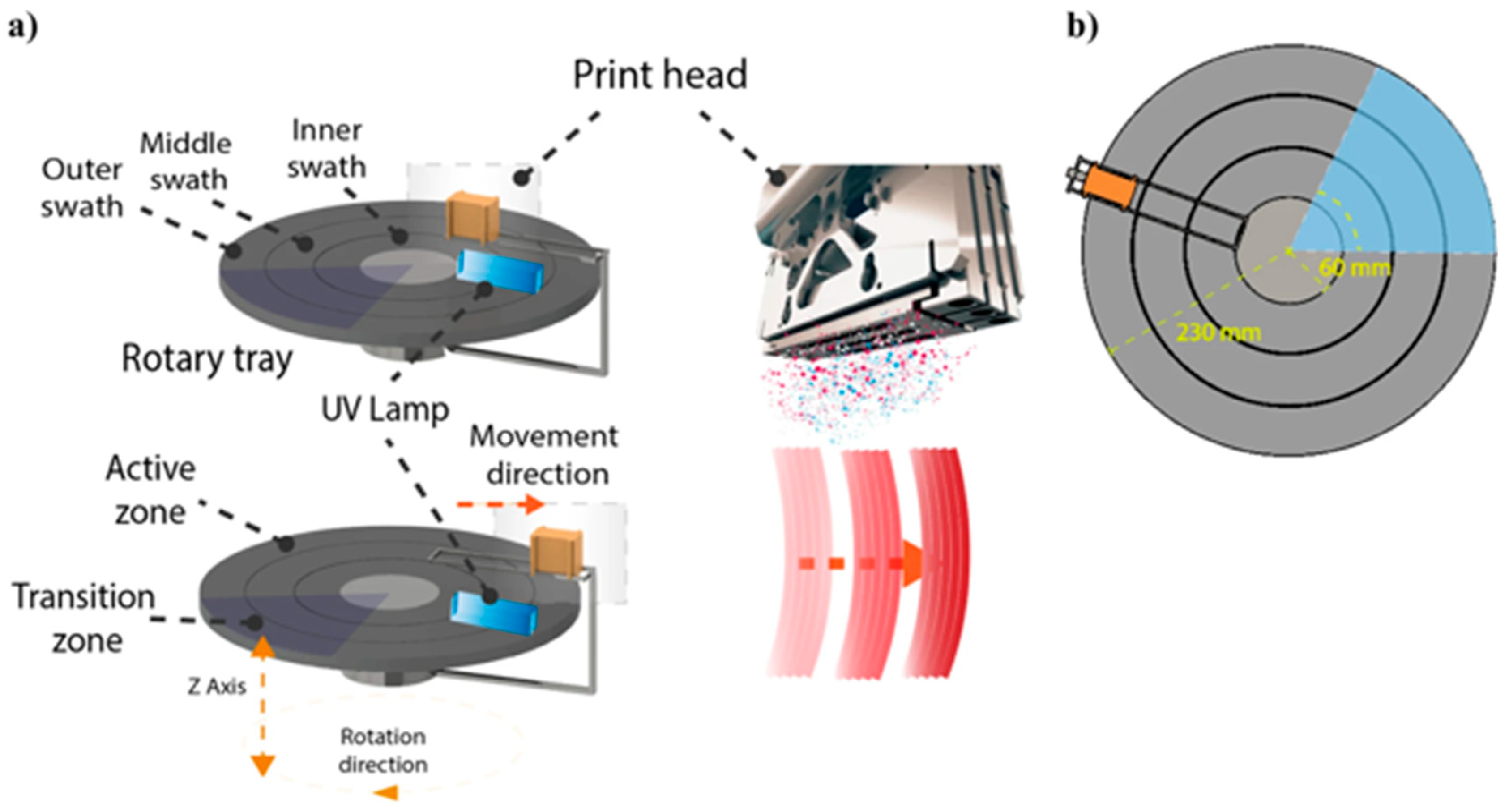
2. Material Jetting (MJT) 3D Printing Technology Overview
3. Literature Review
3.1. Liver
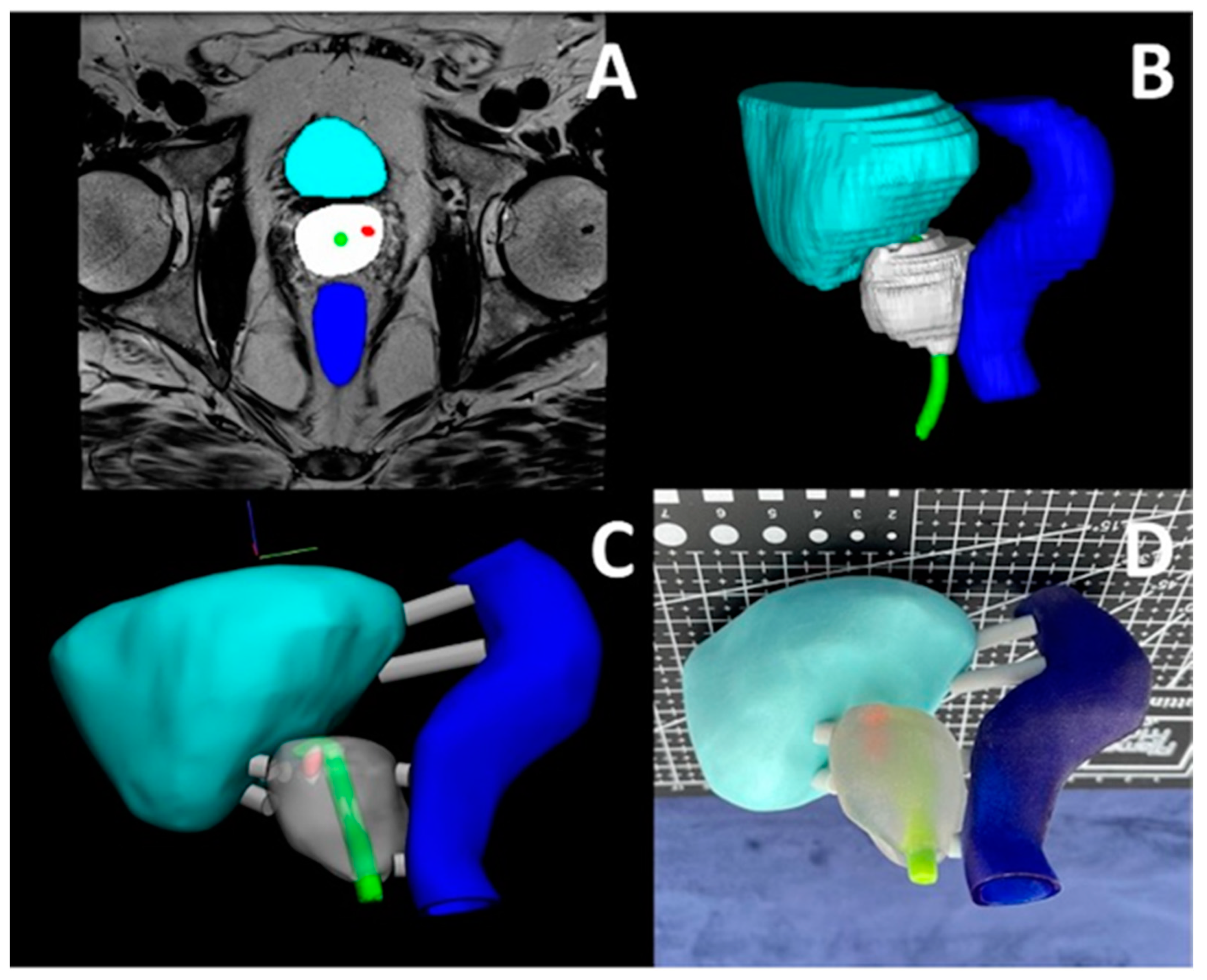
3.2. Prostate, Kidney, and Pelvis
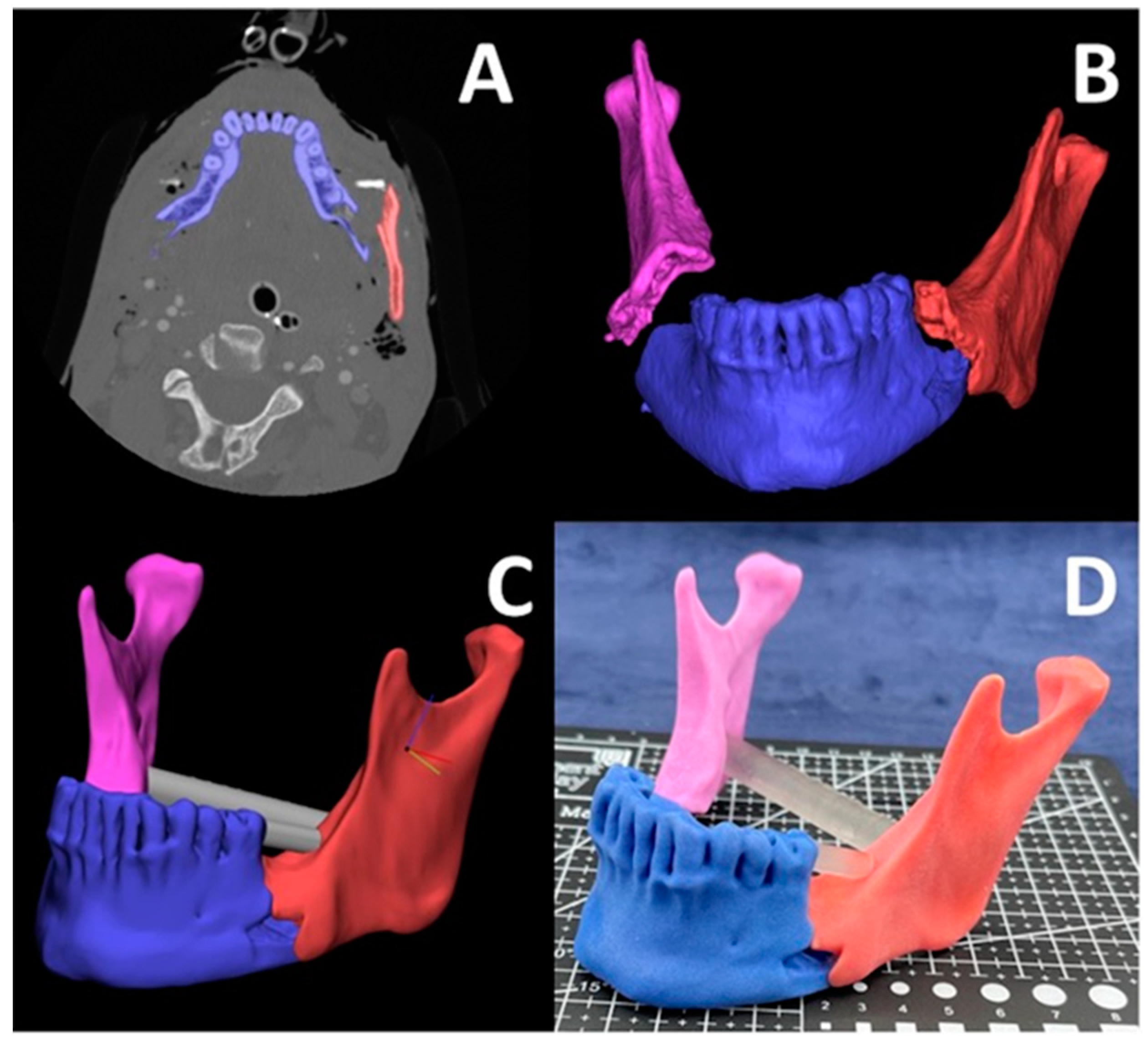
3.3. Oral and Cranio-Maxillofacial
3.4. Ophthalmology
3.5. Phantoms
3.6. Simulators
3.7. Miscellaneous
3.8. University of Cincinnati Radiology 3D Printing Lab Cases
3.9. Mandibular Fracture Reduction
3.10. Complex Hernia Repair
3.11. Prostate Cancer Cryo-Ablation
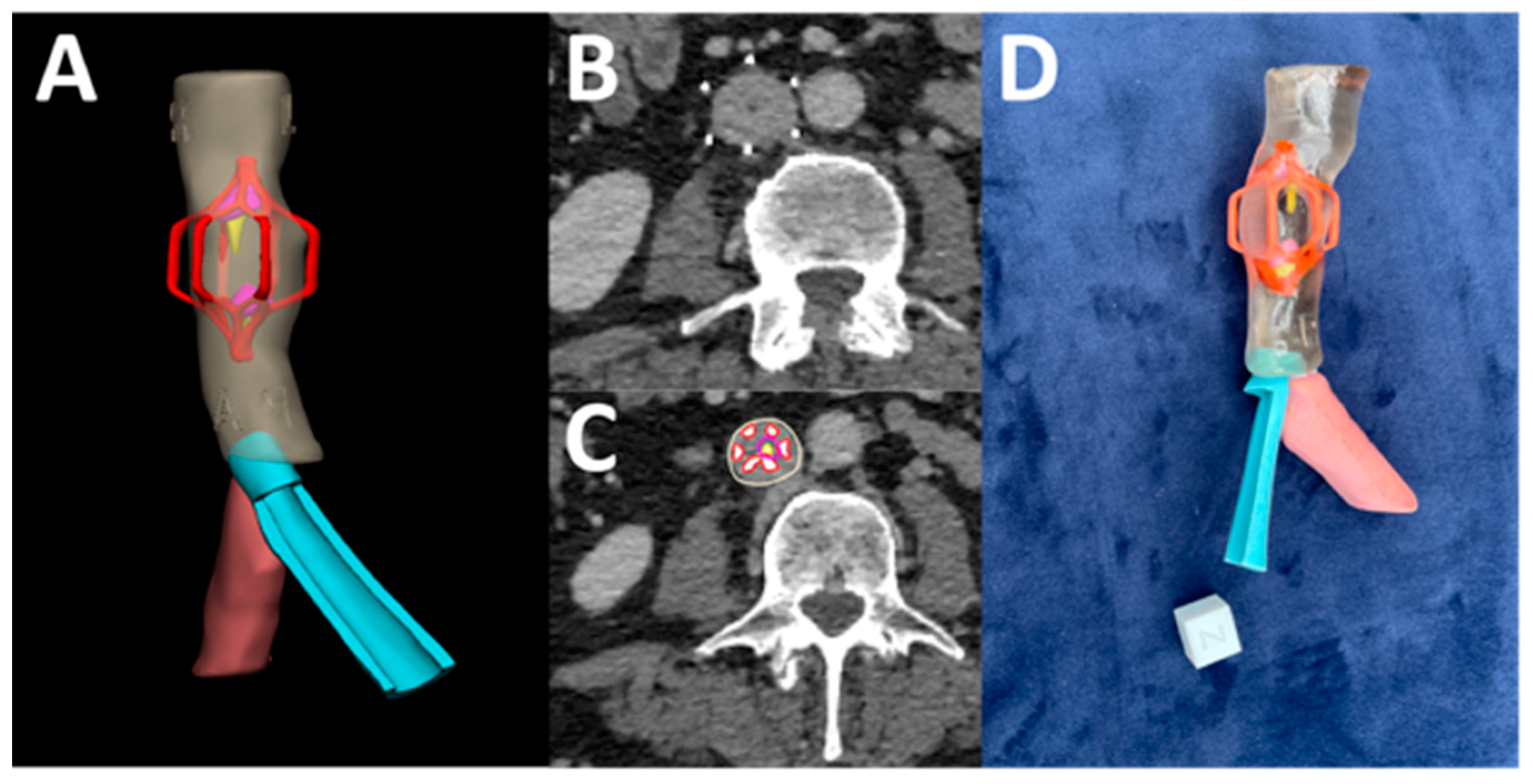
3.12. Complex Inferior Vena Cava (IVC) Filter Removal
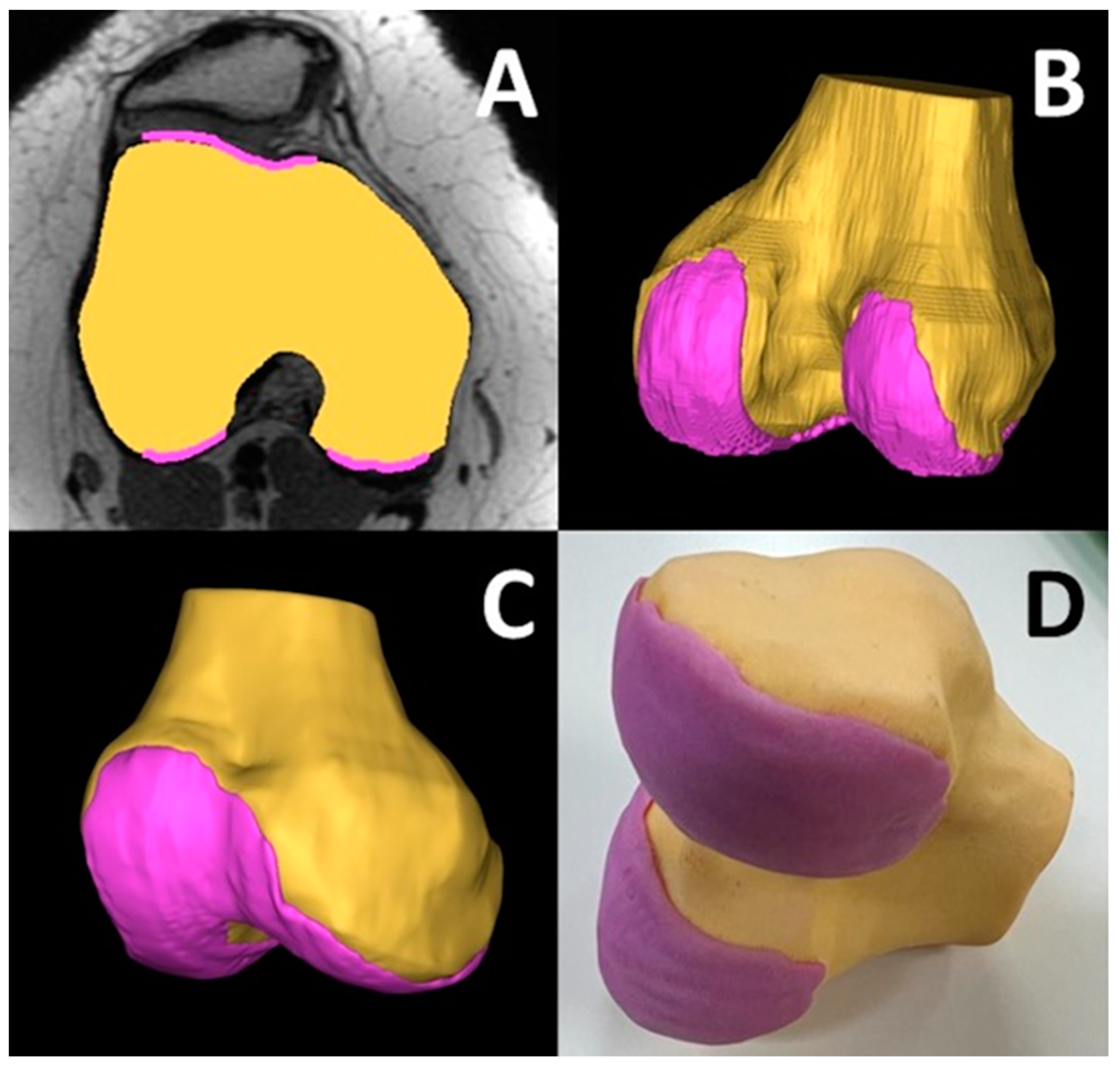
3.13. Patellar Instability Visualization
3.14. Medical Accuracy Studies
3.15. Potential Challenges
3.16. High Cost of Equipment and Materials
3.17. Material Limitations
3.18. Technical Expertise
3.19. Slow Production Speed
3.20. Integration into Clinical Workflow
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitsouras, D.; Liacouras, P.; Imanzadeh, A.; Giannopoulos, A.A.; Cai, T.; Kumamaru, K.K.; George, E.; Wake, N.; Caterson, E.J.; Pomahac, B.; et al. Medical 3D Printing for the Radiologist. RadioGraphics 2015, 35, 1965–1988. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Dhal, K.; Gupta, R.; Tappa, K.; Rybicki, F.J.; Ravi, P. Medical 3D Printing Using Desktop Inverted Vat Photopolymerization: Background, Clinical Applications, and Challenges. Bioengineering 2023, 10, 782. [Google Scholar] [CrossRef]
- Bastawrous, S.; Wu, L.; Liacouras, P.C.; Levin, D.B.; Ahmed, M.T.; Strzelecki, B.; Amendola, M.F.; Lee, J.T.; Coburn, J.; Ripley, B. Establishing 3D Printing at the Point of Care: Basic Principles and Tools for Success. RadioGraphics 2022, 42, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Savini, A.; Savini, G.G. A Short History of 3D Printing, a Technological Revolution Just Started. In Proceedings of the 2015 ICOHTEC/IEEE International History of High-Technologies and Their Socio-Cultural Contexts Conference (HISTELCON), Tel-Aviv, Israel, 18–19 August 2015; pp. 1–8. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. US4575330A, 11 March 1986. [Google Scholar]
- Crump, S.S. Apparatus and Method for Creating Three-Dimensional Objects. US5121329A, 9 June 1992. [Google Scholar]
- Gülcan, O.; Günaydın, K.; Tamer, A. The State of the Art of Material Jetting—A Critical Review. Polymers 2021, 13, 2829. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.C.J.; Forsyth, J.L.; Philbrook, K.F. 3-D Model Maker. US5506607A, 9 April 1996. [Google Scholar]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Material Jetting. In Additive Manufacturing Technologies; Gibson, I., Rosen, D., Stucker, B., Khorasani, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 203–235. [Google Scholar] [CrossRef]
- Benichou, A.; Laufer, L. Tungsten-Carbide/Cobalt Ink Composition for 3D Inkjet Printing. US10913112B2, 9 February 2021. [Google Scholar]
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artés, F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann. Biomed. Eng. 2020, 48, 536–555. [Google Scholar] [CrossRef]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef]
- Kafle, A.; Luis, E.; Silwal, R.; Pan, H.M.; Shrestha, P.L.; Bastola, A.K. 3D/4D Printing of Polymers: Fused Deposition Modelling (FDM), Selective Laser Sintering (SLS), and Stereolithography (SLA). Polymers 2021, 13, 3101. [Google Scholar] [CrossRef]
- Nyberg, E.L.; Farris, A.L.; Hung, B.P.; Dias, M.; Garcia, J.R.; Dorafshar, A.H.; Grayson, W.L. 3D-Printing Technologies for Craniofacial Rehabilitation, Reconstruction, and Regeneration. Ann. Biomed. Eng. 2017, 45, 45–57. [Google Scholar] [CrossRef]
- Kermavnar, T.; Shannon, A.; O’Sullivan, K.J.; McCarthy, C.; Dunne, C.P.; O’Sullivan, L.W. Three-Dimensional Printing of Medical Devices Used Directly to Treat Patients: A Systematic Review. 3D Print. Addit. Manuf. 2021, 8, 366–408. [Google Scholar] [CrossRef]
- Agarwal, P.; Arora, G.; Panwar, A.; Mathur, V.; Srinivasan, V.; Pandita, D.; Vasanthan, K.S. Diverse Applications of Three-Dimensional Printing in Biomedical Engineering: A Review. 3D Print. Addit. Manuf. 2023, 10, 1140–1163. [Google Scholar] [CrossRef]
- Valvez, S.; Oliveira-Santos, M.; Gonçalves, L.; Amaro, A.M.; Piedade, A.P. Preprocedural Planning of Left Atrial Appendage Occlusion: A Review of the Use of Additive Manufacturing. 3D Print. Addit. Manuf. 2023, 11, 333–346. [Google Scholar] [CrossRef]
- Papagelopoulos, P.J.; Savvidou, O.D.; Koutsouradis, P.; Chloros, G.D.; Bolia, I.K.; Sakellariou, V.I.; Kontogeorgakos, V.A.; Mavrodontis, I.I.; Mavrogenis, A.F.; Diamantopoulos, P. Three-Dimensional Technologies in Orthopedics. Orthopedics 2018, 41, 12–20. [Google Scholar] [CrossRef]
- Meglioli, M.; Naveau, A.; Macaluso, G.M.; Catros, S. 3D Printed Bone Models in Oral and Cranio-Maxillofacial Surgery: A Systematic Review. 3D Print. Med. 2020, 6, 30. [Google Scholar] [CrossRef]
- Pucci, J.U.; Christophe, B.R.; Sisti, J.A.; Connolly, E.S.J. Three-Dimensional Printing: Technologies, Applications, and Limitations in Neurosurgery. Biotechnol. Adv. 2017, 35, 521–529. [Google Scholar] [CrossRef]
- Ghazi, A.E.; Teplitz, B.A. Role of 3D Printing in Surgical Education for Robotic Urology Procedures. Transl. Androl. Urol. 2020, 9, 931–941. [Google Scholar] [CrossRef]
- Pugalendhi, A.; Ranganathan, R. A Review of Additive Manufacturing Applications in Ophthalmology. Proc. Inst. Mech. Eng. 2021, 235, 1146–1162. [Google Scholar] [CrossRef]
- Witowski, J.S.; Coles-Black, J.; Zuzak, T.Z.; Pędziwiatr, M.; Chuen, J.; Major, P.; Budzyński, A. 3D Printing in Liver Surgery: A Systematic Review. Telemed. e-Health 2017, 23, 943–947. [Google Scholar] [CrossRef]
- Jakus, A.E.; Huang, Y.-H.; Wake, N. Chapter 16—The Future of Medical 3D Printing in Radiology. In 3D Printing for the Radiologist; Wake, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–214. [Google Scholar] [CrossRef]
- Sireesha, M.; Lee, J.; Kiran, A.S.K.; Babu, V.J.; Kee, B.B.T.; Ramakrishna, S. A Review on Additive Manufacturing and Its Way into the Oil and Gas Industry. RSC Adv. 2018, 8, 22460–22468. [Google Scholar] [CrossRef]
- Alexander, A.E.; Wake, N.; Chepelev, L.; Brantner, P.; Ryan, J.; Wang, K.C. A Guideline for 3D Printing Terminology in Biomedical Research Utilizing ISO/ASTM Standards. 3D Print. Med. 2021, 7, 8. [Google Scholar] [CrossRef]
- Ravi, P.; Chokshi, S. Pre-Processing And Preparation Of Medical 3D Printed Parts. In 3D Printing at Hospitals and Medical Centers; Rybicki, F., Morris, J., Grant, G., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 35–46. [Google Scholar] [CrossRef]
- Golhin, A.P.; Sole, A.S.; Strandlie, A. Color Appearance in Rotational Material Jetting. Int. J. Adv. Manuf. Technol. 2023, 124, 1183–1198. [Google Scholar] [CrossRef]
- Zein, N.N.; Hanouneh, I.A.; Bishop, P.D.; Samaan, M.; Eghtesad, B.; Quintini, C.; Miller, C.; Yerian, L.; Klatte, R. Three-Dimensional Print of a Liver for Preoperative Planning in Living Donor Liver Transplantation. Liver Transplant. 2013, 19, 1304–1310. [Google Scholar] [CrossRef]
- Souzaki, R.; Kinoshita, Y.; Ieiri, S.; Hayashida, M.; Koga, Y.; Shirabe, K.; Hara, T.; Maehara, Y.; Hashizume, M.; Taguchi, T. Three-Dimensional Liver Model Based on Preoperative CT Images as a Tool to Assist in Surgical Planning for Hepatoblastoma in a Child. Pediatr. Surg. Int. 2015, 31, 593–596. [Google Scholar] [CrossRef]
- Soejima, Y.; Taguchi, T.; Sugimoto, M.; Hayashida, M.; Yoshizumi, T.; Ikegami, T.; Uchiyama, H.; Shirabe, K.; Maehara, Y. Three-Dimensional Printing and Biotexture Modeling for Preoperative Simulation in Living Donor Liver Transplantation for Small Infants. Liver Transplant. 2016, 22, 1610–1614. [Google Scholar] [CrossRef]
- Madurska, M.J.; Poyade, M.; Eason, D.; Rea, P.; Watson, A.J.M. Development of a Patient-Specific 3D-Printed Liver Model for Preoperative Planning. Surg. Innov. 2017, 24, 145–150. [Google Scholar] [CrossRef]
- Porpiglia, F.; Bertolo, R.; Checcucci, E.; Amparore, D.; Autorino, R.; Dasgupta, P.; Wiklund, P.; Tewari, A.; Liatsikos, E.; Fiori, C. Development and Validation of 3D Printed Virtual Models for Robot-Assisted Radical Prostatectomy and Partial Nephrectomy: Urologists’ and Patients’ Perception. World J. Urol. 2018, 36, 201–207. [Google Scholar] [CrossRef]
- Bernhard, J.-C.; Isotani, S.; Matsugasumi, T.; Duddalwar, V.; Hung, A.J.; Suer, E.; Baco, E.; Satkunasivam, R.; Djaladat, H.; Metcalfe, C.; et al. Personalized 3D Printed Model of Kidney and Tumor Anatomy: A Useful Tool for Patient Education. World J. Urol. 2016, 34, 337–345. [Google Scholar] [CrossRef]
- Christiansen, A.R.; Shorti, R.M.; Smith, C.D.; Prows, W.C.; Bishoff, J.T. Intraoperative Utilization of Advanced Imaging Modalities in a Complex Kidney Stone Case: A Pilot Case Study. World J. Urol. 2018, 36, 733–743. [Google Scholar] [CrossRef]
- Ajao, M.O.; Clark, N.V.; Kelil, T.; Cohen, S.L.; Einarsson, J.I. Case Report: Three-Dimensional Printed Model for Deep Infiltrating Endometriosis. J. Minim. Invasive Gynecol. 2017, 24, 1239–1242. [Google Scholar] [CrossRef]
- Wake, N.; Bjurlin, M.; Rostami, P.; Chandarana, H.; Huang, W. Three-Dimensional Printing and Augmented Reality: Enhanced Precision for Robotic Assisted Partial Nephrectomy. Urology 2018, 116, 227–228. [Google Scholar] [CrossRef]
- Amparore, D.; Pecoraro, A.; Checcucci, E.; DE Cillis, S.; Piramide, F.; Volpi, G.; Piana, A.; Verri, P.; Granato, S.; Sica, M.; et al. 3D Imaging Technologies in Minimally Invasive Kidney and Prostate Cancer Surgery: Which Is the Urologists’ Perception? Minerva Urol. Nephrol. 2022, 74, 178–185. [Google Scholar] [CrossRef]
- Wang, L.; Ye, X.; Hao, Q.; Chen, Y.; Chen, X.; Wang, H.; Wang, R.; Zhao, Y.; Zhao, J. Comparison of Two Three-Dimensional Printed Models of Complex Intracranial Aneurysms for Surgical Simulation. World Neurosurg. 2017, 103, 671–679. [Google Scholar] [CrossRef]
- Pacione, D.; Tanweer, O.; Berman, P.; Harter, D.H. The Utility of a Multimaterial 3D Printed Model for Surgical Planning of Complex Deformity of the Skull Base and Craniovertebral Junction. J. Neurosurg. 2016, 125, 1194–1197. [Google Scholar] [CrossRef]
- Rose, A.S.; Webster, C.E.; Harrysson, O.L.A.; Formeister, E.J.; Rawal, R.B.; Iseli, C.E. Pre-Operative Simulation of Pediatric Mastoid Surgery with 3D-Printed Temporal Bone Models. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 740–744. [Google Scholar] [CrossRef]
- Mavili, M.E.; Canter, H.I.; Saglam-Aydinatay, B.; Kamaci, S.; Kocadereli, I. Use of Three-Dimensional Medical Modeling Methods for Precise Planning of Orthognathic Surgery. J. Craniofac. Surg. 2007, 18, 740–747. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, Z.; Guan, J.; Zhu, Y.; Liu, Y.; Yang, Z.; Lin, B.; Jiang, Y.; Quan, X.; Ke, Y.; et al. Using Three-Dimensional Printing to Create Individualized Cranial Nerve Models for Skull Base Tumor Surgery. World Neurosurg. 2018, 120, e142–e152. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Elgalal, M.; Loba, P.; Komuński, P.; Arkuszewski, P.; Broniarczyk-Loba, A.; Stefańczyk, L. Clinical Application of 3D Pre-Bent Titanium Implants for Orbital Floor Fractures. J. Cranio-Maxillofac. Surg. 2009, 37, 229–234. [Google Scholar] [CrossRef]
- Park, S.W.; Choi, J.W.; Koh, K.S.; Oh, T.S. Mirror-Imaged Rapid Prototype Skull Model and Pre-Molded Synthetic Scaffold to Achieve Optimal Orbital Cavity Reconstruction. J. Oral Maxillofac. Surg. 2015, 73, 1540–1553. [Google Scholar] [CrossRef]
- Xie, P.; Hu, Z.; Zhang, X.; Li, X.; Gao, Z.; Yuan, D.; Liu, Q. Application of 3-Dimensional Printing Technology to Construct an Eye Model for Fundus Viewing Study. PLoS ONE 2014, 9, e109373. [Google Scholar] [CrossRef]
- Adams, J.W.; Paxton, L.; Dawes, K.; Burlak, K.; Quayle, M.; McMenamin, P.G. 3D Printed Reproductions of Orbital Dissections: A Novel Mode of Visualising Anatomy for Trainees in Ophthalmology or Optometry. Br. J. Ophthalmol. 2015, 99, 1162–1167. [Google Scholar] [CrossRef]
- Ruiters, S.; Sun, Y.; de Jong, S.; Politis, C.; Mombaerts, I. Computer-Aided Design and Three-Dimensional Printing in the Manufacturing of an Ocular Prosthesis. Br. J. Ophthalmol. 2016, 100, 879–881. [Google Scholar] [CrossRef]
- Alam, M.S.; Sugavaneswaran, M.; Arumaikkannu, G.; Mukherjee, B. An Innovative Method of Ocular Prosthesis Fabrication by Bio-CAD and Rapid 3-D Printing Technology: A Pilot Study. Orbit Amst. Neth. 2017, 36, 223–227. [Google Scholar] [CrossRef]
- Sun, M.G.; Rojdamrongratana, D.; Rosenblatt, M.I.; Aakalu, V.K.; Yu, C.Q. 3D Printing for Low Cost, Rapid Prototyping of Eyelid Crutches. Orbit Amst. Neth. 2019, 38, 342–346. [Google Scholar] [CrossRef]
- Gear, J.I.; Long, C.; Rushforth, D.; Chittenden, S.J.; Cummings, C.; Flux, G.D. Development of Patient-Specific Molecular Imaging Phantoms Using a 3D Printer. Med. Phys. 2014, 41, 082502. [Google Scholar] [CrossRef]
- Hatamikia, S.; Oberoi, G.; Unger, E.; Kronreif, G.; Kettenbach, J.; Buschmann, M.; Figl, M.; Knäusl, B.; Moscato, F.; Birkfellner, W. Additively Manufactured Patient-Specific Anthropomorphic Thorax Phantom with Realistic Radiation Attenuation Properties. Front. Bioeng. Biotechnol. 2020, 8, 385. [Google Scholar] [CrossRef]
- Hong, D.; Lee, S.; Kim, T.; Baek, J.H.; Lee, Y.-M.; Chung, K.-W.; Sung, T.-Y.; Kim, N. Development of a Personalized and Realistic Educational Thyroid Cancer Phantom Based on CT Images: An Evaluation of Accuracy between Three Different 3D Printers. Comput. Biol. Med. 2019, 113, 103393. [Google Scholar] [CrossRef]
- Hatamikia, S.; Oberoi, G.; Zacher, A.; Kronreif, G.; Birkfellner, W.; Kettenbach, J.; Ponti, S.; Lorenz, A.; Buschmann, M.; Jaksa, L.; et al. Additively Manufactured Test Phantoms for Mimicking Soft Tissue Radiation Attenuation in CBCT Using Polyjet Technology. Z. Med. Phys. 2023, 33, 168–181. [Google Scholar] [CrossRef]
- Illi, J.; Bernhard, B.; Nguyen, C.; Pilgrim, T.; Praz, F.; Gloeckler, M.; Windecker, S.; Haeberlin, A.; Gräni, C. Translating Imaging Into 3D Printed Cardiovascular Phantoms: A Systematic Review of Applications, Technologies, and Validation. JACC Basic Transl. Sci. 2022, 7, 1050–1062. [Google Scholar] [CrossRef]
- Javan, R.; Ellenbogen, A.L.; Greek, N.; Haji-Momenian, S. A Prototype Assembled 3D-Printed Phantom of the Glenohumeral Joint for Fluoroscopic-Guided Shoulder Arthrography. Skeletal Radiol. 2019, 48, 791–802. [Google Scholar] [CrossRef]
- Shannon, A.; O’Sullivan, K.J.; Clifford, S.; O’Sullivan, L. Assessment and Selection of Filler Compounds for Radiopaque PolyJet Multi-Material 3D Printing for Use in Clinical Settings. Proc. Inst. Mech. Eng. 2022, 236, 740–747. [Google Scholar] [CrossRef]
- Silvestro, E.; Betts, K.N.; Francavilla, M.L.; Andronikou, S.; Sze, R.W. Imaging Properties of Additive Manufactured (3D Printed) Materials for Potential Use for Phantom Models. J. Digit. Imaging 2020, 33, 456–464. [Google Scholar] [CrossRef]
- Ho, B.H.K.; Chen, C.J.; Tan, G.J.S.; Yeong, W.Y.; Tan, H.K.J.; Lim, A.Y.H.; Ferenczi, M.A.; Mogali, S.R. Multi-Material Three Dimensional Printed Models for Simulation of Bronchoscopy. BMC Med. Educ. 2019, 19, 236. [Google Scholar] [CrossRef]
- Hong, D.; Kim, H.; Kim, T.; Kim, Y.-H.; Kim, N. Development of Patient Specific, Realistic, and Reusable Video Assisted Thoracoscopic Surgery Simulator Using 3D Printing and Pediatric Computed Tomography Images. Sci. Rep. 2021, 11, 6191. [Google Scholar] [CrossRef]
- Dissanayaka, N.; Maclachlan, L.R.; Alexander, H.; Redmond, M.; Carluccio, D.; Jules-Vandi, L.; Novak, J.I. Evaluation of 3D Printed Burr Hole Simulation Models Using 8 Different Materials. World Neurosurg. 2023, 176, e651–e663. [Google Scholar] [CrossRef]
- Zammit, D.; Safran, T.; Ponnudurai, N.; Jaberi, M.; Chen, L.; Noel, G.; Gilardino, M.S. Step-Specific Simulation: The Utility of 3D Printing for the Fabrication of a Low-Cost, Learning Needs-Based Rhinoplasty Simulator. Aesthet. Surg. J. 2020, 40, NP340–NP345. [Google Scholar] [CrossRef]
- Alrasheed, A.S.; Nguyen, L.H.P.; Mongeau, L.; Funnell, W.R.J.; Tewfik, M.A. Development and Validation of a 3D-Printed Model of the Ostiomeatal Complex and Frontal Sinus for Endoscopic Sinus Surgery Training. Int. Forum Allergy Rhinol. 2017, 7, 837–841. [Google Scholar] [CrossRef]
- Molinari, G.; Emiliani, N.; Cercenelli, L.; Bortolani, B.; Gironi, C.; Fernandez, I.J.; Presutti, L.; Marcelli, E. Assessment of a Novel Patient-Specific 3D Printed Multi-Material Simulator for Endoscopic Sinus Surgery. Front. Bioeng. Biotechnol. 2022, 10, 974021. [Google Scholar] [CrossRef]
- Narayanan, V.; Narayanan, P.; Rajagopalan, R.; Karuppiah, R.; Rahman, Z.A.A.; Wormald, P.-J.; Van Hasselt, C.A.; Waran, V. Endoscopic Skull Base Training Using 3D Printed Models with Pre-Existing Pathology. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 753–757. [Google Scholar] [CrossRef]
- Rose, A.S.; Kimbell, J.S.; Webster, C.E.; Harrysson, O.L.A.; Formeister, E.J.; Buchman, C.A. Multi-Material 3D Models for Temporal Bone Surgical Simulation. Ann. Otol. Rhinol. Laryngol. 2015, 124, 528–536. [Google Scholar] [CrossRef]
- Wang, X.; Shujaat, S.; Shaheen, E.; Jacobs, R. Quality and Haptic Feedback of Three-Dimensionally Printed Models for Simulating Dental Implant Surgery. J. Prosthet. Dent. 2022, 131, 660–667. [Google Scholar] [CrossRef]
- Cahuana-Bartra, P.; Cahuana-Cárdenas, A.; Brunet-Llobet, L.; Ayats-Soler, M.; Miranda-Rius, J.; Rivera-Baró, A. The Use of 3D Additive Manufacturing Technology in Autogenous Dental Transplantation. 3D Print. Med. 2020, 6, 16. [Google Scholar] [CrossRef]
- Richard, Z.; Jackson, E.; Jung, J.P.; Kanotra, S.P. Feasibility and Potential of Three-Dimensional Printing in Laryngotracheal Stenosis. J. Laryngol. Otol. 2019, 133, 530–534. [Google Scholar] [CrossRef]
- Radzi, S.; Tan, H.K.J.; Tan, G.J.S.; Yeong, W.Y.; Ferenczi, M.A.; Low-Beer, N.; Mogali, S.R. Development of a Three-Dimensional Printed Heart from Computed Tomography Images of a Plastinated Specimen for Learning Anatomy. Anat. Cell Biol. 2020, 53, 48–57. [Google Scholar] [CrossRef]
- Bergquist, J.R.; Morris, J.M.; Matsumoto, J.M.; Schiller, H.J.; Kim, B.D. 3D Printed Modeling Contributes to Reconstruction of Complex Chest Wall Instability. Trauma Case Rep. 2019, 22, 100218. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Huettig, F.; Kraemer-Fernandez, P.; Spintzyk, S. Multi-Material 3D Printing of a Customized Sports Mouth Guard: Proof-of-Concept Clinical Case. Int. J. Environ. Res. Public. Health 2021, 18, 12762. [Google Scholar] [CrossRef]
- Parthasarathy, J.; Jonard, B.; Rees, M.; Selvaraj, B.; Scharschmidt, T. Virtual Surgical Planning and 3D Printing in Pediatric Musculoskeletal Oncological Resections: A Proof-of-Concept Description. Int. J. Comput. Assist. Radiol. Surg. 2023, 18, 95–104. [Google Scholar] [CrossRef]
- Lyons, J.G.; Hudson, T.L.; Krishnamurthy, A.B. Epidemiology of Patellar Dislocations in the United States from 2001 to 2020: Results of a National Emergency Department Database. Phys. Sportsmed. 2022, 52, 26–35. [Google Scholar] [CrossRef]
- Rémy, F.; Chantelot, C.; Fontaine, C.; Demondion, X.; Migaud, H.; Gougeon, F. Inter- and Intraobserver Reproducibility in Radiographic Diagnosis and Classification of Femoral Trochlear Dysplasia. Surg. Radiol. Anat. SRA 1998, 20, 285–289. [Google Scholar] [CrossRef]
- Stepanovich, M.; Bomar, J.D.; Pennock, A.T. Are the Current Classifications and Radiographic Measurements for Trochlear Dysplasia Appropriate in the Skeletally Immature Patient? Orthop. J. Sports Med. 2016, 4, 2325967116669490. [Google Scholar] [CrossRef]
- Nelitz, M.; Lippacher, S.; Reichel, H.; Dornacher, D. Evaluation of Trochlear Dysplasia Using MRI: Correlation between the Classification System of Dejour and Objective Parameters of Trochlear Dysplasia. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 120–127. [Google Scholar] [CrossRef]
- Kazley, J.M.; Banerjee, S. Classifications in Brief: The Dejour Classification of Trochlear Dysplasia. Clin. Orthop. 2019, 477, 2380–2386. [Google Scholar] [CrossRef]
- Beitler, B.G.; Yu, K.E.; Wang, A.; Frumberg, D.B.; Tommasini, S.M.; Wiznia, D.H.; Cooperman, D.R.; Lattanza, L.L.; Fulkerson, J.P. Three-Dimensional Printing of the Patellofemoral Joints of Patellar Instability Patients. Arthrosc. Tech. 2023, 12, e401–e406. [Google Scholar] [CrossRef]
- Chen, L.; Lin, W.-S.; Polido, W.D.; Eckert, G.J.; Morton, D. Accuracy, Reproducibility, and Dimensional Stability of Additively Manufactured Surgical Templates. J. Prosthet. Dent. 2019, 122, 309–314. [Google Scholar] [CrossRef]
- Kim, T.; Lee, S.; Kim, G.B.; Hong, D.; Kwon, J.; Park, J.-W.; Kim, N. Accuracy of a Simplified 3D-Printed Implant Surgical Guide. J. Prosthet. Dent. 2020, 124, 195–201.e2. [Google Scholar] [CrossRef]
- Wang, X.; Shujaat, S.; Shaheen, E.; Jacobs, R. Accuracy of Desktop versus Professional 3D Printers for Maxillofacial Model Production. A Systematic Review and Meta-Analysis. J. Dent. 2021, 112, 103741. [Google Scholar] [CrossRef]
- Akyalcin, S.; Rutkowski, P.; Arrigo, M.; Trotman, C.A.; Kasper, F.K. Evaluation of Current Additive Manufacturing Systems for Orthodontic 3-Dimensional Printing. Am. J. Orthod. Dentofac. Orthop. 2021, 160, 594–602. [Google Scholar] [CrossRef]
- Dorweiler, B.; Baqué, P.E.; Chaban, R.; Ghazy, A.; Salem, O. Quality Control in 3D Printing: Accuracy Analysis of 3D-Printed Models of Patient-Specific Anatomy. Materials 2021, 14, 1021. [Google Scholar] [CrossRef]
- Herschdorfer, L.; Negreiros, W.M.; Gallucci, G.O.; Hamilton, A. Comparison of the Accuracy of Implants Placed with CAD-CAM Surgical Templates Manufactured with Various 3D Printers: An in Vitro Study. J. Prosthet. Dent. 2021, 125, 905–910. [Google Scholar] [CrossRef]
- Lüchtenborg, J.; Willems, E.; Zhang, F.; Wesemann, C.; Weiss, F.; Nold, J.; Sun, J.; Sandra, F.; Bai, J.; Reveron, H.; et al. Accuracy of Additively Manufactured Zirconia Four-Unit Fixed Dental Prostheses Fabricated by Stereolithography, Digital Light Processing and Material Jetting Compared with Subtractive Manufacturing. Dent. Mater. 2022, 38, 1459–1469. [Google Scholar] [CrossRef]
- Naeem, O.A.; Bencharit, S.; Yang, I.-H.; Stilianoudakis, S.C.; Carrico, C.; Tüfekçi, E. Comparison of 3-Dimensional Printing Technologies on the Precision, Trueness, and Accuracy of Printed Retainers. Am. J. Orthod. Dentofac. Orthop. 2022, 161, 582–591. [Google Scholar] [CrossRef]
- Németh, A.; Vitai, V.; Czumbel, M.L.; Szabó, B.; Varga, G.; Kerémi, B.; Hegyi, P.; Hermann, P.; Borbély, J. Clear Guidance to Select the Most Accurate Technologies for 3D Printing Dental Models—A Network Meta-Analysis(✰). J. Dent. 2023, 134, 104532. [Google Scholar] [CrossRef]
- Rouzé l’Alzit, F.; Cade, R.; Naveau, A.; Babilotte, J.; Meglioli, M.; Catros, S. Accuracy of Commercial 3D Printers for the Fabrication of Surgical Guides in Dental Implantology. J. Dent. 2022, 117, 103909. [Google Scholar] [CrossRef]
- Wegmüller, L.; Halbeisen, F.; Sharma, N.; Kühl, S.; Thieringer, F.M. Consumer vs. High-End 3D Printers for Guided Implant Surgery-An In Vitro Accuracy Assessment Study of Different 3D Printing Technologies. J. Clin. Med. 2021, 10, 4894. [Google Scholar] [CrossRef]
- Maurya, N.K.; Rastogi, V.; Singh, P. Comparative Study and Measurement of Form Errors for the Component Printed by FDM and PolyJet Process. Instrum. Mes. Métrologie 2019, 18, 353–359. [Google Scholar] [CrossRef]





| Human Anatomy and Application | 3D Printer Specific Technology with Materials | MJT 3DP Specific Application | Medical Device | Ref. |
|---|---|---|---|---|
| Liver | Objet Connex 350; TangoPlus, VeroBlue, VeroClearPlus, TangoBlackPlus | Pre-operative planning for LDLT | Anatomic Model | [29] |
| Liver | Objet 500 Connex 3; Acrylic Resin | Pre-operative planning for pediatric hepatoblastoma | Anatomic Model | [30] |
| Liver | Objet Connex500; TangoPlus, TangoBlackPlus | Pre-operative planning for LDLT in infants | Anatomic Model | [31] |
| Liver | Objet Eden 350V; TangoPlus, TangoBlack | Feasibility study for printing liver models | Anatomic Model | [32] |
| Prostate | * | Surgical planning for robotic assisted radical prostatectomy | Anatomic Model | [33] |
| Kidney | Objet 500 Connex 3 * | Patient education of kidney and kidney tumor anatomy | Anatomic Model | [34] |
| Kidney | Objet Connex3 * | Intra-operatively assistance in complex kidney stone case | Anatomic Model | [35] |
| Pelvis | Stratasys J750 * | Surgical planning for endometriosis | [36] | |
| Kidney | * | Pre-operative and intra-operative assistance in robotic assisted partial nephrectomy | Anatomic Model | [37] |
| Prostate | * | Feasibility/utility of prostate tumor models | Anatomic Model | [38] |
| Oral; Cranio-maxillofacial | Objet Connex 350; Molding Silicone | Pre-operative planning for complex intracranial aneurysms | Anatomic Model | [39] |
| Oral; Cranio-maxillofacial | Objet 260 Dental Selection; VeroWhite, VeroMagenta, VeroBlack | Pre-operative planning of complex deformities of the skull base and craniovertebral junction | Anatomic Model | [40] |
| Oral; Cranio-maxillofacial | Objet 350 Connex * | Pre-operative planning of pediatric mastoid surgery | Anatomic Model | [41] |
| Oral; Cranio-maxillofacial | Spectrum Z 510 3D Color Printer * | Pre-operative planning of surgery treating mandibular prognathism | Anatomic Model | [42] |
| Oral; Cranio-maxillofacial | Objet 350 Connex 3; VeroCyan, VeroMajenta, VeroYellow | Pre-operative planning of skull base and tumor surgery | Anatomic Model | [43] |
| Ophthalmology | Objet * | Fitting implants pre-operatively for surgery treating orbital floor fractures | Surgical Template; Surgical Guide | [44] |
| Ophthalmology | Projet 660 Pro * | Intra-operative assistance for orbital defect reconstruction | Surgical Template | [45] |
| Ophthalmology | Projet 3510 HD * | Eye model for fundus viewing | Anatomic Model | [46] |
| Ophthalmology | 3D Systems Z650; Visijet C4 Spectrum | Dissection eye model for medical student training | Anatomic Model | [47] |
| Ophthalmology | Objet Connex 350; MED 610 | Eye prosthesis used in patient with acquired anophthalmos | Prosthesis | [48] |
| Ophthalmology | * | Ocular prosthetic in patient case | Prosthesis | [49] |
| Ophthalmology | Objet 30 Prime; MED610 | Eye crutches for blepharoptosis | Prosthesis | [50] |
| Radiology | Objet Eden 500V; VeroClear | Molecular imaging phantoms including liver with liver tumor | Radiologic Phantom | [51] |
| Radiology | Objet 500 Connex 3; Vero Pure White, Flexible Agilus30 Clear | Anthropomorphic thorax phantom | Radiologic Phantom | [52] |
| Radiology | * | Thyroid cancer phantom | Radiologic Phantom | [53] |
| Radiology | Objet 500 Connex 3; VeroClear, TangoPlus, Vero Pure-White | Soft tissue phantoms | Radiologic Phantom | [54] |
| Radiology | * | Cardiovascular phantoms | Radiologic Phantom | [55] |
| Radiology | Tangoplus * | Phantom of glenohumeral joint | Radiologic Phantom | [56] |
| Radiology | MED610, TangoPlus, VeroWhite * | Best MJT filler compound to achieve radiopaqueness | ---------------- | [57] |
| Radiology | VeroClear, Tango * | Imagining properties of MJT materials | ---------------- | [58] |
| Pulmonology | Objet 500 Connex 3; FullCure RGD851, VeroMagenta, FullCure 930, TangoPlus | Bronchoscopic simulator | Surgical Simulator | [59] |
| Pulmonology | Objet 500 Connex 3; Vero Color, Aglius | Thoracoscopic simulator | Surgical Simulator | [60] |
| Neurosurgery | Stratasys J750; SUP706, BoneTM, SkullTM | Burr hole procedure simulator | Surgical Simulator | [61] |
| Plastic surgery | Objet 500; Shore A75, Shore A85 | Rhinoplasty simulator | Surgical Simulator | [62] |
| Otolaryngology | Objet 500 Connex; VeroWhitePlus, TangoPlus | Endoscopic sinus surgical simulator | Surgical Simulator | [63] |
| Otolaryngology | Stratasys J720 Dental; VeroWhitePlus, VeroMagenta, Agilus30 | Endoscopic sinus surgical simulator | Surgical Simulator | [64] |
| Otolaryngology | Objet Connex 500 * | Endoscopic skull base surgical simulator | Surgical Simulator | [65] |
| Otolaryngology | Objet 350 Connex * | Temporal bone surgical simulator | Surgical Simulator | [66] |
| Dental | Objet Connex 350; Acrylic Based Resin | Dental implant surgical simulator | Surgical Simulator | [67] |
| Dental | Objet 30 Prime; MED610 | Fabrication of tooth to be used in transplantation | Implant | [68] |
| Otolaryngology | Objet Connex; Materialize HeartprintTM | Feasibility of models replicating laryngotracheal stenosis | Anatomic Model | [69] |
| Cardiology | Objet 500 Connex 3 * | Heart model to be used in student education | Anatomic Model | [70] |
| Emergency medicine | Objet 500 * | Mapping chest wall stability for thoracotomy | Anatomic Model | [71] |
| Dental | Stratasys J750; Agilus30 | Oral sports mouth guard | Unclassified | [72] |
| Orthopedics | Objet 350 Connex 3 * | Pre-operative planning of surgery treating musculoskeletal tumors | Anatomic Model | [73] |
| Description | Three-Dimensional Printer(s) and Materials | Accuracy Results | Reference |
|---|---|---|---|
| Surgical template accuracy between VP, SLS, and MJT using scanning of printed object and comparing with designed files | (VP—SLA) Form 2; Dental SG Resin (MJT) Objet Eden260VS; MED610 (SLS) ProX DMP 200; LaserForm Co-Cr | MJT was concluded to have the greatest accuracy and highest reproducibility | [80] |
| Surgical guides printed with MJT—PolyJet and multijet—and VP technology compared using four different caliper measurements compared with designed files | (VP – SLA) Form 2; Clear Resin (MJT) Objet 500 Connex3; Vero Magenta (MJT) ProJet 3510 SD; VisiJet Cristal | The guide printed with the Objet 500 Connex3 (MJT) was considered to have greatest accuracy compared with the ProJet 3510 SD (MJT) and VP 3D printer | [81] |
| 35 models of large and small vessel were printed using MEX, and MJT. The models were than analyzed for accuracy using CT scanning and comparing model formed from resulting DICOM with original STL file. | (MEX) Ultimaker 2; Polylactic Acid (PLA) (MJT) Objet 30 Prime; Tango Series | MJT printing technology was considered to be comparable in accuracy with VP | [84] |
| Meta-analysis accuracy study in 2021 comparing accuracy between the various 3DP technologies | - | Revealed that MJT and SLS 3DP offered the lowest absolute mean difference in terms of accuracy (0.09 mm) | [82] |
| Comparison of accuracy between MJT – PolyJet and multijet and VP in mandibular surgical templates | (VP—SLA) Form 2; Dental SG Resin (MJT) Objet Eden260VS; Veroclear (MJT) ProJet 3500; VisiJet Stoneplast | Found that the 3DP technology had no significant effect on the accuracy of guided mandibular implant surgery | [85] |
| Comparison of accuracy between MJT, MEX, and VP 3DP technology for drill guides using scanner | (VP—SLA) Form 3; Dental SG Resin (VP—DLP) Wanhao Duplicator 7 Plus; Freeprint ortho 405 (MJT) Objet30 Prime; MED610, SUP705 (MEX) Ultimaker 3 Extended; Nylon680, ProFillTM polyvinyl alcohol | MJT and VP 3DP offered the greatest accuracy for the drill guides; however, there was no significant difference in accuracy between them | [90] |
| Comparison of 3DP technology accuracy between MJT and VP for retainers using landmark measurements | (VP—SLA)—Form 3 * (VP—DLP)—Moonray * (VP—cDLP)—Envision One cDLM Dental * (MJT)—Objet Eden260VS * | The VP and MJT technologies were concluded to have no significant difference in accuracy for 3DP retainers | [87] |
| Accuracy of dental surgical guides between VP, MEX, SLS, and MJT | (VP—SLA)—Form 2 * (VP—DLP) – Rapid Shape D40 * (VP—DLP)—Cara Print 4.0 * (MJT)—Stratasys J750 * (MEX)—Raise 3D Pro2 * SLS—Prodways P1000 * | VP and MJT technologies were concluded to have no significant difference between each other | [89] |
| Accuracy of dental maxillary and mandibular guides between VP and MJT | (VP—SLA)—Form 2 * (VP—DLP)—Juell 3D * (VP—DLS)—Carbon M2 * (MJT)—Objet Eden 260VS * | No significant difference between guides fabricated with MJT and VP was found | [83] |
| Systematic search of accuracy in full-arch dental models formed from VP, MEX, and MJT | - | The accuracy between the VP and MJT 3DP technologies did not portray a significant difference | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chokshi, S.; Gangatirkar, R.; Kandi, A.; DeLeonibus, M.; Kamel, M.; Chadalavada, S.; Gupta, R.; Munigala, H.; Tappa, K.; Kondor, S.; et al. Medical 3D Printing Using Material Jetting: Technology Overview, Medical Applications, and Challenges. Bioengineering 2025, 12, 249. https://doi.org/10.3390/bioengineering12030249
Chokshi S, Gangatirkar R, Kandi A, DeLeonibus M, Kamel M, Chadalavada S, Gupta R, Munigala H, Tappa K, Kondor S, et al. Medical 3D Printing Using Material Jetting: Technology Overview, Medical Applications, and Challenges. Bioengineering. 2025; 12(3):249. https://doi.org/10.3390/bioengineering12030249
Chicago/Turabian StyleChokshi, Shivum, Raghav Gangatirkar, Anish Kandi, Maria DeLeonibus, Mohamed Kamel, Seetharam Chadalavada, Rajul Gupta, Harshitha Munigala, Karthik Tappa, Shayne Kondor, and et al. 2025. "Medical 3D Printing Using Material Jetting: Technology Overview, Medical Applications, and Challenges" Bioengineering 12, no. 3: 249. https://doi.org/10.3390/bioengineering12030249
APA StyleChokshi, S., Gangatirkar, R., Kandi, A., DeLeonibus, M., Kamel, M., Chadalavada, S., Gupta, R., Munigala, H., Tappa, K., Kondor, S., Burch, M. B., & Ravi, P. (2025). Medical 3D Printing Using Material Jetting: Technology Overview, Medical Applications, and Challenges. Bioengineering, 12(3), 249. https://doi.org/10.3390/bioengineering12030249







