Advancing Gait Analysis: Integrating Multimodal Neuroimaging and Extended Reality Technologies
Abstract
1. Introduction
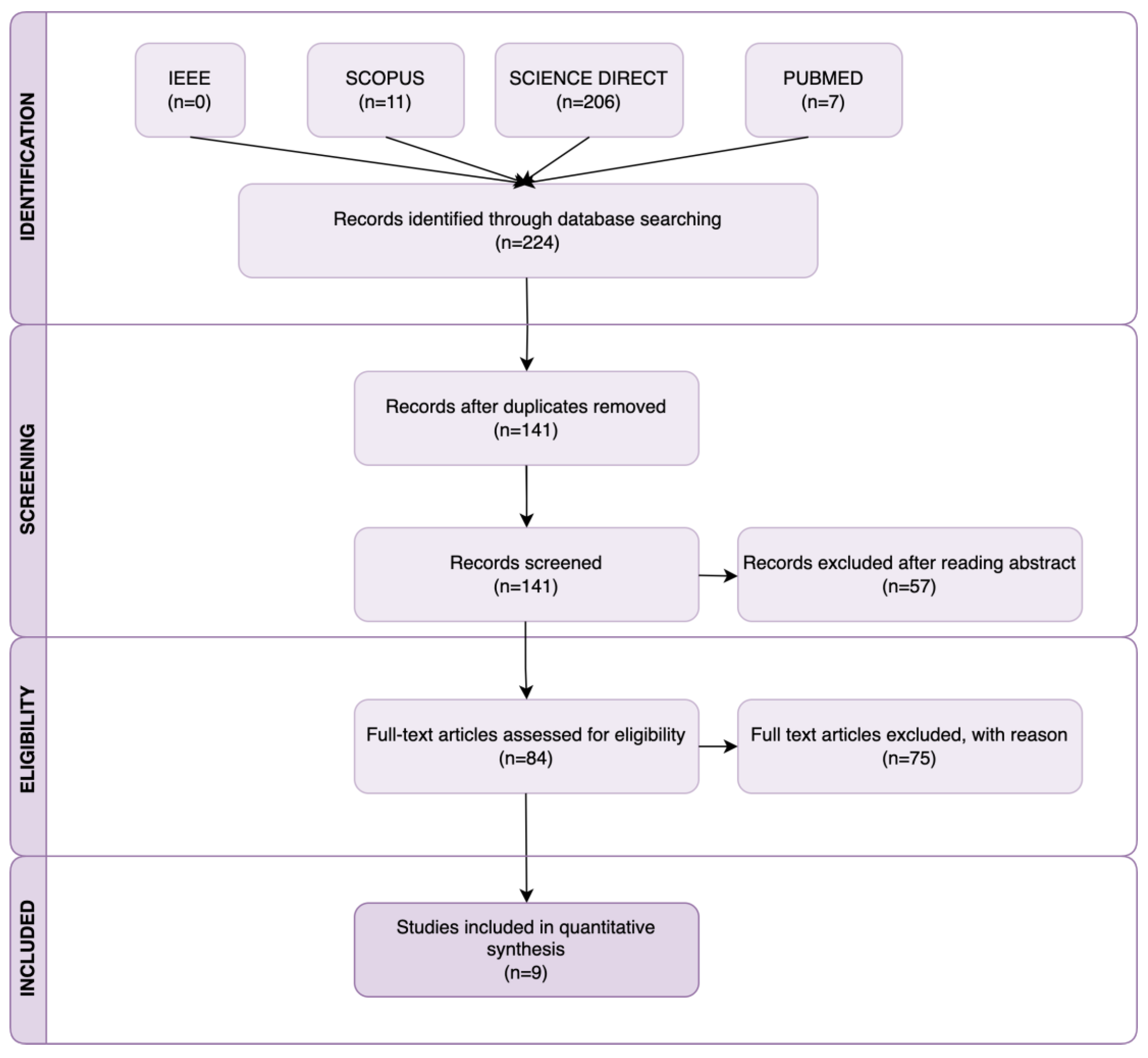
2. Research Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
3. Advanced Gait Analysis Approach and Key Components
3.1. Neuroimaging Techniques
3.2. Extended Reality (XR) Technologies
3.3. Sensor-Based Systems
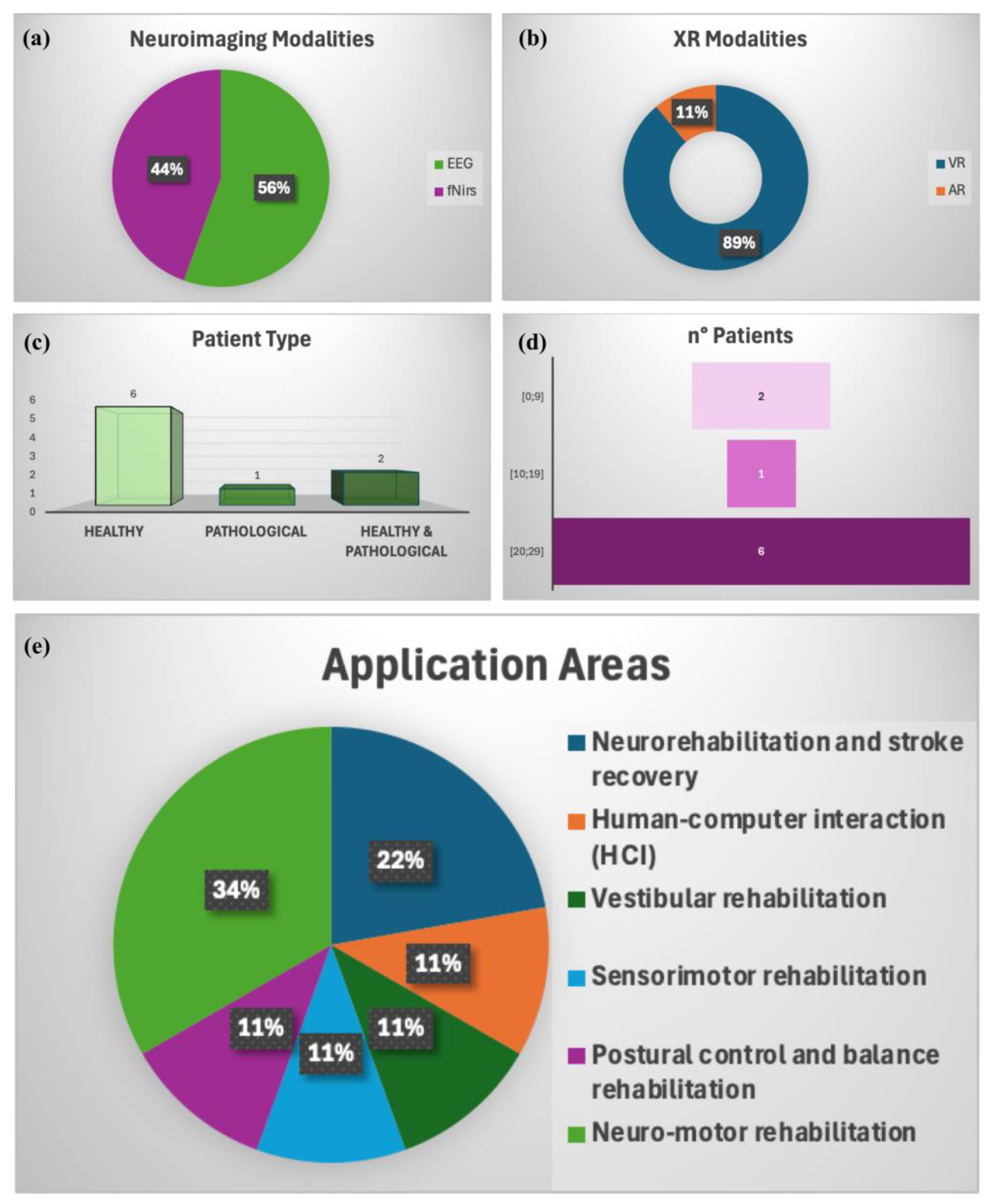
4. Advanced Gait Analysis: Literature Results
5. Discussion
- Comprehensive Analysis: By merging brain activity measurements with physical movement data, researchers can better understand how neurological and mechanical factors influence walking.
- Rehabilitation: XR environments allow for innovative therapeutic interventions, such as retraining gait patterns in individuals with neurological disorders or injuries.
- Personalized Medicine: These approaches enable tailored interventions based on individual gait patterns and neural responses.
- Scientific Exploration: These approaches advance the study of locomotion under various physiological and environmental conditions.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dapp, U.; Vinyard, D.; Golgert, S.; Krumpoch, S.; Freiberger, E. Reference values of gait characteristics in community-dwelling older persons with different physical functional levels. BMC Geriatr. 2022, 22, 713. [Google Scholar] [CrossRef] [PubMed]
- Hulleck, A.A.; Menoth Mohan, D.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front Med. Technol. 2022, 4, 901331. [Google Scholar] [CrossRef]
- Palumbo, A. Microsoft HoloLens 2 in Medical and Healthcare Context: State of the Art and Future Prospects. Sensors 2022, 22, 7709. [Google Scholar] [CrossRef]
- De Keersmaecker, E.; Lefeber, N.; Geys, M.; Jespers, E.; Kerckhofs, E.; Swinnen, E. Virtual reality during gait training: Does it improve gait function in persons with central nervous system movement disorders? A systematic review and meta-analysis. NeuroRehabilitation 2019, 44, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.R.; Huaco Aranguri, A.A.; Sanchez Zevallos, G.A.; Juarez Huanca, C.B.; Huanca Machon, M. Effects of Virtual Reality on Biomechanical Parameters of Gait in Older Adults: A Systematic Review. Arch. Rehabil. Res. Clin. Transl. 2024, 6, 100354. [Google Scholar] [CrossRef] [PubMed]
- Kourtesis, P. A Comprehensive Review of Multimodal XR Applications, Risks, and Ethical Challenges in the Metaverse. Multimodal Technol. Interact. 2024, 8, 98. [Google Scholar] [CrossRef]
- Han, X.; Guffanti, D.; Brunete, A. A Comprehensive Review of Vision-Based Sensor Systems for Human Gait Analysis. Sensors 2025, 25, 498. [Google Scholar] [CrossRef]
- Katmah, R.; Shehhi, A.A.; Jelinek, H.F.; Hulleck, A.A.; Khalaf, K. A Systematic Review of Gait Analysis in the Context of Multimodal Sensing Fusion and AI. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 4189–4202. [Google Scholar] [CrossRef]
- Clark, D.J.; Manini, T.M.; Ferris, D.P.; Hass, C.J.; Brumback, B.A.; Cruz-Almeida, Y.; Pahor, M.; Reuter-Lorenz, P.A.; Seidler, R.D. Multimodal Imaging of Brain Activity to Investigate Walking and Mobility Decline in Older Adults (Mind in Motion Study): Hypothesis, Theory, and Methods. Front. Aging Neurosci. 2019, 11, 358. [Google Scholar] [CrossRef]
- Lorenz, E.A.; Su, X.; Skjæret-Maroni, N. A review of combined functional neuroimaging and motion capture for motor rehabilitation. J. Neuroeng. Rehabil. 2024, 21, 3. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Ono, Y.; Suzuki, T.; Ogihara, Y.; Imai, Y.; Watanabe, A.; Tokikuni, Y.; Sakuraba, S.; Sawamura, D. Regional brain activity and neural network changes in cognitive-motor dual-task interference: A functional near-infrared spectroscopy study. NeuroImage 2024, 297, 120714. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, H.P.; Alcock, L.; Firbank, M.; Wilson, R.; Brown, P.; Maxwell, R.; Bennett, E.; Pavese, N.; Brooks, D.J.; Rochester, L. Developing a novel dual-injection FDG-PET imaging methodology to study the functional neuroanatomy of gait. NeuroImage 2024, 288, 120531. [Google Scholar] [CrossRef]
- Togo, H.; Nakamura, T.; Wakasugi, N.; Takahashi, Y.; Hanakawa, T. Interactions across emotional, cognitive and subcortical motor networks underlying freezing of gait. NeuroImage Clin. 2023, 37, 103342. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiu, Y.; Zhao, H.; Zhan, J.; Chen, R.; Wei, T.; Huang, Z. GaitSlice: A gait recognition model based on spatio-temporal slice features. Pattern Recognit. 2022, 124, 108453. [Google Scholar] [CrossRef]
- Morel, E.; Armand, S.; Assal, F.; Allali, G. Is frontal gait a myth in normal pressure hydrocephalus? J. Neurol. Sci. 2019, 402, 175–179. [Google Scholar] [CrossRef]
- Balaji, E.; Brindha, D.; Balakrishnan, R. Supervised machine learning based gait classification system for early detection and stage classification of Parkinson’s disease. Appl. Soft Comput. 2020, 94, 106494. [Google Scholar] [CrossRef]
- Krasovsky, T.; Holtzer, R.; Jahjah, E.; Fruchter, E. Trait anxiety increases the attentional cost of walking in young adults: A cross-sectional study. J. Affect. Disord. 2024, 362, 716–722. [Google Scholar] [CrossRef]
- Demro, C.; Mueller, B.A.; Kent, J.S.; Burton, P.C.; Olman, C.A.; Schallmo, M.-P.; Lim, K.O.; Sponheim, S.R. The psychosis human connectome project: An overview. NeuroImage 2021, 241, 118439. [Google Scholar] [CrossRef]
- Bu, S.; Pang, H.; Li, X.; Zhao, M.; Wang, J.; Liu, Y.; Yu, H.; Fan, G. Structural and Functional Alterations of Motor-Thalamus in Different Motor Subtype of Parkinson’s Disease: An Individual Study. Acad. Radiol. 2024, 31, 1605–1614. [Google Scholar] [CrossRef]
- Hussain, M.M.; Weslin, D.; Kumari, S.; Umamaheswari, S.; Kamalakannan, K. Enhancing Parkinson’s disease identification using ensemble classifier and data augmentation techniques in machine learning. Clin. eHealth 2023, 6, 150–158. [Google Scholar] [CrossRef]
- Ding, Q.; Ou, Z.; Yao, S.; Wu, C.; Chen, J.; Shen, J.; Lan, Y.; Xu, G. Cortical activation and brain network efficiency during dual tasks: An fNIRS study. NeuroImage 2024, 289, 120545. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Tejada, I.; Czosnyka, M.; Czosnyka, Z.; Juhler, M.; Smielewski, P. Causal relationship between slow waves of arterial, intracranial pressures and blood velocity in brain. Comput. Biol. Med. 2021, 139, 104970. [Google Scholar] [CrossRef]
- Finn, D.; Cardini, F.; Aspell, J.E.; Swami, V.; Todd, J. The impact of body image on social cognition: Fear of negative evaluation mediates the relationship between body surveillance and interpersonal distance in women. Body Image 2024, 51, 101777. [Google Scholar] [CrossRef] [PubMed]
- Katsipis, G.; Tzekaki, E.E.; Andreadou, E.G.; Mouzakidis, C.; Baldimtsi, E.N.; Karathanasi, E.M.; Hassandra, M.; Galanis, E.; Hatzigeorgiadis, A.; Goudas, M.; et al. The effect of physical exercise with cognitive training on inflammation and Alzheimer’s disease biomarkers of Mild Cognitive Impairment patients. Neurosci. Appl. 2024, 3, 104085. [Google Scholar] [CrossRef]
- Robles, C.M.; Anderson, B.; Dukelow, S.P.; Striemer, C.L. Assessment and recovery of visually guided reaching deficits following cerebellar stroke. Neuropsychologia 2023, 188, 108662. [Google Scholar] [CrossRef]
- Ozdogar, A.T.; Ertekin, O.; Kahraman, T.; Yigit, P.; Ozakbas, S. Effect of video-based exergaming on arm and cognitive function in persons with multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2020, 40, 101966. [Google Scholar] [CrossRef]
- Kashikar-Zuck, S.; Briggs, M.S.; Bout-Tabaku, S.; Connelly, M.; Daffin, M.; Guite, J.; Ittenbach, R.; Logan, D.E.; Lynch-Jordan, A.M.; Myer, G.D.; et al. Randomized clinical trial of Fibromyalgia Integrative Training (FIT teens) for adolescents with juvenile fibromyalgia—Study design and protocol. Contemp. Clin. Trials 2021, 103, 106321. [Google Scholar] [CrossRef]
- Djebbara, Z.; King, J.; Ebadi, A.; Nakamura, Y.; Bermudez, J. Contemplative neuroaesthetics and architecture: A sensorimotor exploration. Front. Archit. Res. 2024, 13, 97–111. [Google Scholar] [CrossRef]
- Over, H.; Cook, R. Where do spontaneous first impressions of faces come from? Cognition 2018, 170, 190–200. [Google Scholar] [CrossRef]
- Huang, Y.-A.; Jastorff, J.; Van den Stock, J.; Van de Vliet, L.; Dupont, P.; Vandenbulcke, M. Studying emotion theories through connectivity analysis: Evidence from generalized psychophysiological interactions and graph theory. NeuroImage 2018, 172, 250–262. [Google Scholar] [CrossRef]
- Bègue, I.; Blakemore, R.; Klug, J.; Cojan, Y.; Galli, S.; Berney, A.; Aybek, S.; Vuilleumier, P. Metacognition of visuomotor decisions in conversion disorder. Neuropsychologia 2018, 114, 251–265. [Google Scholar] [CrossRef]
- Di Marco, S.; Fattori, P.; Galati, G.; Galletti, C.; Lappe, M.; Maltempo, T.; Serra, C.; Sulpizio, V.; Pitzalis, S. Preference for locomotion-compatible curved paths and forward direction of self-motion in somatomotor and visual areas. Cortex 2021, 137, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Yu, H.; Lv, Z.; Yang, X.; Hu, C.; Zhang, T. What can “drag & drop” tell? Detecting mild cognitive impairment by hand motor function assessment under dual-task paradigm. Int. J. Hum.-Comput. Stud. 2021, 145, 102547. [Google Scholar] [CrossRef]
- Rossion, B. Twenty years of investigation with the case of prosopagnosia PS to understand human face identity recognition. Part I: Function. Neuropsychologia 2022, 173, 108278. [Google Scholar] [CrossRef]
- Langen, C.D.; Cremers, L.G.M.; de Groot, M.; White, T.; Ikram, M.A.; Niessen, W.J.; Vernooij, M.W. Disconnection due to white matter hyperintensities is associated with lower cognitive scores. NeuroImage 2018, 183, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.B.; Brudvig, J.J.; Lehtimäki, K.K.; Cain, J.T.; White, K.A.; Bragge, T.; Rytkönen, J.; Huhtala, T.; Timm, D.; Vihma, M.; et al. A multimodal approach to identify clinically relevant biomarkers to comprehensively monitor disease progression in a mouse model of pediatric neurodegenerative disease. Prog. Neurobiol. 2020, 189, 101789. [Google Scholar] [CrossRef]
- Androwis, G.J.; Sandroff, B.M.; Niewrzol, P.; Fakhoury, F.; Wylie, G.R.; Yue, G.; DeLuca, J. A pilot randomized controlled trial of robotic exoskeleton-assisted exercise rehabilitation in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 51, 102936. [Google Scholar] [CrossRef]
- Ehgoetz Martens, K.A.; Matar, E.; Phillips, J.R.; Shine, J.M.; Grunstein, R.R.; Halliday, G.M.; Lewis, S.J.G. Narrow doorways alter brain connectivity and step patterns in isolated REM sleep behaviour disorder. NeuroImage Clin. 2022, 33, 102958. [Google Scholar] [CrossRef]
- Sansare, A.; Reimann, H.; Crenshaw, J.; Arcodia, M.; Verma, K.; Lee, S.C.K. Subthreshold electrical noise alters walking balance control in individuals with cerebral palsy. Gait Posture 2023, 106, 47–52. [Google Scholar] [CrossRef]
- Pasman, E.P.; McKeown, M.J.; Garg, S.; Cleworth, T.W.; Bloem, B.R.; Inglis, J.T.; Carpenter, M.G. Brain connectivity during simulated balance in older adults with and without Parkinson’s disease. NeuroImage Clin. 2021, 30, 102676. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.L.; Morse, L.R.; Troy, K.; Nguyen, N.; Battaglino, R.A.; Falci, S.P.; Linnman, C. Resting state functional connectivity differentiation of neuropathic and nociceptive pain in individuals with chronic spinal cord injury. NeuroImage Clin. 2023, 38, 103414. [Google Scholar] [CrossRef] [PubMed]
- Brandon Lin, M.-I.; Cheng, S.-W. Different smartphone tasks and traffic complexity affect pedestrian awareness of co-existing road objects and cerebral oxygenation during shared space walking. Transp. Res. Part F Traffic Psychol. Behav. 2024, 103, 460–479. [Google Scholar] [CrossRef]
- Huang, C.H.; Umegaki, H.; Makino, T.; Uemura, K.; Hayashi, T.; Kitada, T.; Inoue, A.; Shimada, H.; Kuzuya, M. Effect of Various Exercises on Intrinsic Capacity in Older Adults with Subjective Cognitive Concerns. J. Am. Med. Dir. Assoc. 2021, 22, 780–786.e782. [Google Scholar] [CrossRef]
- Walter, C.S.; Hengge, C.R.; Lindauer, B.E.; Schaefer, S.Y. Declines in motor transfer following upper extremity task-specific training in older adults. Exp. Gerontol. 2019, 116, 14–19. [Google Scholar] [CrossRef]
- Žepič, Z.M. Improvement of Cognitive Abilities of Older Employees with Computerized Cognitive Training (CCT). IFAC-Pap 2021, 54, 651–656. [Google Scholar] [CrossRef]
- Tustin, K.; Elze, M.C.; Lumsden, D.E.; Gimeno, H.; Kaminska, M.; Lin, J.-P. Gross motor function outcomes following deep brain stimulation for childhood-onset dystonia: A descriptive report. Eur. J. Paediatr. Neurol. 2019, 23, 473–483. [Google Scholar] [CrossRef]
- Mohd Salah Aljabiri, S.; Hamdan, M.M. Analyzing lower body movements using machine learning to classify autistic children. Biomed. Signal Process. Control 2024, 94, 106288. [Google Scholar] [CrossRef]
- Sarasso, E.; Gardoni, A.; Piramide, N.; Volontè, M.A.; Canu, E.; Tettamanti, A.; Filippi, M.; Agosta, F. Dual-task clinical and functional MRI correlates in Parkinson’s disease with postural instability and gait disorders. Park. Relat. Disord. 2021, 91, 88–95. [Google Scholar] [CrossRef]
- Zuleger, T.M.; Slutsky-Ganesh, A.B.; Kim, H.; Anand, M.; Warren, S.M.; Grooms, D.R.; Yuan, W.; Riley, M.A.; Gore, R.K.; Myer, G.D.; et al. Differential neural mechanisms for movement adaptations following neuromuscular training in young female athletes with a history of sports-related concussion. Neuroscience 2024, 558, 70–80. [Google Scholar] [CrossRef]
- Cockx, H.; Oostenveld, R.; Tabor, M.; Savenco, E.; van Setten, A.; Cameron, I.; van Wezel, R. fNIRS is sensitive to leg activity in the primary motor cortex after systemic artifact correction. NeuroImage 2023, 269, 119880. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Smith-MacDonald, L.; Brown, M.R.G.; VanDehy, J.; Grunnet-Jepsen, R.; Ordek, V.P.; Kruger, S.; Ayres Gerhart, A.; van Veelen, N.; Nijdam, M.J.; et al. The Redesign and Validation of Multimodal Motion-Assisted Memory Desensitization and Reconsolidation Hardware and Software: Mixed Methods, Modified Delphi–Based Validation Study. JMIR Hum. Factors 2022, 9, e33682. [Google Scholar] [CrossRef]
- Akanmu, A.; Okunola, A.; Jebelli, H.; Ammar, A.; Afolabi, A. Cognitive load assessment of active back-support exoskeletons in construction: A case study on construction framing. Adv. Eng. Inform. 2024, 62, 102905. [Google Scholar] [CrossRef]
- Mahesh, T.R.; Vinoth, K.V.; Bhardwaj, R.; Khan, S.B.; Alkhaldi, N.A.; Victor, N.; Verma, A. An artificial intelligence-based decision support system for early and accurate diagnosis of Parkinson’s Disease. Decis. Anal. J. 2024, 10, 100381. [Google Scholar] [CrossRef]
- Qu, J.; Cui, L.; Guo, W.; Bu, L.; Wang, Z. Development of a novel machine learning-based approach for brain function assessment and integrated software solution. Adv. Eng. Inform. 2024, 60, 102461. [Google Scholar] [CrossRef]
- Salisbury, J.P.; Keshav, N.U.; Sossong, A.D.; Sahin, N.T. Concussion Assessment with Smartglasses: Validation Study of Balance Measurement Toward a Lightweight, Multimodal, Field-Ready Platform. JMIR Mhealth Uhealth 2018, 6, e15. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Chen, I.H.; Hsu, W.-C.; Tseng, H.-Y.; Wang, R.-Y. Effect of exergaming versus combined exercise on cognitive function and brain activation in frail older adults: A randomised controlled trial. Ann. Phys. Rehabil. Med. 2021, 64, 101492. [Google Scholar] [CrossRef]
- Castilla, A.; Berthoz, A.; Urukalo, D.; Zaoui, M.; Perrochon, A.; Kronovsek, T. Age and sex impact on visuospatial working memory (VSWM), mental rotation, and cognitive strategies during navigation. Neurosci. Res. 2022, 183, 84–96. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, Y.; Bu, L. Functional near-infrared spectroscopy in the assessment of rehabilitation efficacy of virtual reality products for people with cognitive disorders. Int. J. Ind. Ergon. 2023, 97, 103500. [Google Scholar] [CrossRef]
- Ouellet, É.; Boller, B.; Corriveau-Lecavalier, N.; Cloutier, S.; Belleville, S. The Virtual Shop: A new immersive virtual reality environment and scenario for the assessment of everyday memory. J. Neurosci. Methods 2018, 303, 126–135. [Google Scholar] [CrossRef]
- Fishbein, P.; Hutzler, Y.; Ratmansky, M.; Treger, I.; Dunsky, A. A Preliminary Study of Dual-Task Training Using Virtual Reality: Influence on Walking and Balance in Chronic Poststroke Survivors. J. Stroke Cerebrovasc. Dis. 2019, 28, 104343. [Google Scholar] [CrossRef] [PubMed]
- Manuli, A.; Maggio, M.G.; Stagnitti, M.C.; Aliberti, R.; Cannavò, A.; Casella, C.; Milardi, D.; Bruschetta, A.; Naro, A.; Calabrò, R.S. Is intensive gait training feasible and effective at old age? A retrospective case-control study on the use of Lokomat Free-D in patients with chronic stroke. J. Clin. Neurosci. 2021, 92, 159–164. [Google Scholar] [CrossRef]
- Potvin-Desrochers, A.; Martinez-Moreno, A.; Clouette, J.; Parent-L’Ecuyer, F.; Lajeunesse, H.; Paquette, C. Upregulation of the parietal cortex improves freezing of gait in Parkinson’s disease. J. Neurol. Sci. 2023, 452, 120770. [Google Scholar] [CrossRef] [PubMed]
- Lench, D.H.; Doolittle, J.D.; Ramakrishnan, V.; Rowland, N.; Revuelta, G.J. Subthalamic functional connectivity associated with freezing of gait dopa-response. Park. Relat. Disord. 2024, 118, 105952. [Google Scholar] [CrossRef] [PubMed]
- Whittier, T.T.; Weller, Z.D.; Fling, B.W. Novel applications of Bayesian inference clarify sensorimotor uncertainty during stepping movements. Neuropsychologia 2022, 173, 108310. [Google Scholar] [CrossRef]
- Nishida, D.; Mizuno, K.; Yamada, E.; Hanakawa, T.; Liu, M.; Tsuji, T. The neural correlates of gait improvement by rhythmic sound stimulation in adults with Parkinson’s disease—A functional magnetic resonance imaging study. Park. Relat. Disord. 2021, 84, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, E.; Agosta, F.; Piramide, N.; Canu, E.; Volontè, M.A.; Filippi, M. Brain activity of the emotional circuit in Parkinson’s disease patients with freezing of gait. NeuroImage Clin. 2021, 30, 102649. [Google Scholar] [CrossRef]
- De Rosa, A.; Guizzardi, G.; Moncada, M.; Roldán, P.; Ferrés, A.; Topczewski, T.E.; Somma, T.; Cavallo, L.M.; González, J.; Enseñat, J.; et al. Ultrasound-Oriented Surgical Planning (“UOSP”) for Intracranial Lesions: A Systematic Integration to the Standard Preoperative Planning. World Neurosurg. 2023, 170, e766–e776. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Cole, R.C.; Espinoza, A.I.; Brown, D.; Cavanagh, J.F.; Narayanan, N.S. Frontal theta and beta oscillations during lower-limb movement in Parkinson’s disease. Clin. Neurophysiol. 2020, 131, 694–702. [Google Scholar] [CrossRef]
- Ragothaman, A.; Mancini, M.; Nutt, J.G.; Wang, J.; Fair, D.A.; Horak, F.B.; Miranda-Dominguez, O. Motor networks, but also non-motor networks predict motor signs in Parkinson’s disease. NeuroImage Clin. 2023, 40, 103541. [Google Scholar] [CrossRef]
- Tang, X.; Guo, R.; Zhang, C.; Qian, X. A causal counterfactual graph neural network for arising-from-chair abnormality detection in parkinsonians. Med. Image Anal. 2024, 97, 103266. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Yin, B. Affective foundations in AI-human interactions: Insights from evolutionary continuity and interspecies communications. Comput. Hum. Behav. 2024, 161, 108406. [Google Scholar] [CrossRef]
- Karnath, H.-O.; Sperber, C.; Rorden, C. Reprint of: Mapping human brain lesions and their functional consequences. NeuroImage 2019, 190, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Levi, D.M. Learning to see in depth. Vis. Res. 2022, 200, 108082. [Google Scholar] [CrossRef]
- Goyal, J.; Khandnor, P.; Aseri, T.C. Classification, Prediction, and Monitoring of Parkinson’s disease using Computer Assisted Technologies: A Comparative Analysis. Eng. Appl. Artif. Intell. 2020, 96, 103955. [Google Scholar] [CrossRef]
- Souza Silva, W.; Aravind, G.; Sangani, S.; Lamontagne, A. Healthy young adults implement distinctive avoidance strategies while walking and circumventing virtual human vs. non-human obstacles in a virtual environment. Gait Posture 2018, 61, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Tolley, C.; Mc Ardle, R.; Beswick, E.; Slight, S.P. Key Considerations When Developing and Implementing Digital Technology for Early Detection of Dementia-Causing Diseases Among Health Care Professionals: Qualitative Study. J. Med. Internet Res. 2023, 25, e46711. [Google Scholar] [CrossRef]
- Bluett, B.; Banks, S.; Cordes, D.; Bayram, E.; Mishra, V.; Cummings, J.; Litvan, I. Neuroimaging and neuropsychological assessment of freezing of gait in Parkinson’s disease. Alzheimers Dement. 2018, 4, 387–394. [Google Scholar] [CrossRef]
- Górriz, J.M.; Ramírez, J.; Ortíz, A.; Martínez-Murcia, F.J.; Segovia, F.; Suckling, J.; Leming, M.; Zhang, Y.-D.; Álvarez-Sánchez, J.R.; Bologna, G.; et al. Artificial intelligence within the interplay between natural and artificial computation: Advances in data science, trends and applications. Neurocomputing 2020, 410, 237–270. [Google Scholar] [CrossRef]
- Nakai, N.; Sato, M.; Yamashita, O.; Sekine, Y.; Fu, X.; Nakai, J.; Zalesky, A.; Takumi, T. Virtual reality-based real-time imaging reveals abnormal cortical dynamics during behavioral transitions in a mouse model of autism. Cell Rep. 2023, 42, 112258. [Google Scholar] [CrossRef]
- Hazany, S.; Bagrodia, N.; Chu, R., Jr.; Shaw, S. Results of a 2-week novel robotic rehabilitation program in 18 children with prior hemispherectomy. J. Clin. Neurosci. 2023, 108, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.M.; Perdikis, D.; Blickensdörfer, A.; Stefanovski, L.; Liu, Q.; Maith, O.; Dinkelbach, H.Ü.; Baladron, J.; Hamker, F.H.; Ritter, P. Virtual deep brain stimulation: Multiscale co-simulation of a spiking basal ganglia model and a whole-brain mean-field model with The Virtual Brain. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lacroix, C.; Soeiro, T.; Le Marois, M.; Guilhaumou, R.; Cassé-Perrot, C.; Jouve, E.; Röhl, C.; Belzeaux, R.; Micallef, J.; Blin, O. Innovative approaches in CNS clinical drug development: Quantitative systems pharmacology. Therapies 2021, 76, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-Y.; Onuma, Y.; Złahoda-Huzior, A.; Kageyama, S.; Dudek, D.; Wang, Q.; Lim, R.P.; Garg, S.; Poon, E.K.W.; Puskas, J.; et al. Merging virtual and physical experiences: Extended realities in cardiovascular medicine. Eur. Heart J. 2023, 44, 3311–3322. [Google Scholar] [CrossRef] [PubMed]
- Asoodar, M.; Janesarvatan, F.; Yu, H.; de Jong, N. Theoretical foundations and implications of augmented reality, virtual reality, and mixed reality for immersive learning in health professions education. Adv. Simul. 2024, 9, 36. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, L.; Fang, L.; Zhang, H.; Li, X.; Hong, Y.; Chen, S.; Zhang, Y.; Zheng, B.; Wu, J.; et al. Effectiveness of repetitive transcranial magnetic stimulation combined with intelligent Gait-Adaptability Training in improving lower limb function and brain symmetry after subacute stroke: A preliminary study. J. Stroke Cerebrovasc. Dis. 2024, 33, 107961. [Google Scholar] [CrossRef]
- Gomaa, Y.S.; Awad, M.I.; Emara, T.; Elbokl, A.; Al-Yahya, E.; ElMeligie, M.M. Role of virtual reality in examining the effect of fear of falling (FOF) on postural stability in individuals without and with Parkinson’s disease in Egypt: A mixed-methods feasibility study protocol. BMJ Open 2024, 14, e080592. [Google Scholar] [CrossRef]
- Maas, S.A.; Göcking, T.; Stojan, R.; Voelcker-Rehage, C.; Kutz, D.F. Synchronization of Neurophysiological and Biomechanical Data in a Real-Time Virtual Gait Analysis System (GRAIL): A Proof-of-Principle Study. Sensors 2024, 24, 3779. [Google Scholar] [CrossRef]
- Daşdemir, Y. Locomotion techniques with EEG signals in a virtual reality environment. Displays 2023, 80, 102538. [Google Scholar] [CrossRef]
- Stojan, R.; Mack, M.; Bock, O.; Voelcker-Rehage, C. Inefficient frontal and parietal brain activation during dual-task walking in a virtual environment in older adults. NeuroImage 2023, 273, 120070. [Google Scholar] [CrossRef]
- Nishimoto, R.; Fujiwara, S.; Kutoku, Y.; Ogata, T.; Mihara, M. Effect of dual-task interaction combining postural and visual perturbations on cortical activity and postural control ability. Neuroimage 2023, 280, 120352. [Google Scholar] [CrossRef] [PubMed]
- Piazza, C.; Pirovano, I.; Mastropietro, A.; Genova, C.; Gagliardi, C.; Turconi, A.C.; Malerba, G.; Panzeri, D.; Maghini, C.; Reni, G.; et al. Development and Preliminary Testing of a System for the Multimodal Analysis of Gait Training in a Virtual Reality Environment. Electronics 2021, 10, 2838. [Google Scholar] [CrossRef]
- Peterson, S.M.; Ferris, D.P. Group-level cortical and muscular connectivity during perturbations to walking and standing balance. NeuroImage 2019, 198, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Hoppes, C.W.; Sparto, P.J.; Whitney, S.L.; Furman, J.M.; Huppert, T.J. Changes in cerebral activation in individuals with and without visual vertigo during optic flow: A functional near-infrared spectroscopy study. NeuroImage Clin. 2018, 20, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Nordin, A.D. Mobile Electroencephalography for Studying Neural Control of Human Locomotion. Front. Hum. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Khan, M.S.; Charissis, V.; Sakellariou, S. Exploring the Development Requirements for Virtual Reality Gait Analysis. Multimodal Technol. Interact. 2019, 3, 24. [Google Scholar] [CrossRef]

| Database Name | URL | Date Accessed |
|---|---|---|
| IEEEXplore | https://ieeexplore.ieee.org/Xplore/home.jsp | 14 December 2024 |
| Science Direct | https://www.sciencedirect.com/ | 14 December 2024 |
| Scopus | https://www.scopus.com/ | 14 December 2024 |
| Pubmed | https://pubmed.ncbi.nlm.nih.gov/ | 14 December 2024 |
| Reference | Year | Aim | Methodology | Participants (n, Type) |
|---|---|---|---|---|
| Zhang et al. [86] | 2024 | To explore the efficacy of combining rTMS with gait-adaptive training to enhance lower limb function and regulatory mechanisms in subacute stroke. | -Neuroimaging: EEG (actiCHamp) -Extended Reality: AR -Sensor-Based Systems: C-Mill smart gait training system (C-Mill, Motekforce Link BV) | 27 patients with subacute hemiparesis (18–75 years) |
| Gomaa et al. [87] | 2024 | To develop an assessment method for FOF while in motion and walking within virtual environments. | -Neuroimaging: EEG (ANT Neuro, Hengelo, the Netherlands) -Extended Reality: VR (Meta Quest 3) -Sensor-Based Systems: Postural IMU; Trigno wireless EMG system (Delsys, Natick, MA, USA) | 10 to 30 participants, people without PD and people with PD |
| Maas et al. [88] | 2024 | To develop and validate a setup that allows for the simultaneous collection and real-time synchronization of brain activity (via mobile EEG and fNIRS), kinetic, and kinematic gait measurements. | -Neuroimaging: fNIRS (two 8 × 8 NIRSport 2.0 systems (NIRx Medical Technologies, Glen Head, NY, USA); EEG (LiveAmp, Brain Products GmbH, Gilchingen, Germany) -Extended Reality: VR -Sensor-Based Systems: Marker-based, passive, optical motion detection system (VICON Motion Systems Ltd.; Oxford, UK), two ground reaction force plates (Motek Medical; Utrecht, the Netherlands), and an external EMG measuring system (Cometa; Bareggio, Italy) | 3 volunteers (1 M, 2 F, 22–37 years) |
| Daşdemir et al. [89] | 2023 | To investigate changes in objective brain activity (EEG) and subjective simulatory sickness questionnaire (SSQ) scores according to an individual’s susceptibility to VR locomotion. | -Neuroimaging: EEG (Emotiv EPOC Flex) -Extended Reality: VR (HTC-Vive Lighthouses) -Sensor-Based Systems: n.d. | 32 volunteers (21 M, 11 F, aged 18–30) |
| Stojan et al. [90] | 2023 | To assess brain activity in the PFC and parietal lobe and to investigate whether higher PFC activation during DT walking in older adults is related to compensation, dedifferentiation, or neural inefficiency. | -Neuroimaging: fNIRS (NIRSport systems, NIRx Medical Technologies, Glen Head, NY, USA) -Extended Reality: VR (D-Flow, Motekforce Link, Amsterdam, the Netherlands) -Sensor-Based Systems: Vicon Nexus (v2.10) | 56 healthy older adults (30 F, aged 64 –79) |
| Nishimoto et al. [91] | 2023 | To investigate postural control performance under different visual conditions using a virtual reality system, simultaneously measuring cortical activities with a functional near-infrared spectroscopy system. | -Neuroimaging: 50-channel NIRS system (OMM 3000; Shimadzu Corporation, Kyoto, Japan) -Extended Reality: VR (HTC Vive Pro, HTC America Inc., Seattle, WA, USA) -Sensor-Based Systems: Wireless surface EMG (WEB-1000; NIHON KOHDEN Corporation, Tokyo, Japan) | 24 healthy participants (11 M, 13 F, aged 19–42 years) |
| Piazza et al. [92] | 2021 | To set up and test a system for the multimodal analysis of the gait pattern during the VR gait training of pediatric populations by analyzing the EEG correlates as well as the kinematic and kinetic parameters of the gait. | -Neuroimaging: EEG system (eegoTMmylab (ANT Neuro, Hengelo, The Netherlands)) -Extended Reality: VR GRAIL (Motek Medical, Houten, The Netherlands) -Sensor-Based Systems: Vicon motion capture system (Oxford Metrics, Oxford, UK) | 5 healthy adult volunteers (mean age = 30.9 years; 2 M) 4 children (mean age = 11.2 years; 1 M healthy child; 3 children with a diagnosis of unilateral CP, 2 M) |
| Peterson et al. [93] | 2019 | To quantify differences in group-level corticomuscular connectivity responses to sensorimotor perturbations during walking and standing. | -Neuroimaging: 136-channel EEG (BioSemi Active II, BioSemi, Amsterdam, NL) -Extended Reality: VR (Oculus Rift DK2, Oculus, Redmond, WA, USA) -Sensor-Based Systems: 8 lower leg EMG channels (Vicon, Los Angeles, CA, USA) | 30 healthy young adults (15 F, 15 M, aged 22.5 ± 4.8 years) |
| Hoppes et al. [94] | 2018 | To determine if individuals with visual vertigo have different cerebral activation during optic flow compared with control subjects. | -Neuroimaging: fNIRS (CW6 real-time system; TechEn, Inc.; Milford, MA, USA) -Extended Reality: VR -Sensor-Based Systems: Ground reaction forces | 15 healthy controls (5 M, 10 F, aged 18–65) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramigna, V.; Palumbo, A.; Perri, G. Advancing Gait Analysis: Integrating Multimodal Neuroimaging and Extended Reality Technologies. Bioengineering 2025, 12, 313. https://doi.org/10.3390/bioengineering12030313
Gramigna V, Palumbo A, Perri G. Advancing Gait Analysis: Integrating Multimodal Neuroimaging and Extended Reality Technologies. Bioengineering. 2025; 12(3):313. https://doi.org/10.3390/bioengineering12030313
Chicago/Turabian StyleGramigna, Vera, Arrigo Palumbo, and Giovanni Perri. 2025. "Advancing Gait Analysis: Integrating Multimodal Neuroimaging and Extended Reality Technologies" Bioengineering 12, no. 3: 313. https://doi.org/10.3390/bioengineering12030313
APA StyleGramigna, V., Palumbo, A., & Perri, G. (2025). Advancing Gait Analysis: Integrating Multimodal Neuroimaging and Extended Reality Technologies. Bioengineering, 12(3), 313. https://doi.org/10.3390/bioengineering12030313





