Berg Balance Scale Scoring System for Balance Evaluation by Leveraging Attention-Based Deep Learning with Wearable IMU Sensors
Abstract
1. Introduction
2. Materials and Methods
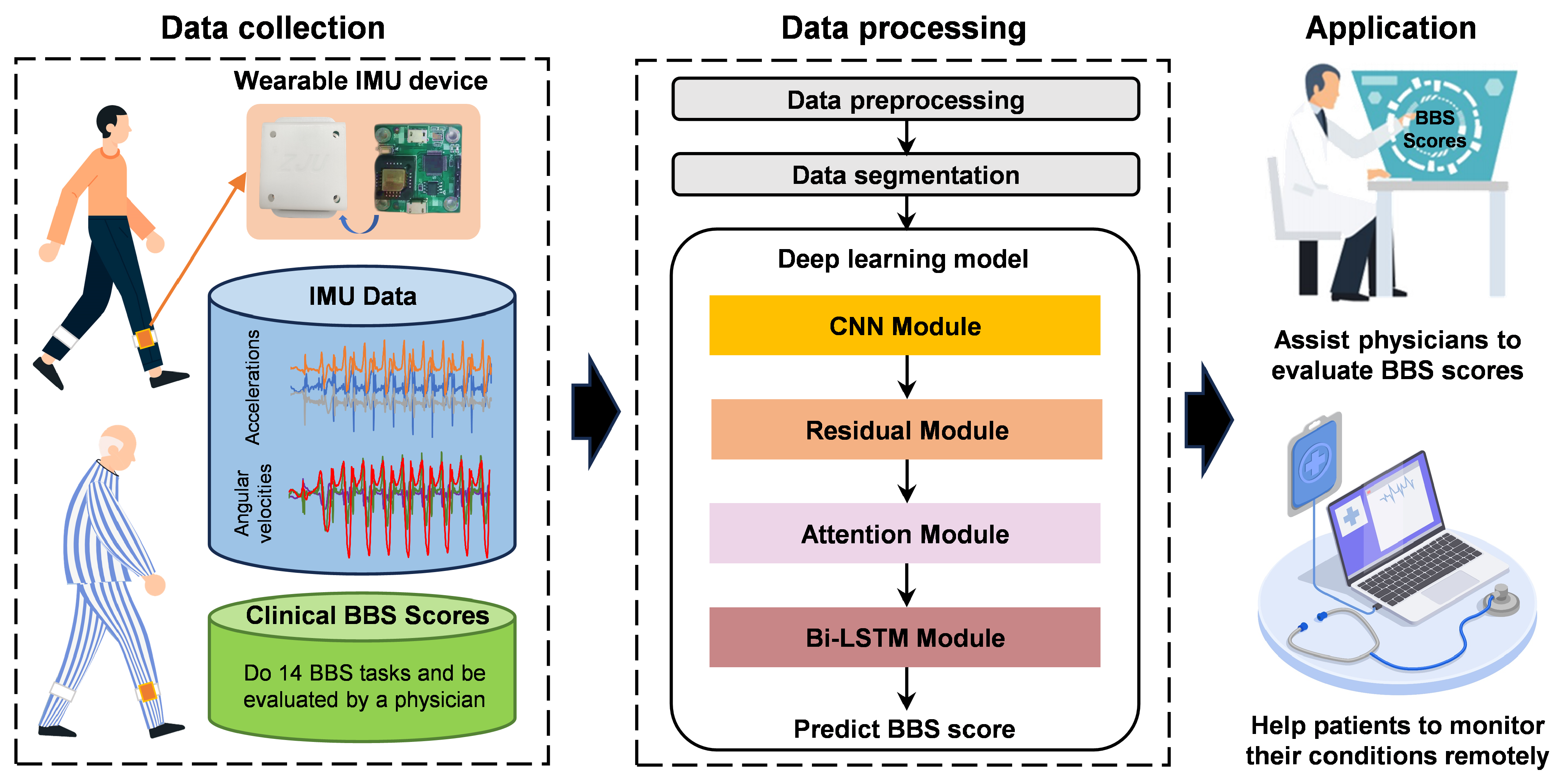
2.1. System Overview
2.2. Data Collection
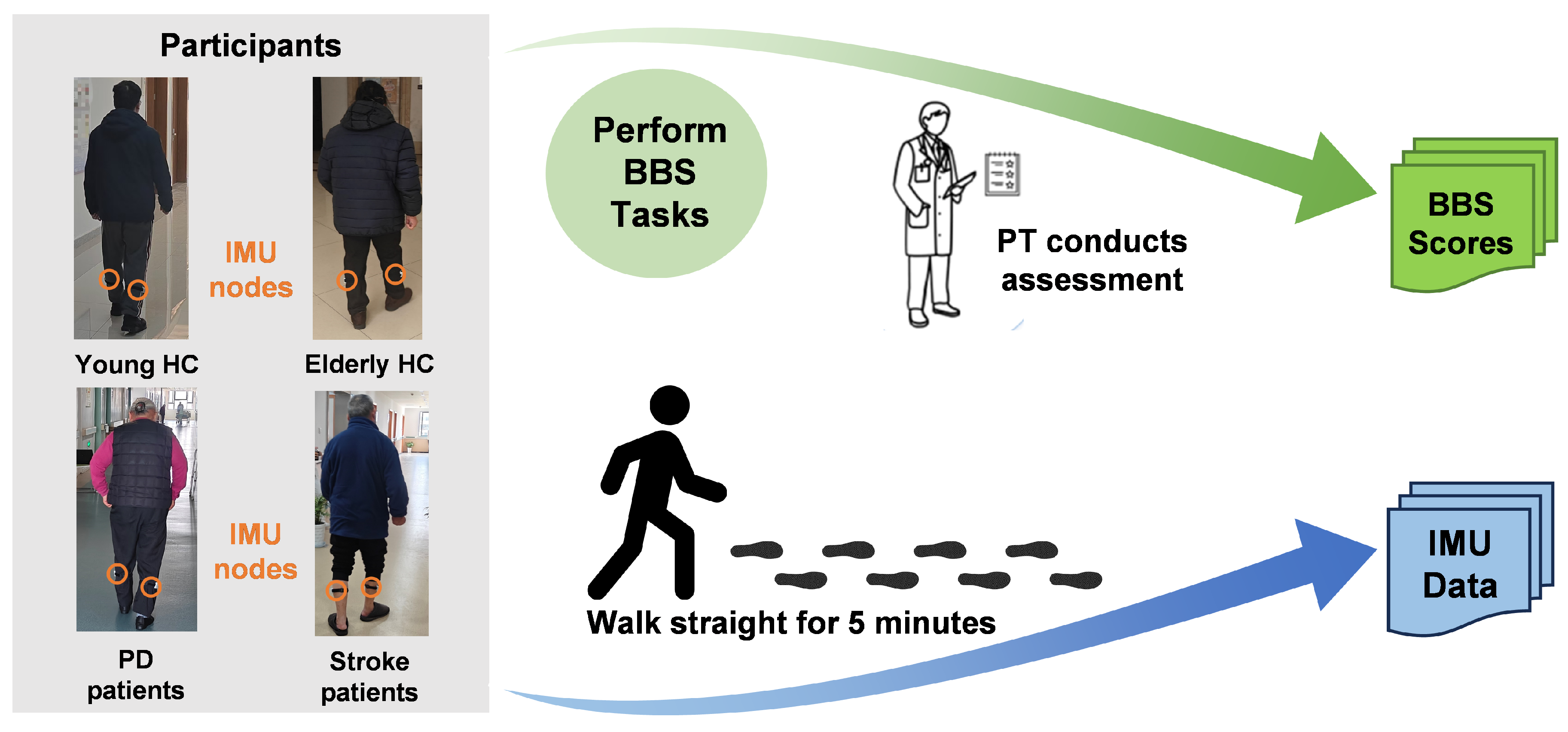
2.2.1. Information of Participants
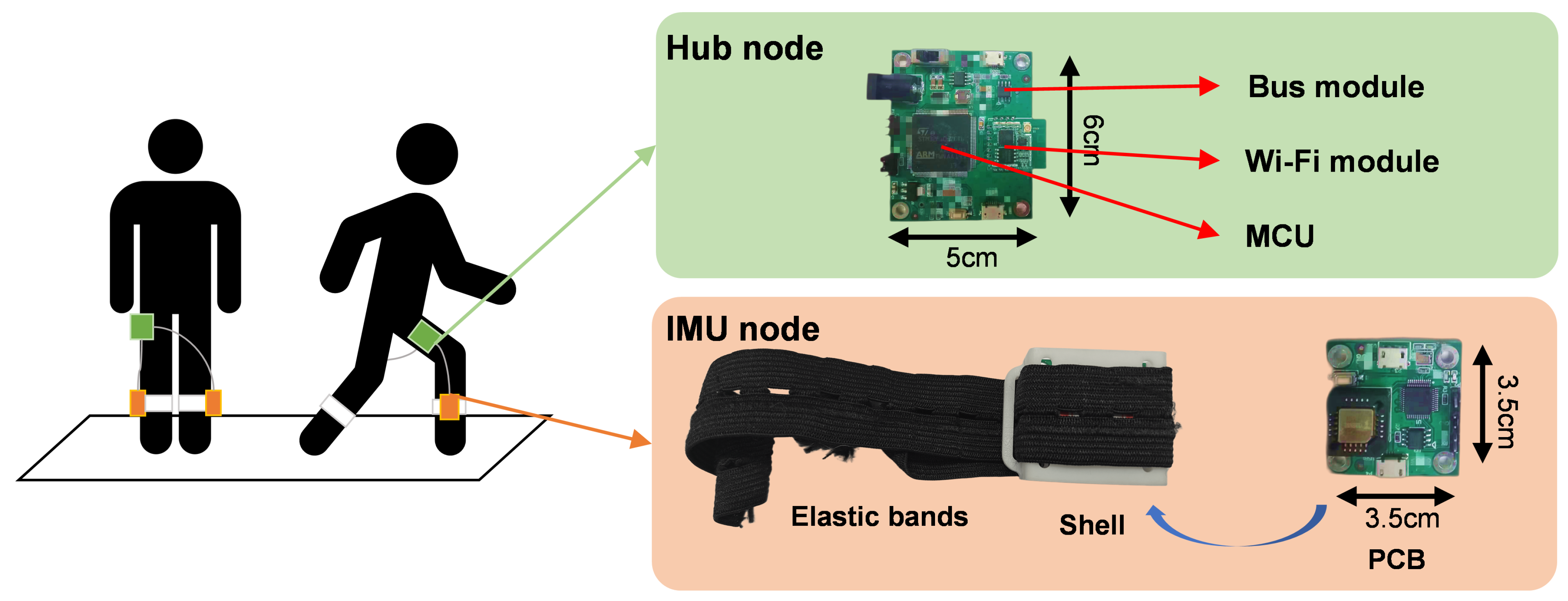
2.2.2. Hardware Components of the IMU Device
2.2.3. Task Protocol
2.3. Data Preprocessing
2.3.1. IMU Data Filtering and Standardization
2.3.2. BBS Normalization
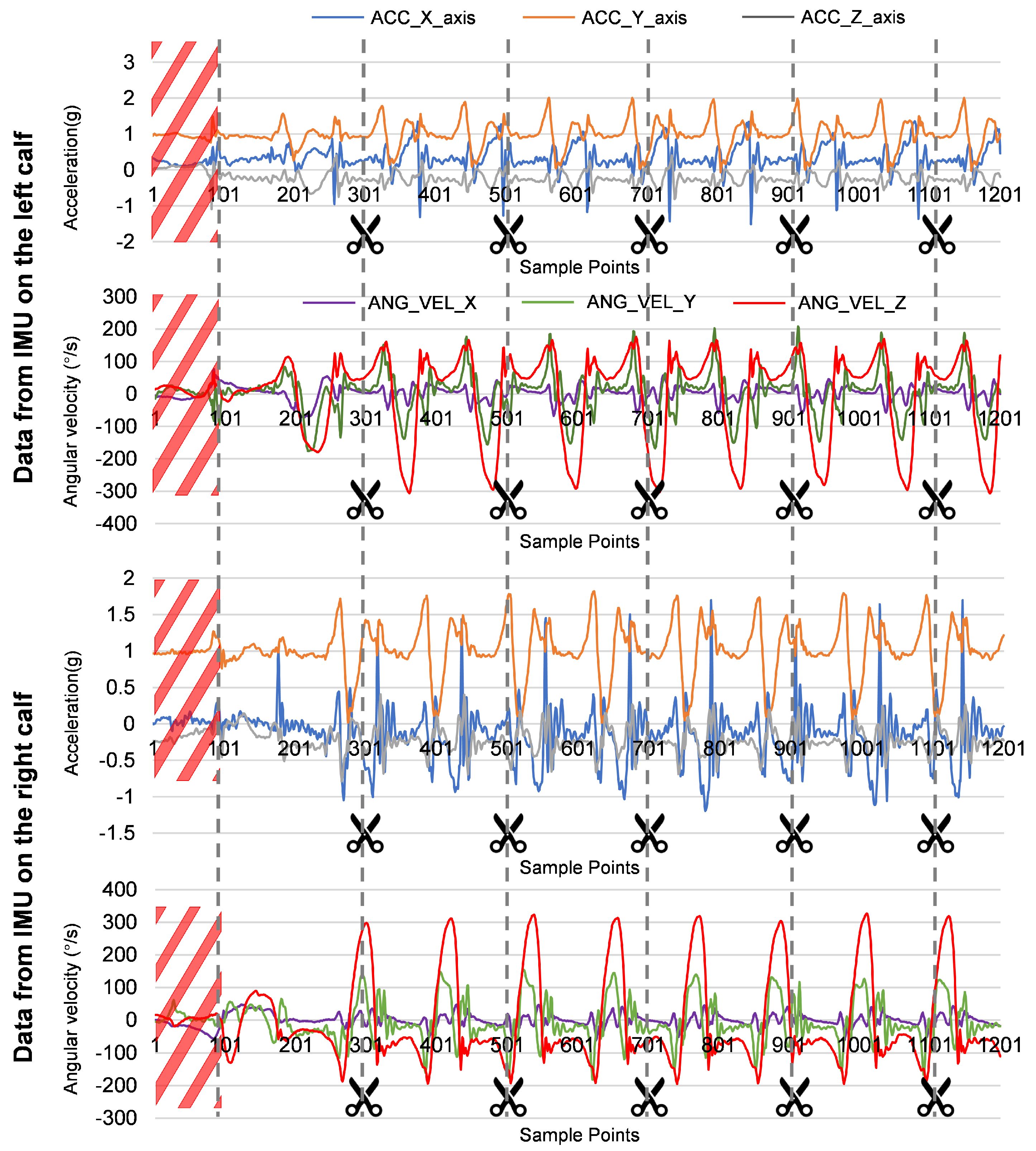
2.3.3. Gait Data Segmentation
2.4. Deep Learning Model Architecture
3. Experiments and Results
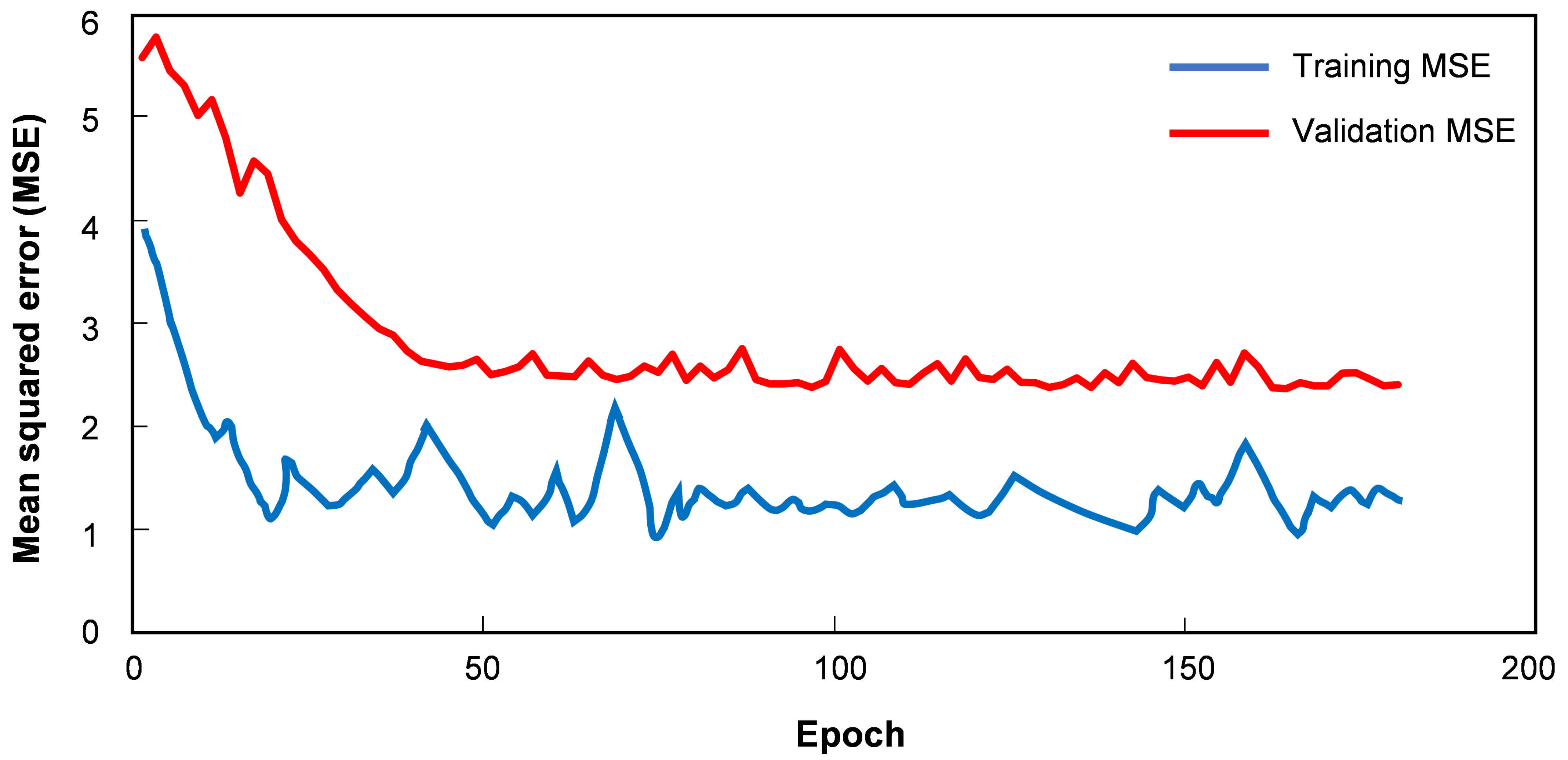
3.1. Training Details
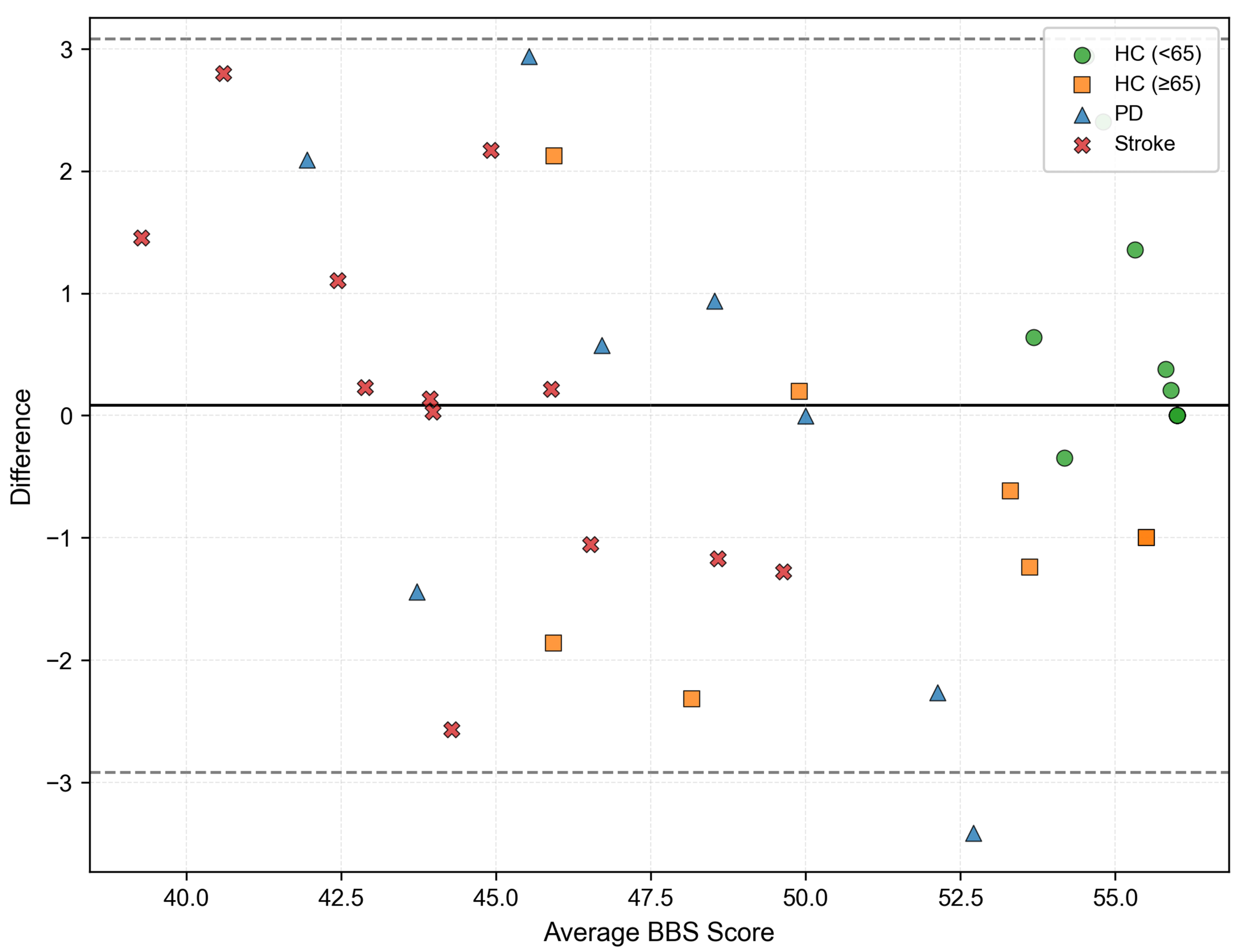
3.2. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beck Jepsen, D.; Robinson, K.; Ogliari, G.; Montero-Odasso, M.; Kamkar, N.; Ryg, J.; Masud, T. Predicting Falls in Older Adults: An Umbrella Review of Instruments Assessing Gait, Balance, and Functional Mobility. BMC Geriatr. 2022, 22, 615. [Google Scholar]
- Felius, R.A.W.; Geerars, M.; Bruijn, S.M.; Wouda, N.C.; Van Dieën, J.H.; Punt, M. Reliability of IMU-Based Balance Assessment in Clinical Stroke Rehabilitation. Gait Posture 2022, 98, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, D.; Liu, Y.; Wang, J.; Xiao, Q. Virtual Reality for Limb Motor Function, Balance, Gait, Cognition and Daily Function of Stroke Patients: A Systematic Review and Meta-Analysis. J. Adv. Nurs. 2021, 77, 3255–3273. [Google Scholar]
- Muir, S.W.; Berg, K.; Chesworth, B.; Speechley, M. Use of the Berg Balance Scale for predicting multiple falls in community-dwelling elderly people: A prospective study. Phys. Ther. 2008, 88, 449–459. [Google Scholar] [PubMed]
- Zimbelman, J.L.; Juraschek, S.P.; Zhang, X.; Lin, V.W.H. Physical therapy workforce in the United States: Forecasting nationwide shortages. PM&R 2010, 2, 1021–1029. [Google Scholar]
- Bacciu, D.; Chessa, S.; Gallicchio, C.; Micheli, A.; Pedrelli, L.; Ferro, E.; Parodi, O. A learning system for automatic Berg Balance Scale score estimation. Eng. Appl. Artif. Intell. 2017, 66, 60–74. [Google Scholar] [CrossRef]
- Johnson, L.; Fry, A.; Dehbandi, B.; Rubin, L.; Halem, M.; Barachant, A.; Putrino, D. An automated, electronic assessment tool can accurately classify older adult postural stability. J. Biomech. 2019, 93, 6–10. [Google Scholar]
- Han, Y.; Liu, X.; Zhang, N.; Zhang, X.; Zhang, B.; Wang, S.; Liu, T.; Yi, J. Automatic Assessments of Parkinsonian Gait with Wearable Sensors for Human Assistive Systems. Sensors 2023, 23, 2104. [Google Scholar] [CrossRef]
- Similä, H.; Mäntyjärvi, J.; Merilahti, J.; Lindholm, M.; Ermes, M. Accelerometry-based Berg Balance Scale score estimation. IEEE J. Biomed. Health Inform. 2013, 18, 1114–1121. [Google Scholar]
- Badura, P.; Pietka, E. Automatic Berg Balance Scale assessment system based on accelerometric signals. Biomed. Signal Process. Control. 2016, 24, 114–119. [Google Scholar] [CrossRef]
- Shahzad, A.; Ko, S.; Lee, S.; Lee, J.A.; Kim, K. Quantitative assessment of balance impairment for fall-risk estimation using wearable triaxial accelerometer. IEEE Sens. J. 2017, 17, 6743–6751. [Google Scholar]
- Lin, B.S.; Zhang, Z.; Peng, C.W.; Lin, C.C.; Lin, C.H.; Lin, B.S. Automatic assessment system based on IMUs and machine learning for predicting Berg Balance Scale. IEEE Sens. J. 2022, 22, 19919–19930. [Google Scholar]
- Kong, D.; Huang, W.; Zhao, L.; Ding, J.; Wu, H.; Yang, G. Mining knowledge from unlabeled data for fault diagnosis: A multi-task self-supervised approach. Mech. Syst. Signal Process. 2024, 211, 111189. [Google Scholar]
- Lv, H.; Yang, G.; Zhou, H.; Huang, X.; Yang, H.; Pang, Z. Teleoperation of collaborative robot for remote dementia care in home environments. IEEE J. Transl. Eng. Health Med. 2020, 8, 1–10. [Google Scholar]
- Zhou, H.; Yang, G.; Wang, B.; Li, X.; Wang, R.; Huang, X.; Wang, X.V. An attention-based deep learning approach for inertial motion recognition and estimation in human-robot collaboration. J. Manuf. 2023, 67, 97–110. [Google Scholar]
- Mannini, A.; Trojaniello, D.; Cereatti, A.; Sabatini, A.M. A machine learning framework for gait classification using inertial sensors: Application to elderly, post-stroke and Huntington’s disease patients. Sensors 2016, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Abdul Jabbar, K.; Sarvestan, J.; Zia Ur Rehman, R.; Lord, S.; Kerse, N.; Teh, R.; Del Din, S. Validation of an Algorithm for Measurement of Sedentary Behaviour in Community-Dwelling Older Adults. Sensors 2023, 23, 4605. [Google Scholar] [CrossRef]
- Romijnders, R.; Warmerdam, E.; Hansen, C.; Schmidt, G.; Maetzler, W. A Deep Learning Approach for Gait Event Detection from a Single Shank-Worn IMU: Validation in Healthy and Neurological Cohorts. Sensors 2022, 22, 3859. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Li, Q.; Liu, T.; Yi, J. IMU-based gait normalcy index calculation for clinical evaluation of impaired gait. IEEE J. Biomed. Health Inform. 2020, 25, 3–12. [Google Scholar]
- Keogh, A.; Argent, R.; Anderson, A.; Caulfield, B.; Johnston, W. Assessing the usability of wearable devices to measure gait and physical activity in chronic conditions: A systematic review. J. Neuroeng. Rehabilitat. 2021, 18, 138. [Google Scholar]
- Lin, B.S.; Lee, I.J.; Wang, S.P.; Chen, J.L.; Lin, B.S. Residual neural network and long short-term memory–based algorithm for estimating the motion trajectory of inertial measurement units. IEEE Sens. J. 2022, 22, 6910–6919. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, B.; Zhu, P.; Li, P.; Zuo, W.; Hu, Q. ECA-Net: Efficient channel attention for deep convolutional neural networks. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Seattle, WA, USA, 14–19 June 2020; pp. 11534–11542. [Google Scholar]
- Hu, J.; Shen, L.; Sun, G. Squeeze-and-excitation networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 7132–7141. [Google Scholar]
- Lin, C.H.; Peng, C.W.; Lee, I.J.; Wu, C.M.; Lin, B.S. Analysis of gait data based on deep learning to predict multiple balance scale scores. IEEE Sens. J. 2023, 24, 920–930. [Google Scholar] [CrossRef]

| HC (≤65) (n = 12) | HC (≥65) (n = 8) | PD (n = 8) | Stroke (n = 12) | |
|---|---|---|---|---|
| Age (years) | 38.74 ± 12.43 | 72.38 ± 4.57 | 56.85 ± 8.41 | 60.73 ± 5.66 |
| Height (m) | 1.70 ± 0.18 | 1.66 ± 0.31 | 1.58 ± 0.26 | 1.63 ± 0.04 |
| Weight (kg) | 70.31 ± 8.24 | 60.68 ± 2.32 | 49.31 ± 4.83 | 55.71 ± 7.94 |
| H&Y Staging | - | - | 1.50 ± 0.76 | - |
| UPDRS Part III | - | - | 20.8 ± 5.6 | - |
| Modified Rankin Scale | - | - | - | 1.67 ± 0.89 |
| BBS Scores | 55.67 ± 0.75 | 50.63 ± 3.67 | 47.63 ± 3.04 | 44.50 ± 2.47 |
| Task Index | Description |
|---|---|
| 1 | Move from sitting to standing |
| 2 | Stand up unsupported |
| 3 | Sit unsupported |
| 4 | Move from a standing to a sitting position |
| 5 | Transfer from one chair to another |
| 6 | Stand up with eyes closed |
| 7 | Stand with two feet together |
| 8 | Reach forward with an outstretched arm |
| 9 | Pick an object up off the floor |
| 10 | Turn and look behind |
| 11 | Turn around in a complete circle |
| 12 | Alternate placing each foot onto the stool |
| 13 | Stand unsupported with one foot in front |
| 14 | Stand on one leg for as long as one can |
| RMSE | MAE | |
|---|---|---|
| CNN | 2.0561 | 1.5898 |
| Bi-LSTM | 2.5789 | 2.0345 |
| CNN + Bi-LSTM | 1.8866 | 1.5606 |
| Bi-LSTM (with attention) | 1.8101 | 1.3819 |
| CNN + Bi-LSTM (with attention) | 1.5333 | 1.1627 |
| Subgroup | RMSE | MAE |
|---|---|---|
| Young healthy people | 1.1892 | 0.6897 |
| Elderly healthy people | 1.4702 | 1.2943 |
| Patients with PD | 2.0328 | 1.7075 |
| Patients with stroke | 1.4923 | 1.1847 |
| Johnson et al. [7] | Shahzad et al. [11] | Lin et al. [12] | Lin et al. [24] | This Work | |
|---|---|---|---|---|---|
| Device | Kinect2 | IMU | IMU | IMU | IMU |
| Participants | 43 | 23 | 136 | 136 | 40 |
| Participant category | HC | HC and Patients | HC | HC | HC and Patients |
| Test task | Task 5 and task 6 in BBS | TUGT, FTSS and AST tasks 1 | Task 12 and task 14 in BBS | Walking 15m | Walking |
| Model | Neural network | Lasso regression | Random forest | CNN + LSTM | CNN + Bi-LSTM (with attention) |
| BBS RMSE | N/A | 1.9700 | N/A | N/A | 1.5333 |
| BBS MAE | 1.1677 | 1.4400 | 1.2700 | 1.4300 | 1.1627 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Zhou, H.; Lyu, H.; Wu, H.; Tian, S.; Yang, G. Berg Balance Scale Scoring System for Balance Evaluation by Leveraging Attention-Based Deep Learning with Wearable IMU Sensors. Bioengineering 2025, 12, 395. https://doi.org/10.3390/bioengineering12040395
Lu Z, Zhou H, Lyu H, Wu H, Tian S, Yang G. Berg Balance Scale Scoring System for Balance Evaluation by Leveraging Attention-Based Deep Learning with Wearable IMU Sensors. Bioengineering. 2025; 12(4):395. https://doi.org/10.3390/bioengineering12040395
Chicago/Turabian StyleLu, Zhangli, Huiying Zhou, Honghao Lyu, Haiteng Wu, Shaohua Tian, and Geng Yang. 2025. "Berg Balance Scale Scoring System for Balance Evaluation by Leveraging Attention-Based Deep Learning with Wearable IMU Sensors" Bioengineering 12, no. 4: 395. https://doi.org/10.3390/bioengineering12040395
APA StyleLu, Z., Zhou, H., Lyu, H., Wu, H., Tian, S., & Yang, G. (2025). Berg Balance Scale Scoring System for Balance Evaluation by Leveraging Attention-Based Deep Learning with Wearable IMU Sensors. Bioengineering, 12(4), 395. https://doi.org/10.3390/bioengineering12040395









