Development of 3D Cell-Based Fluorescent Reporter Assay for Screening of Drugs Downregulating Telomerase Reverse Transcriptase
Abstract
1. Introduction
2. Materials and Methods
2.1. Drug Reagents
2.2. Plasmid Construction
| p1500 bp-hTERT-EGFP | p330 bp-hTERT-EGFP |
| 5′-CCCCGCGTCAAGCTTCGAATTCTGC-3′ | 5′-CCCCGCGTCAAGCTTCGAATTCTGC-3′ |
| 5′-AAGGGGCGGAACTAATGCATGGCGGTAATAC-3′ | 5′-CGGACCCGGGAACTAATGCATGG-3′ |
| 5′-GCATTAGTTCCGCCCCTTTGCCCTAG-3′ | 5′-AAGCTTGACGCGGGGGTGGC-3′ |
| 5′-AAGCTTGACGCGGGGGTGGC-3′ | 5′-TGCATTAGTTCCCGGGTCCGCC-3′ |
2.3. Cell Lines and Cell Culture
2.4. Generation of Stable EGFP Reporting Cells
2.5. Real-Time PCR for mRNA Quantification
| hTERT primers | 5′-CGGAAGAGTGTCTGGAGCAA-3′ 5′-GGATGAAGCGGAGTCTGGA-3′ |
| EGFP primers | 5′-GCTGACCCTGAAGTTCATCTG-3′ 5′-CACCTTGATGCCGTTCTTCT-3′ |
| β-actin primers | 5′-CAGGTCATCACCATTGGCAATGAGC-3′ 5′-CGGATGTCCACGTCACACTTCATGA-3′ |
2.6. Fluorescence Imaging
2.7. Two-Dimensional Cell Culture
2.8. Three-Dimensional Cell Culture and Fluorescence Intensity Reading
2.9. Three-Dimensional Cytotoxicity Assay
2.10. Three-Dimensional Reporter Assay of hTERT Downregulation by Drugs
2.11. Logistic Regression
2.12. Statistical Analysis
3. Results
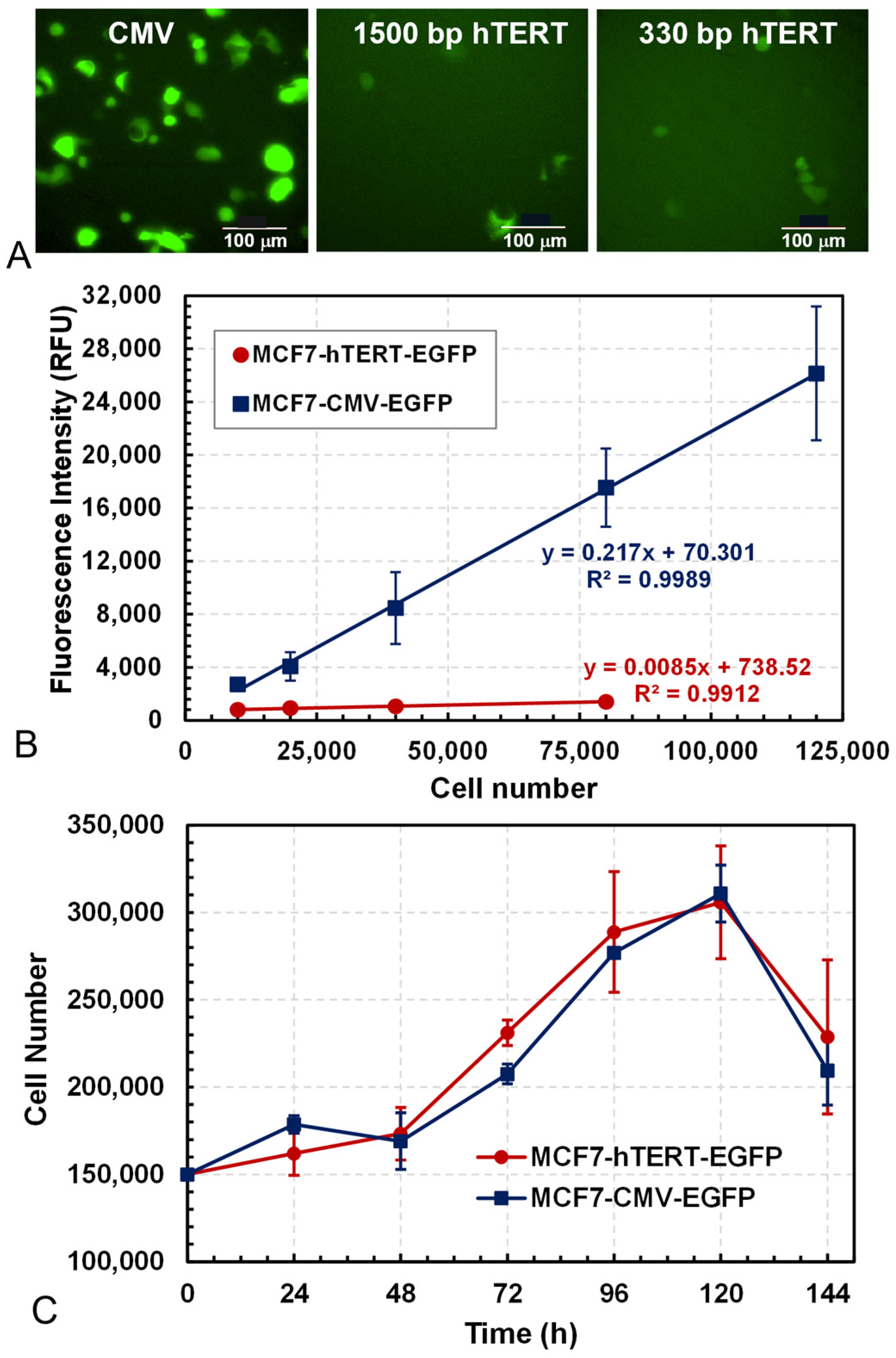
3.1. Characterization of Fluorescent Reporting Cell Lines
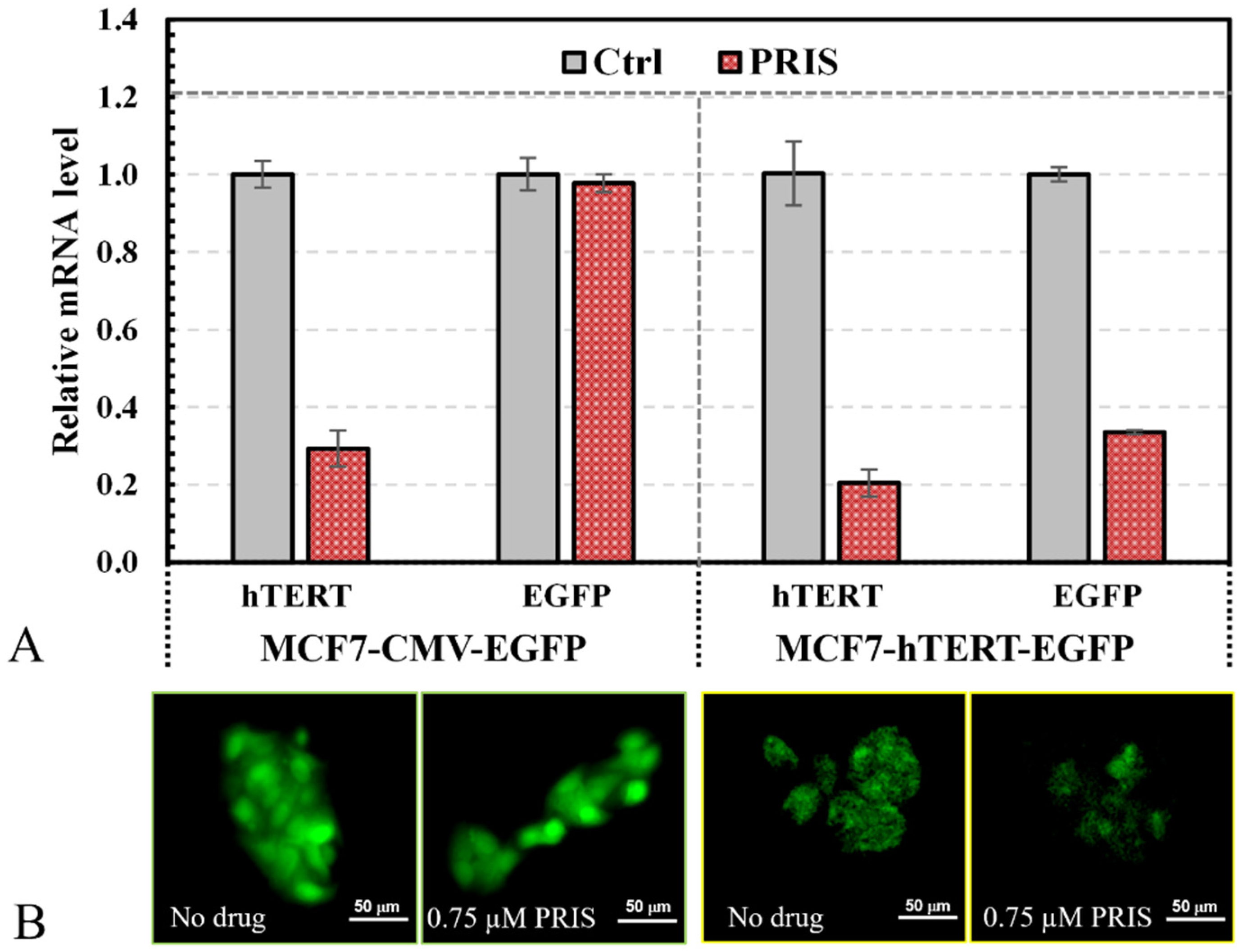
3.2. Validation of Reporting Cell Pair with Pristimerin
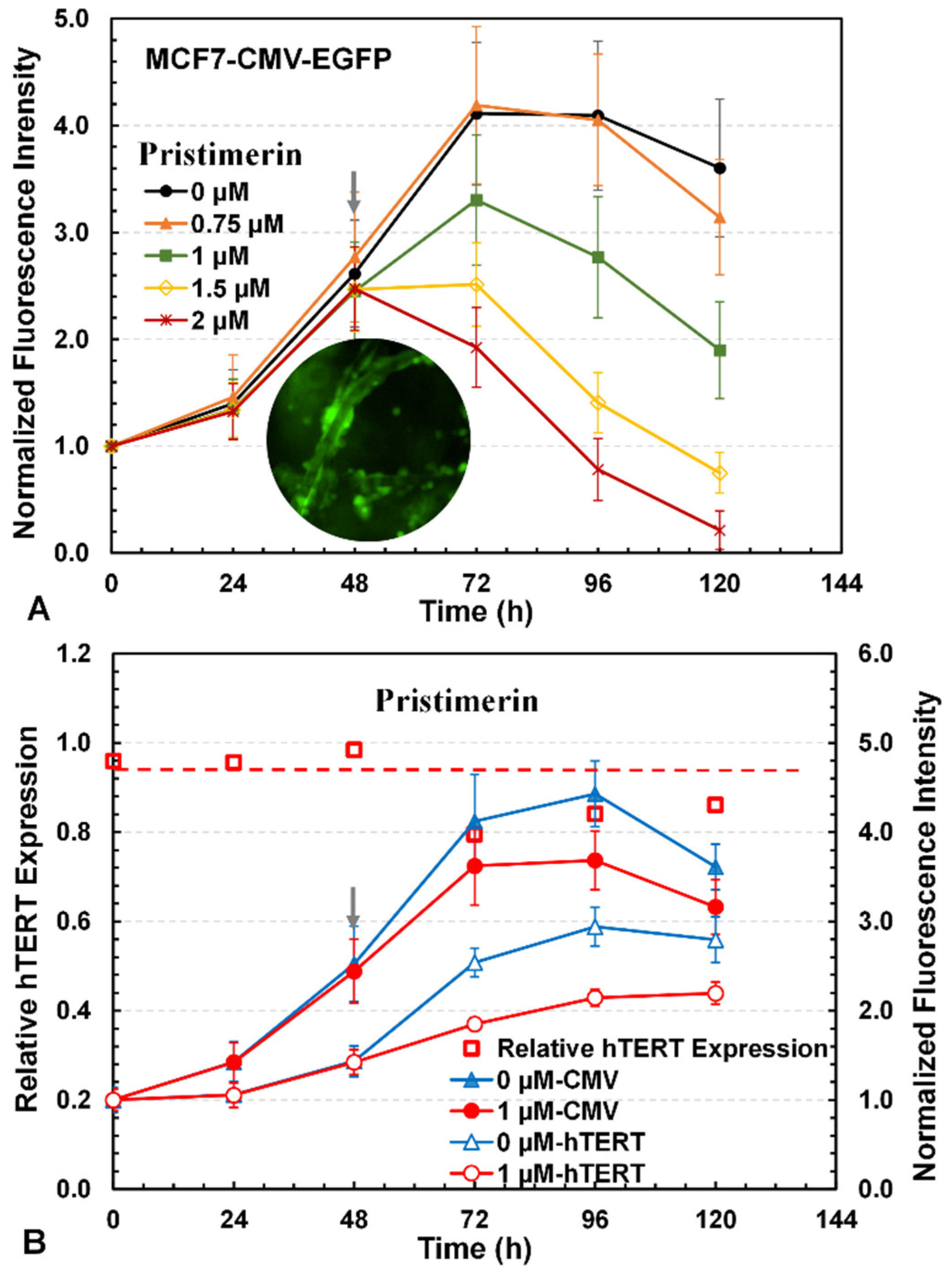
3.3. Three-Dimensional Reporter Assay for Screening of hTERT Repressors
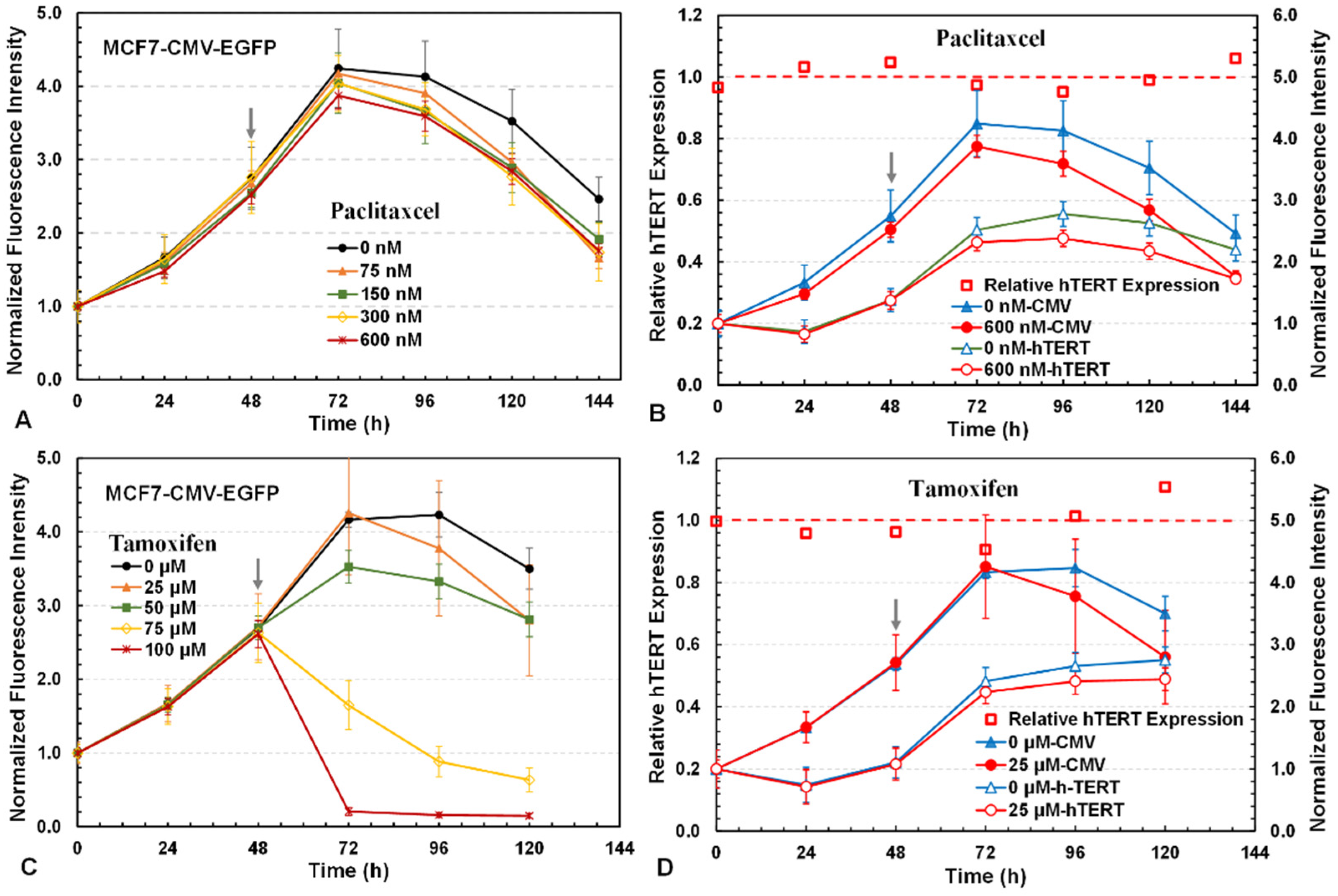
3.4. Investigation of Drug Cytotoxicity and hTERT Repressors with 3D Reporter Assay
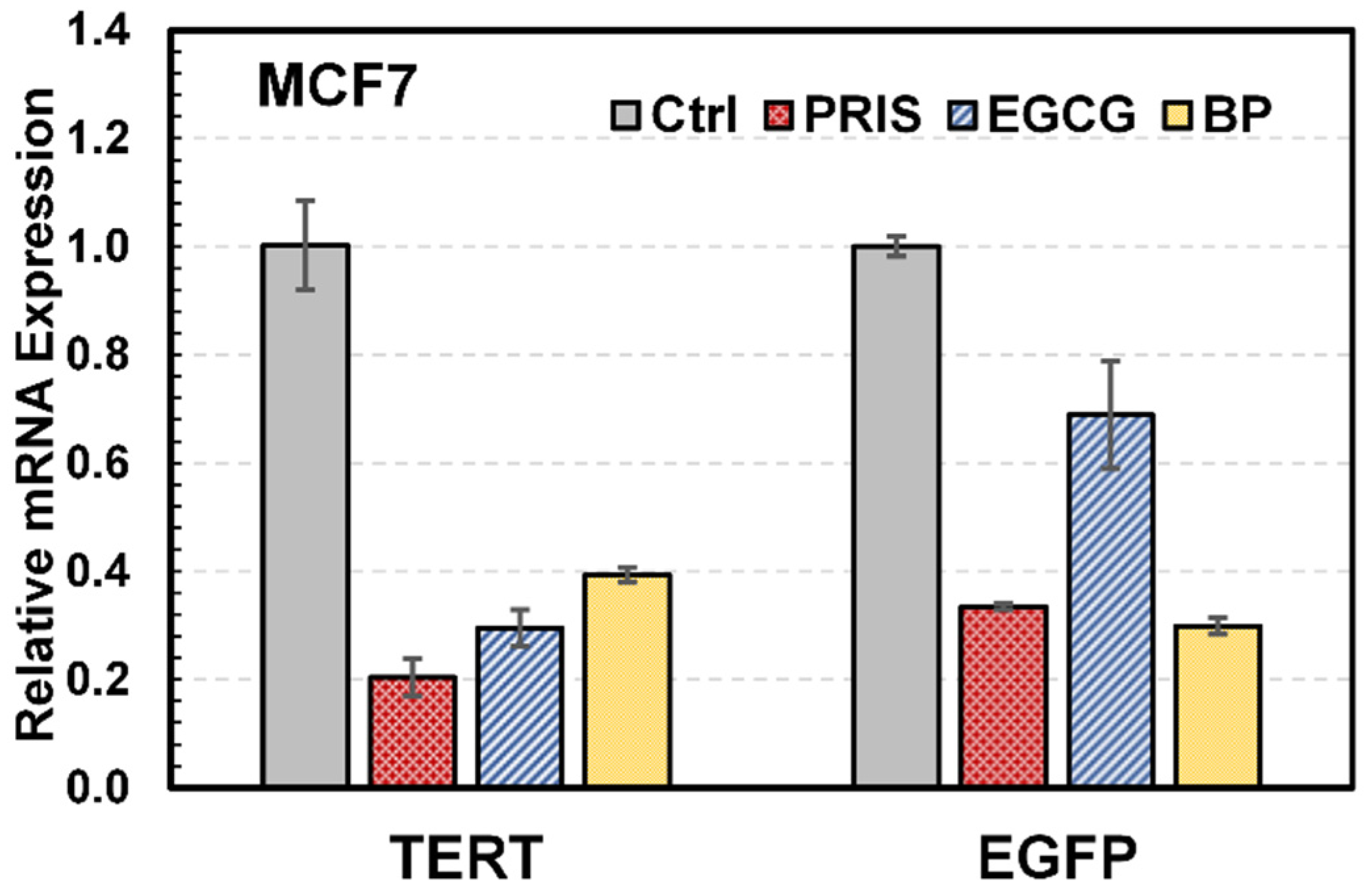
3.5. Validation of 3D Assay with qRT-PCR
3.6. Binary Logistic Regression Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef]
- Smith, E.M.; Pendlebury, D.F.; Nandakumar, J. Structural biology of telomeres and telomerase. Cell. Mol. Life Sci. 2020, 77, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, health, and hallmarks of aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Engin, A. The connection between cell fate and telomere. Protein Kinase-Mediat. Decis. Life Death 2021, 1275, 71–100. [Google Scholar] [CrossRef]
- Robinson, N.J.; Schiemann, W.P. Telomerase in cancer: Function, regulation, and clinical translation. Cancers 2022, 14, 808. [Google Scholar] [CrossRef]
- Dratwa, M.; Wysoczańska, B.; Łacina, P.; Kubik, T.; Bogunia-Kubik, K. TERT—Regulation and roles in cancer formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef]
- Indran, I.R.; Hande, M.P.; Pervaiz, S. HTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res. 2011, 71, 266–276. [Google Scholar] [CrossRef]
- Hasanau, T.N.; Pisarev, E.P.; Kisil, O.V.; Zvereva, M.E. The TERT promoter: A key player in the fight for cancer cell immortality. Biochemistry 2023, 88 (Suppl. 1), S21–S38. [Google Scholar] [CrossRef]
- Horikawa, I.; Cable, P.L.; Afshari, C.; Barrett, J.C. Cloning and characterization of the promoter region of human telomerase reverse transcriptase gene. Cancer Res. 1999, 59, 826–830. [Google Scholar]
- Wick, M.; Zubov, D.; Hagen, G. Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT). Gene 1999, 232, 97–106. [Google Scholar] [CrossRef]
- Takakura, M.; Kyo, S.; Kanaya, T.; Hirano, H.; Takeda, J.; Yutsudo, M.; Inoue, M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999, 59, 551–557. [Google Scholar]
- Cong, Y.-S.; Wen, J.; Bacchetti, S. The human telomerase catalytic subunit hTERT: Organization of the gene and characterization of the promoter. Hum. Mol. Genet. 1999, 8, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Kumar, M.S.; Peters, G.; Mayur, Y. Targeting telomerase for its advent in cancer therapeutics. Med. Res. Rev. 2020, 40, 1871–1919. [Google Scholar] [CrossRef]
- Guterres, A.N.; Villanueva, J. Targeting telomerase for cancer therapy. Oncogene 2020, 39, 5811–5824. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, G.; Chen, Y.; Yang, Q.; Hao, T.; Zhao, L.; Zhao, L.; Cong, Y.; Diao, A.; Yu, P. Inhibitor of the human telomerase reverse trancriptase (hTERT) gene promoter induces cell apoptosis via a mitochondrial-dependent pathway. Eur. J. Med. Chem. 2018, 145, 370–378. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Shih, J.W.; Lin, J.J. Establishing cell-based reporter systems for the analysis of hTERT expression. Methods Mol. Biol. 2007, 405, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Li, W.-C.; Shih, J.-W.; Hong, K.-F.; Pan, Y.-R.; Lin, J.-J. The tea polyphenols EGCG and EGC repress mRNA expression of human telomerase reverse transcriptase (hTERT) in carcinoma cells. Cancer Lett. 2006, 236, 80–88. [Google Scholar] [CrossRef]
- Zang, R.; Li, D.; Tang, I.C.; Wang, J.; Yang, S.T. Cell-based assays in high-throughput screening for drug discovery. Int. J. Biotechnol. Wellness Ind. 2012, 1, 31–51. [Google Scholar] [CrossRef]
- Badr-Eldin, S.M.; Aldawsari, H.M.; Kotta, S.; Deb, P.K.; Venugopala, K.N. Three-dimensional in vitro cell culture models for efficient drug discovery: Progress so far and future prospects. Pharmaceuticals 2022, 15, 926. [Google Scholar] [CrossRef]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D cell culture models as recapitulators of the tumor microenvironment for the screening of anti-cancer drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.T. High-throughput 3-D cell-based proliferation and cytotoxicity assays for drug screening and bioprocess development. J. Biotechnol. 2011, 151, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Wu, Y.; Zang, R.; Yang, S.T. A fluorescent 3D cell culture assay for high throughput screening of cancer drugs down-regulating survivin. J. Biotechnol. 2019, 289, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Zang, R.; Xin, X.; Zhang, F.; Li, D.; Yang, S. An engineered mouse embryonic stem cell model with survivin as a molecular marker and EGFP as the reporter for high throughput screening of embryotoxic chemicals in vitro. Biotechnol. Bioeng. 2019, 116, 1656–1668. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Z.; Zhang, F.; Yang, S.T. Two-color fluorescent proteins reporting survivin regulation in breast cancer cells for high throughput drug screening. Biotechnol. Bioeng. 2022, 119, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, Z.; Hu, C.; Xing, N.; Xia, Y.; Pang, B. Pristimerin suppressed breast cancer progression via miR-542-5p/DUB3 axis. OncoTargets Ther. 2020, 13, 6651. [Google Scholar] [CrossRef]
- Li, J.; Guo, Q.; Lei, X.; Zhang, L.; Su, C.; Liu, Y.; Zhou, W.; Chen, H.; Wang, H.; Wang, F.; et al. Pristimerin induces apoptosis and inhibits proliferation, migration in H1299 Lung Cancer Cells. J. Cancer 2020, 11, 6348–6355. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Liu, Y.; Pindolia, K.; Gautam, S.C. Inhibition of hTERT/telomerase contributes to the antitumor activity of pristimerin in pancreatic ductal adenocarcinoma cells. Oncol. Rep. 2015, 34, 518–524. [Google Scholar] [CrossRef]
- Li, J.-J.; Yan, Y.-Y.; Sun, H.-M.; Liu, Y.; Su, C.-Y.; Chen, H.-B.; Zhang, J.-Y. Anti-Cancer Effects of Pristimerin and the Mechanisms: A Critical Review. Front. Pharmacol. 2019, 10, 746. [Google Scholar] [CrossRef]
- Liu, Y.B.; Gao, X.; Deeb, D.; Pindolia, K.; Gautam, S.C. Role of telomerase in anticancer activity of pristimerin in prostate cancer cells. J. Exp. Ther. Oncol. 2015, 11, 41–49. [Google Scholar]
- Deeb, D.; Gao, X.; Liu, Y.B.; Zhang, Y.; Shaw, J.; Valeriote, F.A.; Gautam, S.C. Inhibition of hTERT in pancreatic cancer cells by pristimerin involves suppression of epigenetic regulators of gene transcription. Oncol. Rep. 2017, 37, 1914–1920. [Google Scholar] [CrossRef]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Chen, B.-H.; Hsieh, C.-H.; Tsai, S.-Y.; Wang, C.-Y.; Wang, C.-C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Q.; Li, K.; Chen, L.; Li, W.; Hou, M.; Liu, T.; Yang, J.; Lindvall, C.; Björkholm, M.; et al. Telomerase reverse transcriptase promotes epithelial–mesenchymal transition and stem cell-like traits in cancer cells. Oncogene 2013, 32, 4203–4213. [Google Scholar] [CrossRef]
- Berletch, J.B.; Liu, C.; Love, W.K.; Andrews, L.G.; Katiyar, S.K.; Tollefsbol, T.O. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J. Cell. Biochem. 2008, 103, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-W.; Lin, C.-C.; Yu, Y.-L.; Lin, P.-C.; Wu, M.-T.; Chen, C.-J.; Chang, W.; Lin, S.-Z.; Chen, Y.-L.S.; Harn, H.-J. n-Butylidenephthalide induced apoptosis in the A549 human lung adenocarcinoma cell line by coupled down-regulation of AP-2α and telomerase activity. Acta Pharmacol. Sin. 2009, 30, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Yang, S.-T. Development of a dual fluorescence system for simultaneous detection of two cell populations in a 3D coculture. Process. Biochem. 2019, 86, 144–150. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2012, 65, 157–170. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- IzuMi, M.; Miyazawa, H.; Kamakura, T.; Yamaguchi, I.; Endo, T.; Hanaoka, F. Blasticidin S-resistance gene (bsr): A novel selectable marker for mammalian cells. Exp. Cell Res. 1991, 197, 229–233. [Google Scholar] [CrossRef]
- Svidritskiy, E.; Ling, C.; Ermolenko, D.N.; Korostelev, A.A. Blasticidin S inhibits translation by trapping deformed tRNA on the ribosome. Proc. Natl. Acad. Sci. USA 2013, 110, 12283–12288. [Google Scholar] [CrossRef]
- Barbuti, A.M.; Chen, Z.-S. Paclitaxel through the ages of anticancer therapy: Exploring its role in chemoresistance and radiation therapy. Cancers 2015, 7, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.K.; Zhou, C.; Malloy, K.M.; Sun, L.; Zhong, Y.; Gehrig, P.A.; Bae-Jump, V.L. Metformin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and modulation of the mTOR pathway. Gynecol. Oncol. 2012, 125, 458–469. [Google Scholar] [CrossRef]
- Shafer, A.; Zhou, C.; Gehrig, P.A.; Boggess, J.F.; Bae-Jump, V.L. Rapamycin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and induction of apoptosis. Int. J. Cancer 2010, 126, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Mandlekar, S.; Kong, A.N. Mechanisms of tamoxifen-induced apoptosis. Apoptosis 2001, 6, 469–477. [Google Scholar] [PubMed]
- Wang, Z.; Kyo, S.; Maida, Y.; Takakura, M.; Tanaka, M.; Yatabe, N.; Kanaya, T.; Nakamura, M.; Koike, K.; Hisamoto, K.; et al. Tamoxifen regulates human telomerase reverse transcriptase (hTERT) gene expression differently in breast and endometrial cancer cells. Oncogene 2002, 21, 3517–3524. [Google Scholar] [CrossRef]
- Zhou, C.; Steplowski, T.A.; Dickens, H.K.; Malloy, K.M.; Gehrig, P.A.; Boggess, J.F.; Bae-Jump, V.L. Estrogen induction of telomerase activity through regulation of the mitogen-activated protein kinase (MAPK) dependent pathway in human endometrial cancer cells. PLoS ONE 2013, 8, e55730. [Google Scholar] [CrossRef]
- Boggess, J.F.; Zhou, C.; Bae-Jump, V.L.; Gehrig, P.A.; Whang, Y.E. Estrogen-receptor-dependent regulation of telomerase activity in human endometrial cancer cell lines. Gynecol. Oncol. 2006, 103, 417–424. [Google Scholar] [CrossRef]
- Ramlee, M.K.; Wang, J.; Toh, W.X.; Li, S. Transcription regulation of the human telomerase reverse transcriptase (hTERT) gene. Genes 2016, 7, 50. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Ahmadian, S.; Zarghami, N.; Kahroba, H. Fundamental insights into the interaction between telomerase/TERT and intracellular signaling pathways. Biochimie 2021, 181, 12–24. [Google Scholar] [CrossRef]
- Lamb, R.; Ozsvari, B.; Bonuccelli, G.; Smith, D.L.; Pestell, R.G.; Martinez-Outschoorn, U.E.; Clarke, R.B.; Sotgia, F.; Lisanti, M.P. Dissecting tumor metabolic heterogeneity: Telomerase and large cell size metabolically define a sub-population of stem-like, mitochondrial-rich, cancer cells. Oncotarget 2015, 6, 21892–21905. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Otero, J.; Ray, P.; Paulmurugan, R.; Hoffman, A.R.; Gambhir, S.S.; Biswal, S.; A Ulaner, G. Visualization of telomerase reverse transcriptase (hTERT) promoter activity using a trimodality fusion reporter construct. J. Nucl. Med. 2006, 47, 270–277. [Google Scholar] [PubMed]
- Fujiki, T.; Katakura, Y.; Miura, T.; Shirahata, S. Application of hTERT Promoter to Cancer Therapy. In Animal Cell Technology: From Target to Market; Lindner-Olsson, E., Chatzissavidou, N., Lüllau, E., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 562–564. [Google Scholar] [CrossRef]
- Gu, J.; Kagawa, S.; Takakura, M.; Kyo, S.; Inoue, M.; A Roth, J.; Fang, B. Tumor-specific transgene expression from the human telomerase reverse transcriptase promoter enables targeting of the therapeutic effects of the Bax gene to cancers. Cancer Res. 2000, 60, 5359–5364. [Google Scholar] [PubMed]
- Xin, X.; Yang, H.; Zhang, F.; Yang, S.-T. 3D cell coculture tumor model: A promising approach for future cancer drug discovery. Proc. Biochem. 2019, 78, 148–160. [Google Scholar]
- Eskiocak, U.; Işeri, O.D.; Kars, M.D.; Biçer, A.; Gunduz, U. Effect of doxorubicin on telomerase activity and apoptotic gene expression in doxorubicin-resistant and-sensitive MCF-7 cells. Chemotherapy 2008, 54, 209–216. [Google Scholar] [CrossRef]
- Lanvers-Kaminsky, C.; Winter, B.; Koling, S.; Frodermann, B.; Braun, Y.; Schaefer, K.-L.; Diallo, R.; Koenemann, S.; Wai, D.; Willich, N.; et al. Doxorubicin modulates telomerase activity in Ewing’s sarcoma in vitro and in vivo. Oncol. Rep. 2005, 14, 751–758. [Google Scholar] [CrossRef]
- Lee, B.-J.; Lee, B.-H.; Wang, S.-G.; Lee, J.-C.; Roh, H.-J.; Goh, E.-K.; Kim, C.-M.; Jun, E.-S. Change of the expression of human telomerase reverse transcriptase mRNA and human telomerase RNA after cisplatin and 5-fluorouracil exposure in head and neck squamous cell carcinoma cell lines. J. Korean Med. Sci. 2007, 22, S73–S78. [Google Scholar] [CrossRef]
- Guo, X.L.; Ma, N.N.; Zhou, F.G.; Zhang, L.; Bu, X.X.; Sun, K.; Song, J.-R.; Li, R.; Zhang, B.-H.; Wu, M.-C.; et al. Up-regulation of hTERT expression by low-dose cisplatin contributes to chemotherapy resistance in human hepatocellular cancer cells. Oncol. Rep. 2009, 22, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.P.; Wang, X.W.; Xie, H. Effects of cisplatin on telomerase activity and telomere length in BEL-7404 human hepatoma cells. Cell Res. 2002, 12, 55–62. [Google Scholar] [CrossRef]
- Sperandei, S. Understanding logistic regression analysis. Biochem. Medica 2014, 24, 12–18. [Google Scholar] [CrossRef]
- Chen, C.; Yue, D.; Lei, L.; Wang, H.; Lu, J.; Zhou, Y.; Liu, S.; Ding, T.; Guo, M.; Xu, L. Promoter-Operating Targeted Expression of Gene Therapy in Cancer: Current Stage and Prospect. Mol. Ther. Nucleic Acids 2018, 11, 508–514. [Google Scholar] [CrossRef]
- Davis, J.J.; Wang, L.; Dong, F.; Zhang, L.; Guo, W.; Teraishi, F.; Xu, K.; Ji, L.; Fang, B. Oncolysis and suppression of tumor growth by a GFP-expressing oncolytic adenovirus controlled by an hTERT and CMV hybrid promoter. Cancer Gene Ther. 2006, 13, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Bowman, K.R.; Kim, J.H.; Lim, C.S. Narrowing the field: Cancer-specific promoters for mitochondrially-targeted p53-BH3 fusion gene therapy in ovarian cancer. J. Ovarian Res. 2019, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gerbi, S.A. Making ends meet: Targeted integration of DNA fragments by genome editing. Chromosoma 2018, 127, 405–420. [Google Scholar] [CrossRef]
- Smith, K. Theoretical mechanisms in targeted and random integration of transgene DNA. Reprod. Nutr. Dev. 2001, 41, 465–485. [Google Scholar] [CrossRef][Green Version]
- Würtele, H.; E Little, K.C.; Chartrand, P. Illegitimate DNA integration in mammalian cells. Gene Ther. 2003, 10, 1791–1799. [Google Scholar] [CrossRef]
- Volpicella, M.; Leoni, C.; Costanza, A.; Fanizza, I.; Placido, A.; Ceci, L.R. Genome Walking by Next Generation Sequencing Approaches. Biology 2012, 1, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, J.; Almeida, A.; Pópulo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef]
- Lee, D.D.; Leão, R.; Komosa, M.; Gallo, M.; Zhang, C.H.; Lipman, T.; Remke, M.; Heidari, A.; Nunes, N.M.; Apolónio, J.D.; et al. DNA hypermethylation within TERT promoter upregulates TERT expression in cancer. J. Clin. Investig. 2018, 129, 223–229. [Google Scholar] [CrossRef]



| 0 h | 24 h | 48 h | 72 h | 96 h | 120 h | 144 h | P0 | P1 | P2 | M | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blasticidin | 0.98 | 0.99 | 0.98 | 1.01 | 0.93 | 0.84 | - | 0.99 | −0.016 | −0.099 | −6.992 |
| Cisplatin | 0.97 | 1.04 | 1.06 | 0.94 | 0.98 | 0.82 | - | 1.02 | −0.066 | −0.123 | −7.573 |
| Pristimerin | 0.96 | 0.96 | 0.98 | 0.80 | 0.84 | 0.86 | - | 0.97 | −0.148 | −0.115 | −5.295 |
| Doxorubicin | 0.95 | 0.95 | 0.98 | 0.99 | 1.01 | 0.93 | 0.90 | 0.96 | 0.012 | −0.044 | −3.877 |
| Paclitaxcel | 0.97 | 1.03 | 1.05 | 0.97 | 0.95 | 0.99 | 1.06 | 1.01 | −0.044 | 0.010 | 0.973 |
| Tamoxifen | 1.00 | 0.96 | 0.96 | 0.91 | 1.01 | 1.11 | - | 0.97 | −0.013 | 0.088 | 5.522 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, F.; Qin, Z.; Yang, S.-T. Development of 3D Cell-Based Fluorescent Reporter Assay for Screening of Drugs Downregulating Telomerase Reverse Transcriptase. Bioengineering 2025, 12, 335. https://doi.org/10.3390/bioengineering12040335
Li Y, Zhang F, Qin Z, Yang S-T. Development of 3D Cell-Based Fluorescent Reporter Assay for Screening of Drugs Downregulating Telomerase Reverse Transcriptase. Bioengineering. 2025; 12(4):335. https://doi.org/10.3390/bioengineering12040335
Chicago/Turabian StyleLi, You, Fengli Zhang, Zhen Qin, and Shang-Tian Yang. 2025. "Development of 3D Cell-Based Fluorescent Reporter Assay for Screening of Drugs Downregulating Telomerase Reverse Transcriptase" Bioengineering 12, no. 4: 335. https://doi.org/10.3390/bioengineering12040335
APA StyleLi, Y., Zhang, F., Qin, Z., & Yang, S.-T. (2025). Development of 3D Cell-Based Fluorescent Reporter Assay for Screening of Drugs Downregulating Telomerase Reverse Transcriptase. Bioengineering, 12(4), 335. https://doi.org/10.3390/bioengineering12040335








