Progress in Development of Functional Biological and Synthetic Blood Products to Augment Transfusable Blood Supply in Operational Medicine
Abstract
1. Introduction
2. Traditional Whole Blood Donations
3. Walking Blood Bank
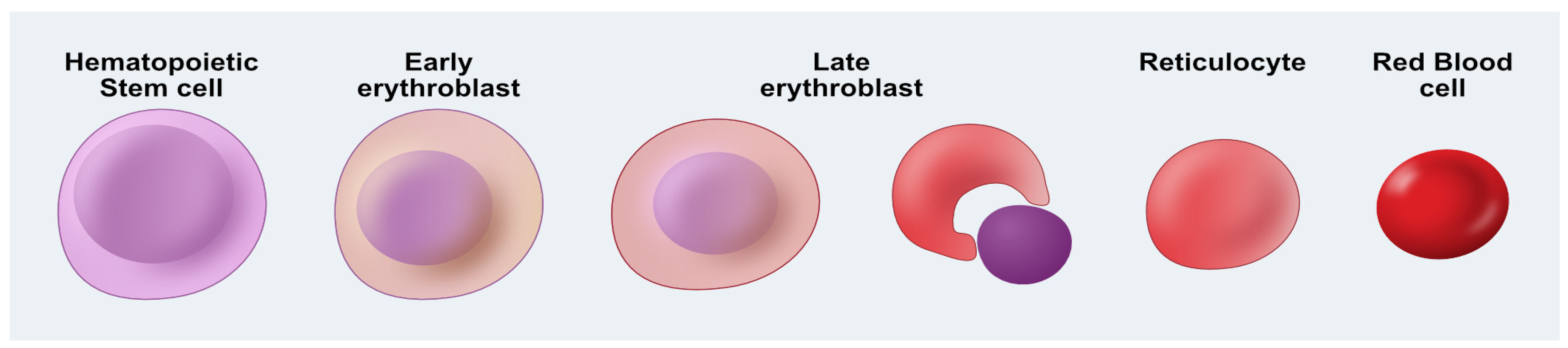
4. Manufactured Blood—Erythropoiesis In Vitro
5. Synthetic Blood Products
6. Future Prospects: Opportunities for Blood Bioengineering
7. Summary
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RBC | Red blood cell |
| WBB | Walking blood bank |
| HSC | Hematopoietic stem cell |
| HBOC | Hemoglobin based oxygen carrier |
| PFC | Perfluoro-Chemicals |
| IPSCs | Induced Pluripotent Stem Cells |
| UCB | Umbilical cord blood |
| SCF | Stem cell factor |
| Hb | Hemoglobin |
| IL-3 | Interleukin-3 |
| EPO | Erythropoietin |
| 2,3-DPG | 2,3-diphosphoglycerate |
| Oct4 | Octamer-binding transcription factor 4 |
| KLF1 | Krüppel-like factor 1 |
| Sox2 | SRY-box 2 |
| NANOG | Nanog homeobox |
References
- Cazzola, M. Introduction to a review series on transfusion medicine. Blood 2019, 133, 1793–1794. [Google Scholar] [CrossRef]
- Spinella, P.C. Warm fresh whole blood transfusion for severe hemorrhage: U.S. military and potential civilian applications. Crit. Care Med. 2008, 36, S340–S345. [Google Scholar] [CrossRef]
- Kaur, P.; Bedi, R.K.; Mittal, K.; Sood, T. Exploring the unseen effect of COVID 19 pandemic on blood transfusion services in a tertiary care centre. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapheresis 2023, 62, 103569. [Google Scholar] [CrossRef] [PubMed]
- Chiem, C.; Alghamdi, K.; Nguyen, T.; Han, J.H.; Huo, H.; Jackson, D. The Impact of COVID-19 on Blood Transfusion Services: A Systematic Review and Meta-Analysis. Transfus. Med. Hemother. 2021, 49, 107–118. [Google Scholar] [CrossRef]
- Stanworth, S.J.; New, H.V.; Apelseth, T.O.; Brunskill, S.; Cardigan, R.; Doree, C.; Germain, M.; Goldman, M.; Massey, E.; Prati, D.; et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020, 7, e756–e764. [Google Scholar] [CrossRef]
- McGann, P.T.; Weyand, A.C. Lessons learned from the COVID-19 pandemic blood supply crisis. J. Hosp. Med. 2022, 17, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Anania, K.; DeCicco, A.; Hamm, J.A. Toward Resiliency in the Joint Blood Supply Chain. Rand Health Q. 2019, 8, 9. [Google Scholar] [PubMed]
- McCoy, C.C.; Brenner, M.; Duchesne, J.; Roberts, D.; Ferrada, P.; Horer, T.; Kauvar, D.; Khan, M.; Kirkpatrick, A.; Ordonez, C.; et al. Back to the Future: Whole Blood Resuscitation of the Severely Injured Trauma Patient. Shock 2021, 56, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chandler, M.H.; Roberts, M.; Sawyer, M.; Myers, G. The US military experience with fresh whole blood during the conflicts in Iraq and Afghanistan. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.M.; Spinella, P.C. Blood transfusion management in the severely bleeding military patient. Curr. Opin. Anaesthesiol. 2018, 31, 207–214. [Google Scholar] [CrossRef]
- Ti, C.R. The strategic vulnerability of NATO blood supply logistics: A case study of Estonian national defence. Def. Secur. Anal. 2022, 38, 369–388. [Google Scholar] [CrossRef]
- Spinella, P.C.; Dunne, J.; Beilman, G.J.; O’Connell, R.J.; Borgman, M.A.; Cap, A.P.; Rentas, F. Constant challenges and evolution of US military transfusion medicine and blood operations in combat. Transfusion 2012, 52, 1146–1153. [Google Scholar] [CrossRef]
- Shankar, A.; Akulwar, A.V.; Singh, Y.; Sirohi, Y.S.; Chari, V. Blood Transfusion Practices in Military Medicine. Med. J. Armed Forces India 2009, 65, 30–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, V.V.; Swiatkowski, S.A. Scientific aspects of supplying blood to distant military theaters. Curr. Opin. Hematol. 2007, 14, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Sussman, J.S.; Dabrowska, A. The U.S. Blood Supply and the COVID-19 Response: In Brief; R46375; Congressional Research Service: Washington, DC, USA, 2020. [Google Scholar]
- Drew, V.J.; Barro, L.; Seghatchian, J.; Burnouf, T. Towards pathogen inactivation of red blood cells and whole blood targeting viral DNA/RNA: Design, technologies, and future prospects for developing countries. Blood Transfus. Trasfus. Sangue 2017, 15, 512–521. [Google Scholar] [CrossRef]
- Dorle, A.; Gajbe, U.; Singh, B.R.; Noman, O.; Dawande, P. A Review of Amelioration of Awareness About Blood Donation Through Various Effective and Practical Strategies. Cureus 2023, 15, e46892. [Google Scholar] [CrossRef]
- Food and Drug Administration. Requirements for blood and blood components intended for transfusion or for further manufacturing use. Final rule. Fed. Regist. 2015, 80, 29841–29906. [Google Scholar]
- Gurney, J.M.; Cap, A.P.; Holcomb, J.B.; Staudt, A.M.; Tadlock, M.D.; Polk, T.M.; Davis, C.; Corley, J.B.; Schreiber, M.A.; Beckett, A.; et al. The thin red line: Blood planning factors and the enduring need for a robust military blood system to support combat operations. J. Trauma Acute Care Surg. 2024, 97, S31–S36. [Google Scholar] [CrossRef]
- Mitra, R.; Mishra, N.; Rath, G.P. Blood groups systems. Indian J. Anaesth. 2014, 58, 524–528. [Google Scholar] [CrossRef]
- Lee, R.I. A Simple and Rapid Method for the Selection of Suitable Donors for Transfusion by the Determination of Blood Groups. Br. Med. J. 1917, 2, 684–685. [Google Scholar] [CrossRef]
- Lewisohn, R. The citrate method of blood transfusion in retrospect. Acta Haematol. 1958, 20, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C. A Call to Arms: Wartime Blood Donor Recruitment. Transfus. Med. Rev. 2018, 32, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Robertson, O.H. Transfusion with Preserved Red Blood Cells. Br. Med. J. 1918, 1, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, R.; Goldman, M.; Germain, M.; Lozano, M.; Collaborative, B. Preoperative Autologous Blood Donation: Waning Indications in an Era of Improved Blood Safety. Transfus. Med. Rev. 2015, 29, 268–275. [Google Scholar] [CrossRef]
- Elkbuli, A.; Sutherland, M.; Ehrlich, H.; Santiesteban, L.; Liu, H.; Ang, D.; McKenney, M. The Effects of COVID-19 Pandemic on Trauma Registry and Performance Improvement Operations and Workforce Nationwide: A Survey of Trauma Center Association of America Members. J. Surg. Res. 2022, 273, 24–33. [Google Scholar] [CrossRef]
- Carson, J.L.; Stanworth, S.J.; Guyatt, G.; Valentine, S.; Dennis, J.; Bakhtary, S.; Cohn, C.S.; Dubon, A.; Grossman, B.J.; Gupta, G.K.; et al. Red Blood Cell Transfusion: 2023 AABB International Guidelines. JAMA 2023, 330, 1892–1902. [Google Scholar] [CrossRef]
- Hinojosa-Laborde, C.; Hudson, I.L.; Ross, E.; Xiang, L.; Ryan, K.L. Pathophysiology of Hemorrhage as It Relates to the Warfighter. Physiology 2022, 37, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Olsen, C.; Comes, R.; McDaniel, S.; Carrillo, M.; Wilson, R.; Hanson, M. Military Blood Supply and Distribution in USCENTCOM. Mil. Med. 2024, 189, 249–252. [Google Scholar] [CrossRef]
- Cook, L.S. Therapeutic phlebotomy: A review of diagnoses and treatment considerations. J. Infus. Nurs. Off. Publ. Infus. Nurses Soc. 2010, 33, 81–88. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Montagnana, M.; Franchini, M.; Guidi, G.C. Phlebotomy issues and quality improvement in results of laboratory testing. Clin. Lab. 2006, 52, 217–230. [Google Scholar]
- Degueldre, J.; Dessy, E.; T’Sas, F.; Deneys, V.; Pattyn, N. Minimal tactical impact and maximal donor safety after a buddy transfusion: A study on elite soldier performances in both laboratory and field environments. Transfusion 2021, 61 (Suppl. S1), S32–S42. [Google Scholar] [CrossRef] [PubMed]
- Degueldre, J.; Dessy, E.; T’Sas, F.; Deneys, V. A systematic review of indications when and how a military walking blood bank could bridge blood product unavailability. Blood Transfus. Trasfus. Sangue 2024, 22, 395–404. [Google Scholar] [CrossRef]
- Naumann, D.N.; Boulton, A.J.; Sandhu, A.; Campbell, K.; Charlton, W.; Gurney, J.M.; Martin, M.J.; Scorer, T.; Doughty, H. Fresh whole blood from walking blood banks for patients with traumatic hemorrhagic shock: A systematic review and meta-analysis. J. Trauma Acute Care Surg. 2020, 89, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Erika Paola, B.; Justin, C.; Kelly, H.; Leslie, G.; Samantha, N.; Adriene, M.; Eric, E.; Brian, E.; Susannah, N.; Donald, H.J. Walking blood bank: A plan to ensure self-sufficiency in an era of blood shortage. Trauma Surg. Acute Care Open 2024, 9, e001151. [Google Scholar] [CrossRef]
- Bahr, M.; Cap, A.P.; Dishong, D.; Yazer, M.H. Practical Considerations for a Military Whole Blood Program. Mil. Med. 2020, 185, e1032–e1038. [Google Scholar] [CrossRef]
- Martinaud, C.; Scorer, T.; Lozano, M.; Miles, A.; Fitchett, G.; Ba, A.; Wikman, A.; Nimberger-Hansson, P.; Enbuske, S.; Bohonek, M.; et al. International Forum on Walking Blood Bank Programmes: Summary. Vox Sang. 2021, 116, 924–929. [Google Scholar] [CrossRef]
- Spinella, P.C.; Perkins, J.G.; Grathwohl, K.W.; Beekley, A.C.; Holcomb, J.B. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J. Trauma 2009, 66, S69–S76. [Google Scholar] [CrossRef]
- Fisher, A.D.; Paulson, M.W.; McKay, J.T.; Bynum, J.; Flarity, K.M.; Howell, M.; Bebarta, V.S.; Schauer, S.G. Blood Product Administration During the Role 1 Phase of Care: The Prehospital Trauma Registry Experience. Mil. Med. 2022, 187, e70–e75. [Google Scholar] [CrossRef]
- Wheeler, A.R.; Cuenca, C.; Fisher, A.D.; April, M.D.; Shackelford, S.A.; Schauer, S.G. Development of prehospital assessment findings associated with massive transfusion. Transfusion 2020, 60 (Suppl. S3), S70–S76. [Google Scholar] [CrossRef]
- Bresnick, E.H.; Hewitt, K.J.; Mehta, C.; Keles, S.; Paulson, R.F.; Johnson, K.D. Mechanisms of erythrocyte development and regeneration: Implications for regenerative medicine and beyond. Development 2018, 145, dev151423. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.M. Red Blood Cell Population Dynamics. Clin. Lab. Med. 2015, 35, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Zivot, A.; Lipton, J.M.; Narla, A.; Blanc, L. Erythropoiesis: Insights into pathophysiology and treatments in 2017. Mol. Med. 2018, 24, 11. [Google Scholar] [CrossRef]
- Moras, M.; Lefevre, S.D.; Ostuni, M.A. From Erythroblasts to Mature Red Blood Cells: Organelle Clearance in Mammals. Front. Physiol. 2017, 8, 1076. [Google Scholar] [CrossRef]
- Stevens-Hernandez, C.J.; Flatt, J.F.; Kupzig, S.; Bruce, L.J. Reticulocyte Maturation and Variant Red Blood Cells. Front. Physiol. 2022, 13, 834463. [Google Scholar] [CrossRef]
- Douay, L.; Lapillonne, H.; Turhan, A.G. Stem cells—a source of adult red blood cells for transfusion purposes: Present and future. Crit. Care Clin. 2009, 25, 383–398. [Google Scholar] [CrossRef]
- Cervellera, C.F.; Mazziotta, C.; Di Mauro, G.; Iaquinta, M.R.; Mazzoni, E.; Torreggiani, E.; Tognon, M.; Martini, F.; Rotondo, J.C. Immortalized erythroid cells as a novel frontier for in vitro blood production: Current approaches and potential clinical application. Stem Cell Res. Ther. 2023, 14, 139. [Google Scholar] [CrossRef]
- Baghbaderani, B.A.; Tian, X.; Neo, B.H.; Burkall, A.; Dimezzo, T.; Sierra, G.; Zeng, X.; Warren, K.; Kovarcik, D.P.; Fellner, T.; et al. cGMP-Manufactured Human Induced Pluripotent Stem Cells Are Available for Pre-clinical and Clinical Applications. Stem Cell Rep. 2015, 5, 647–659. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, K.H.; Kim, H.H.; Kim, H.S. Current Advances in Red Blood Cell Generation Using Stem Cells from Diverse Sources. Stem Cells Int. 2019, 2019, 9281329. [Google Scholar] [CrossRef]
- Huang, X.; Shah, S.; Wang, J.; Ye, Z.; Dowey, S.N.; Tsang, K.M.; Mendelsohn, L.G.; Kato, G.J.; Kickler, T.S.; Cheng, L. Extensive ex vivo expansion of functional human erythroid precursors established from umbilical cord blood cells by defined factors. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Kobari, L.; Yates, F.; Oudrhiri, N.; Francina, A.; Kiger, L.; Mazurier, C.; Rouzbeh, S.; El-Nemer, W.; Hebert, N.; Giarratana, M.C.; et al. Human induced pluripotent stem cells can reach complete terminal maturation: In vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica 2012, 97, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, H.; Kobari, L.; Mazurier, C.; Tropel, P.; Giarratana, M.C.; Zanella-Cleon, I.; Kiger, L.; Wattenhofer-Donze, M.; Puccio, H.; Hebert, N.; et al. Red blood cell generation from human induced pluripotent stem cells: Perspectives for transfusion medicine. Haematologica 2010, 95, 1651–1659. [Google Scholar] [CrossRef]
- Yang, C.T.; Ma, R.; Axton, R.A.; Jackson, M.; Taylor, A.H.; Fidanza, A.; Marenah, L.; Frayne, J.; Mountford, J.C.; Forrester, L.M. Activation of KLF1 Enhances the Differentiation and Maturation of Red Blood Cells from Human Pluripotent Stem Cells. Stem Cells 2017, 35, 886–897. [Google Scholar] [CrossRef]
- Pellegrin, S.; Severn, C.E.; Toye, A.M. Towards manufactured red blood cells for the treatment of inherited anemia. Haematologica 2021, 106, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Solves, P.; Carbonell-Uberos, F.; Mirabet, V.; Roig, R. CD34+ cell content for selecting umbilical cord blood units for cryopreservation. Transfusion 2007, 47, 552–553. [Google Scholar] [CrossRef]
- Rousseau, G.F.; Giarratana, M.C.; Douay, L. Large-scale production of red blood cells from stem cells: What are the technical challenges ahead? Biotechnol. J. 2014, 9, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, Y.; Ma, F.; Tsuji, K. Generation of red blood cells from human embryonic/induced pluripotent stem cells for blood transfusion. Int. J. Hematol. 2012, 95, 610–616. [Google Scholar] [CrossRef]
- Bayley, R.; Ahmed, F.; Glen, K.; McCall, M.; Stacey, A.; Thomas, R. The productivity limit of manufacturing blood cell therapy in scalable stirred bioreactors. J. Tissue Eng. Regen. Med. 2018, 12, e368–e378. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.; Vermette, P. Bioreactors for tissue mass culture: Design, characterization, and recent advances. Biomaterials 2005, 26, 7481–7503. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, S.; Baek, E.J. Current status of red blood cell manufacturing in 3D culture and bioreactors. Blood Res. 2023, 58, S46–S51. [Google Scholar] [CrossRef]
- Levy, M.J.; Garfinkel, E.M.; May, R.; Cohn, E.; Tillett, Z.; Wend, C.; Sikorksi, R.A.; Troncoso, R., Jr.; Jenkins, J.L.; Chizmar, T.P.; et al. Implementation of a prehospital whole blood program: Lessons learned. J. Am. Coll. Emerg. Physicians Open 2024, 5, e13142. [Google Scholar] [CrossRef] [PubMed]
- Indelen, C.; Uygun Kizmaz, Y.; Kar, A.; Shander, A.; Kirali, K. The cost of one unit blood transfusion components and cost-effectiveness analysis results of transfusion improvement program. Turk Gogus Kalp Damar Cerrahisi Derg. 2021, 29, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S. Artificial blood. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2008, 12, 140–144. [Google Scholar] [CrossRef]
- Haldar, R.; Gupta, D.; Chitranshi, S.; Singh, M.K.; Sachan, S. Artificial Blood: A Futuristic Dimension of Modern Day Transfusion Sciences. Cardiovasc. Hematol. Agents Med. Chem. 2019, 17, 11–16. [Google Scholar] [CrossRef]
- Barbosa, F.T.; Juca, M.J.; Castro, A.A.; Duarte, J.L.; Barbosa, L.T. Artificial oxygen carriers as a possible alternative to red cells in clinical practice. Sao Paulo Med. J. Rev. Paul. Med. 2009, 127, 97–100. [Google Scholar] [CrossRef]
- Frietsch, T.; Lenz, C.; Waschke, K.F. Artificial oxygen carriers. Eur. J. Anaesthesiol. 1998, 15, 571–584. [Google Scholar] [CrossRef]
- Moradi, S.; Jahanian-Najafabadi, A.; Roudkenar, M.H. Artificial Blood Substitutes: First Steps on the Long Route to Clinical Utility. Clin. Med. Insights. Blood Disord. 2016, 9, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Greenburg, A.G. Toward 21st century blood component replacement therapeutics: Artificial oxygen carriers, platelet substitutes, recombinant clotting factors, and others. Artif. Cells Blood Substit. Immobil. Biotechnol. 2006, 34, 537–550. [Google Scholar] [CrossRef]
- Jägers, J.; Wrobeln, A.; Ferenz, K.B. Perfluorocarbon-based oxygen carriers: From physics to physiology. Pflügers Arch.-Eur. J. Physiol. 2021, 473, 139–150. [Google Scholar] [CrossRef]
- Waxman, K. Perfluorocarbons as blood substitutes. Ann. Emerg. Med. 1986, 15, 1423–1424. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Huang, Y.; Chen, X.; Wu, M.; Sun, J.; Jing, X. Hemoglobin conjugated micelles based on triblock biodegradable polymers as artificial oxygen carriers. Biomaterials 2009, 30, 5077–5085. [Google Scholar] [CrossRef] [PubMed]
- Cabrales, P.; Intaglietta, M. Blood substitutes: Evolution from noncarrying to oxygen- and gas-carrying fluids. ASAIO J. 2013, 59, 337–354. [Google Scholar] [CrossRef]
- Sangkum, L.; Yang, Z.; Liu, H. Applications of Blood Substitutes in Transplantation Surgery: Where Are We Now? J. Anesth. Transl. Med. 2023, 2, 6–9. [Google Scholar] [CrossRef]
- Latson, G.W. Perftoran (Vidaphor)—Introduction to Western Medicine. Shock 2019, 52, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R. Blood substitutes. Artificial oxygen carriers: Perfluorocarbon emulsions. Crit Care 1999, 3, R93–R97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, M.; Liu, H.; Jahr, J.S. Perfluorocarbon-based oxygen carriers: What is new in 2024? J. Anesth. Transl. Med. 2024, 3, 10–13. [Google Scholar] [CrossRef]
- Sen Gupta, A. Hemoglobin-based Oxygen Carriers: Current State-of-the-art and Novel Molecules. Shock 2019, 52, 70–83. [Google Scholar] [CrossRef]
- Chang, T.M. Blood substitutes based on nanobiotechnology. Trends Biotechnol. 2006, 24, 372–377. [Google Scholar] [CrossRef]
- Stowell, C.P.; Levin, J.; Spiess, B.D.; Winslow, R.M. Progress in the development of RBC substitutes. Transfusion 2001, 41, 287–299. [Google Scholar] [CrossRef]
- Benitez Cardenas, A.S.; Samuel, P.P.; Olson, J.S. Current Challenges in the Development of Acellular Hemoglobin Oxygen Carriers by Protein Engineering. Shock 2019, 52, 28–40. [Google Scholar] [CrossRef]
- Alayash, A.I. Setbacks in blood substitutes research and development: A biochemical perspective. Clin. Lab. Med. 2010, 30, 381–389. [Google Scholar] [CrossRef]
- Dimino, M.L.; Palmer, A.F. High O2 affinity hemoglobin-based oxygen carriers synthesized via polymerization of hemoglobin with ring-opened 2-chloroethyl-beta-D-fructopyranoside and 1-o-octyl-beta-D-glucopyranoside. Biotechnol. Bioeng. 2007, 97, 462–472. [Google Scholar] [CrossRef]
- Alayash, A.I. Mechanisms of Toxicity and Modulation of Hemoglobin-based Oxygen Carriers. Shock 2019, 52, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Savla, C.; Palmer, A.F. Lyophilized annelid mega-hemoglobin retains its’ quaternary structure and oxygen equilibrium properties after room temperature storage for over 6 months. PLoS ONE 2022, 17, e0263996. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, H.; Xiong, Y.; Liu, Z.Z.; Patzak, A.; Georgieva, R. Novel Hemoglobin Particles—Promising New-Generation Hemoglobin-Based Oxygen Carriers. Artif. Organs 2014, 38, 708–714. [Google Scholar] [CrossRef]
- Munoz, C.J.; Lucas, D.; Martinez, J.; Ricario, M.; O’Boyle, Q.T.; Pires, I.S.; Palmer, A.F.; Cabrales, P. Toxic side-effects of diaspirin cross-linked human hemoglobin are attenuated by the apohemoglobin-haptoglobin complex. Biomed. Pharmacother. 2024, 174, 116569. [Google Scholar] [CrossRef]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Synthesis, Characterization, and Applications of Dendrimer-Encapsulated Nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Jahr, J.S.; Williams, J.P. Blood Component Requirements and Erythrocyte Transfusion and Mortality Related to Hemoglobin Deficit in Phase III Trial of Hemoglobin-Based Oxygen Carrier: HBOC-201. Am. J. Ther. 2022, 29, e279–e286. [Google Scholar] [CrossRef]
- Eastman, A.L.; Minei, J.P. Comparison of Hemoglobin-based Oxygen Carriers to Stored Human Red Blood Cells. Crit. Care Clin. 2009, 25, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, S.J.; Arezou Sadighi, A.; Randall, J.H. Crosslinked, Polymerized, and PEG-Conjugated Hemoglobin-Based Oxygen Carriers: Clinical Safety and Efficacy of Recent and Current Products. Curr. Drug Discov. Technol. 2012, 9, 158–165. [Google Scholar] [CrossRef]
- Svergun, D.I.; Ekstrom, F.; Vandegriff, K.D.; Malavalli, A.; Baker, D.A.; Nilsson, C.; Winslow, R.M. Solution structure of poly(ethylene) glycol-conjugated hemoglobin revealed by small-angle X-ray scattering: Implications for a new oxygen therapeutic. Biophys. J. 2008, 94, 173–181. [Google Scholar] [CrossRef]
- Lowe, K.C.; Farrell, K.; Ferguson, E.M.P.; James, V. Current Perceived Risks of Transfusion in the UK and Relevance to the Future Acceptance of Blood Substitutes. Artif. Cells Blood Substit. Biotechnol. 2001, 29, 179–189. [Google Scholar] [CrossRef]
- Varnado, C.L.; Mollan, T.L.; Birukou, I.; Smith, B.J.Z.; Henderson, D.P.; Olson, J.S. Development of Recombinant Hemoglobin-Based Oxygen Carriers. Antioxid. Redox Signal. 2012, 18, 2314–2328. [Google Scholar] [CrossRef]
- Alayash, A.I. Oxidation reactions of cellular and acellular hemoglobins: Implications for human health. Front. Med. Technol. 2022, 4, 1068972. [Google Scholar] [CrossRef] [PubMed]
- Jahr, J.S.; MacKinnon, K.; Baum, V.C.; Alayash, A.I. Hemoglobin-based oxygen carriers: Biochemical, biophysical differences, and safety. Transfusion 2025, 65, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Delpy, E.; Zal, F.; Leize-Zal, E.; Huck, O. Therapeutic Potential of Hemoglobin Derived from the Marine Worm Arenicola marina (M101): A Literature Review of a Breakthrough Innovation. Mar. Drugs 2021, 19, 376. [Google Scholar] [CrossRef] [PubMed]
- Kalmer, M.; Grasshoff, M.; Maié, T.; Pannen, K.; Toledo, M.A.S.; Vieri, M.; Olschok, K.; Lemanzyk, R.; Lazarevic, J.; Junge, B.; et al. Deciphering the complex clonal heterogeneity of polycythemia vera and the response to interferon alpha. Blood Adv. 2025, bloodadvances.2024012600. [Google Scholar] [CrossRef]
- Menon, V.; Ghaffari, S. Erythroid enucleation: A gateway into a “bloody” world. Exp. Hematol. 2021, 95, 13–22. [Google Scholar] [CrossRef]
- Timmins, N.E.; Nielsen, L.K. Blood cell manufacture: Current methods and future challenges. Trends Biotechnol. 2009, 27, 415–422. [Google Scholar] [CrossRef]
- Kolotilin, I. Plant-produced recombinant cytokines IL-37b and IL-38 modulate inflammatory response from stimulated human PBMCs. Sci. Rep. 2022, 12, 19450. [Google Scholar] [CrossRef] [PubMed]
- Szeto, T.H.; Drake, P.M.W.; Teh, A.Y.H.; Falci Finardi, N.; Clegg, A.G.; Paul, M.J.; Reljic, R.; Ma, J.K.C. Production of Recombinant Proteins in Transgenic TobaccoTobacco Plants. In Recombinant Proteins in Plants: Methods and Protocols; Schillberg, S., Spiegel, H., Eds.; Springer: New York, NY, USA, 2022; pp. 17–48. [Google Scholar]
- Rahfeld, P.; Withers, S.G. Toward universal donor blood: Enzymatic conversion of A and B to O type. J. Biol. Chem. 2020, 295, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Osborn, M.J.; Belanto, J.J.; Tolar, J.; Voytas, D.F. Gene editing and its application for hematological diseases. Int. J. Hematol. 2016, 104, 18–28. [Google Scholar] [CrossRef]
- Lawicki, S.; Covin, R.B.; Powers, A.A. The Kidd (JK) Blood Group System. Transfus. Med. Rev. 2017, 31, 165–172. [Google Scholar] [CrossRef]
- Delorme, B.; Chateauvieux, S.; Charbord, P. The Concept of Mesenchymal Stem Cells. Regen. Med. 2006, 1, 497–509. [Google Scholar] [CrossRef]
- Koller, M.R.; Palsson, B.O. Review: Tissue engineering: Reconstitution of human hematopoiesis ex vivo. Biotechnol. Bioeng. 1993, 42, 909–930. [Google Scholar] [CrossRef]
- Panoskaltsis, N.; Mantalaris, A.; Wu, J.H.D. Engineering a mimicry of bone marrow tissue ex vivo. J. Biosci. Bioeng. 2005, 100, 28–35. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Tun, T.; Miyoshi, H.; Aung, T.; Takahashi, S.; Shimizu, R.; Kuroha, T.; Yamamoto, M.; Ohshima, N. Effect of Growth Factors on Ex Vivo Bone Marrow Cell Expansion Using Three-Dimensional Matrix Support. Artif. Organs 2002, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]

| Ineligibility for Blood Donations | ||
|---|---|---|
| Drug users who used needles to take drugs. | ||
| Social Disqualifications | Engaged in sex for money. | |
| Recent tattoos and piercing work. | ||
| HIV-1 | HIV-2 | |
| Hepatitis-B | Hepatitis-C | |
| Chagas disease | Babesiosis | |
| Medical Disqualifications | Psoriasis | Creutzfeldt-Jakob disease |
| HTLV-1 | HTLV-2 | |
| Syphilis | Herpes (HSV) | |
| Lymphoma, leukemia | Anemia | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada, A., III; Furmanski, O.; Klarmann, G.J.; Scheidt, N.; Ho, V.B. Progress in Development of Functional Biological and Synthetic Blood Products to Augment Transfusable Blood Supply in Operational Medicine. Bioengineering 2025, 12, 256. https://doi.org/10.3390/bioengineering12030256
Estrada A III, Furmanski O, Klarmann GJ, Scheidt N, Ho VB. Progress in Development of Functional Biological and Synthetic Blood Products to Augment Transfusable Blood Supply in Operational Medicine. Bioengineering. 2025; 12(3):256. https://doi.org/10.3390/bioengineering12030256
Chicago/Turabian StyleEstrada, Armando, III, Orion Furmanski, George J. Klarmann, Nathan Scheidt, and Vincent B. Ho. 2025. "Progress in Development of Functional Biological and Synthetic Blood Products to Augment Transfusable Blood Supply in Operational Medicine" Bioengineering 12, no. 3: 256. https://doi.org/10.3390/bioengineering12030256
APA StyleEstrada, A., III, Furmanski, O., Klarmann, G. J., Scheidt, N., & Ho, V. B. (2025). Progress in Development of Functional Biological and Synthetic Blood Products to Augment Transfusable Blood Supply in Operational Medicine. Bioengineering, 12(3), 256. https://doi.org/10.3390/bioengineering12030256







