Comparing the Accuracy of Markerless Motion Analysis and Optoelectronic System for Measuring Gait Kinematics of Lower Limb
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
- -
- Five healthy subjects without motor disabilities, with no history of serious injuries (e.g., ligament or musculoskeletal injuries, neurological disorders, fractures) and no history of surgical procedures on the extremities or trunk (4 males/1 females; mean age: 9.24–SD 3.98; age range: 5.4–15.0).
- -
- Five subjects with a diagnosis of cerebral palsy (CP) and right hemiplegia, able to walk more than 30 m without assistance, orthotics, or aids (3 males/2 females; mean age: 11.44–SD 3.55; age range: 7.6–15.9).
- -
- Five subjects with a diagnosis of cerebral palsy and left hemiplegia, able to walk more than 30 m without assistance, orthotics, or aids (2 males/3 females; mean age: 10.88–SD 3.16; age range: 6.8–14.5).
- -
- Five subjects with a diagnosis of spastic paraparesis, able to walk more than 30 m without assistance, orthotics, or aids (3 males/2 females; mean age: 12.44–SD 3.46; age range: 9.4–17.7).
2.2. Data Collection
2.3. Data Processing
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Washabaugh, E.P.; Shanmugam, T.A.; Ranganathan, R.; Krishnan, C. Comparing the accuracy of open-source pose estimation methods for measuring gait kinematics. Gait Posture 2022, 97, 188–195. [Google Scholar] [CrossRef]
- Viehweger, E.; Pfund, L.Z.; Hélix, M.; Rohon, M.A.; Jacquemier, M.; Scavarda, D.; Jouve, J.L.; Bollini, G.; Loundou, A.; Simeoni, M.C. Influence of clinical and gait analysis experience on reliability of observational gait analysis (Edinburgh Gait Score Reliability). Ann. Phys. Rehabil. Med. 2010, 53, 535–546. [Google Scholar] [CrossRef]
- Brunnekreef, J.J.; Van Uden, C.J.T.; Van Moorsel, S.; Kooloos, J.G.M. Reliability of videotaped observational gait analysis in patients with orthopedic impairments. BMC Musculoskelet. Disord. 2005, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Ma’touq, J.; Hu, T.; Haddadin, S. Sub-millimetre accurate human hand kinematics: From surface to skeleton. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Cappozzo, A.; Della Croce, U.; Leardini, A.; Chiari, L. Human movement analysis using stereophotogrammetry. Part 1: Theoretical background. Gait Posture 2005, 21, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; Wiley Online Library: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Krishnan, C.; Washabaugh, E.P.; Seetharaman, Y. A low cost real-time motion tracking approach using webcam technology. J. Biomech. 2015, 48, 544–548. [Google Scholar] [CrossRef]
- Colyer, S.L.; Evans, M.; Cosker, D.P.; Salo, A.I.T. A Review of the Evolution of Vision-Based Motion Analysis and the Integration of Advanced Computer Vision Methods Towards Developing a Markerless System. Sport. Med. Open 2018, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Takimoto, H.; Yamasaki, T.; Higashi, A. Validity of time series kinematical data as measured by a markerless motion capture system on a flatland for gait assessment. J. Biomech. 2018, 71, 281–285. [Google Scholar] [CrossRef]
- Clark, R.A.; Mentiplay, B.F.; Hough, E.; Pua, Y.H. Three-dimensional cameras and skeleton pose tracking for physical function assessment: A review of uses, validity, current developments and Kinect alternatives. Gait Posture 2019, 68, 193–200. [Google Scholar] [CrossRef]
- Shotton, B.J.; Sharp, T.; Kipman, A.; Fitzgibbon, A.; Finocchio, M.; Blake, A.; Cook, R.M. Moore “Real-Time Human Pose from Single Depth Images”. In Proceedings of the 2011 Conference on Computer Vision and Pattern Recognitio, Washington, DC, USA, 20–25 June 2011; pp. 129–1304. [Google Scholar]
- Ye, M.; Zhang, Q.; Wang, L.; Zhu, J. A Survey on Human Motion Analysis from Depth Data. In Time-of-Flight and Depth Imaging. Sensors, Algorithms, and Applications; Springer: Berlin/Heidelberg, Germany, 2013; Volume 8200, pp. 149–187. [Google Scholar]
- Francia, C.; Motta, F.; Donno, L.; Covarrubias, M.; Dornini, C.; Madella, A.; Galli, M. Validation of a MediaPipe System for Markerless Motion Analysis During Virtual Reality Rehabilitation. In International Conference on Extended Reality; LNCS; Springer Nature: Cham, Switzerland, 2024; Volume 15029, pp. 40–49. [Google Scholar] [CrossRef]
- Cao, Z.; Simon, T.; Wei, S.E.; Sheikh, Y. Realtime multi-person 2D pose estimation using part affinity fields. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 1302–1310. [Google Scholar] [CrossRef]
- Leonid Sigal Related Concepts. In Computer Vision; Cambridge University Press: Cambridge, UK, 2021; pp. 753–754. [CrossRef]
- Cao, Z.; Hidalgo, G.; Simon, T.; Wei, S.E.; Sheikh, Y. OpenPose: Realtime Multi-Person 2D Pose Estimation Using Part Affinity Fields. IEEE Trans. Pattern Anal. Mach. Intell. 2021, 43, 172–186. [Google Scholar] [CrossRef]
- Simon, T.; Joo, H.; Matthews, I.; Sheikh, Y. Hand keypoint detection in single images using multiview bootstrapping. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; Volume 2017. [Google Scholar] [CrossRef]
- Wei, S.-E.; Ramakrishna, V.; Kanada, T.; Sheikh, Y. Convolutional pose machines. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Gu, X.; Deligianni, F.; Lo, B.; Chen, W.; Yang, G.Z. Markerless gait analysis based on a single RGB camera. In Proceedings of the 2018 IEEE 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Las Vegas, NV, USA, 4–7 March 2018; Volume 2018, pp. 42–45. [Google Scholar] [CrossRef]
- Yamamoto, M.; Shimatani, K.; Hasegawa, M.; Kurita, Y.; Ishige, Y.; Takemura, H. Accuracy of Temporo-Spatial and Lower Limb Joint Kinematics Parameters Using OpenPose for Various Gait Patterns with Orthosis. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2666–2675. [Google Scholar] [CrossRef] [PubMed]
- Stenum, J.; Rossi, C.; Roemmich, R.T. Two-dimensional video-based analysis of human gait using pose estimation. PLoS Comput. Biol. 2021, 17, e1008935. [Google Scholar] [CrossRef]
- Mehdizadeh, S.; Nabavi, H.; Sabo, A.; Arora, T.; Iaboni, A.; Taati, B. Concurrent validity of human pose tracking in video for measuring gait parameters in older adults: A preliminary analysis with multiple trackers, viewing angles, and walking directions. J. Neuroeng. Rehabil. 2021, 18, 139. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Tateuchi, H.; Hashiguchi, T.; Ichihashi, N. Verification of validity of gait analysis systems during treadmill walking and running using human pose tracking algorithm. Gait Posture 2021, 85, 290–297. [Google Scholar] [CrossRef]
- Guo, R.; Shao, X.; Zhang, C.; Qian, X. Sparse Adaptive Graph Convolutional Network for Leg Agility Assessment in Parkinson’s Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2837–2848. [Google Scholar] [CrossRef]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef]
- Duffell, L.D.; Jordan, S.J.; Cobb, J.P.; McGregor, A.H. Gait adaptations with aging in healthy participants and people with knee-joint osteoarthritis. Gait Posture 2017, 57, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.B., III; Õunpuu, S.; Tyburski, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 2016, 10, 575–587. [Google Scholar] [CrossRef]
- Zeni, J.A.; Richards, J.G.; Higginson, J.S. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 2008, 27, 710–714. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An introduction to the bootstrap. Monographs on Statistics and Applied Probability. Monogr. Stat. Appl. Probab. 1993, 57, 436. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.B.; Messenger, N.; Buckley, J.G. Forefoot, rearfoot and shank coupling: Effect of variations in speed and mode of gait. Gait Posture 2007, 25, 295–302. [Google Scholar] [CrossRef]
- Nakano, N.; Sakura, T.; Ueda, K.; Omura, L.; Kimura, A.; Iino, Y.; Fukashiro, S.; Yoshioka, S. Evaluation of 3D Markerless Motion Capture Accuracy Using OpenPose with Multiple Video Cameras. Front. Sport. Act. Living 2020, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Mentiplay, B.F.; Perraton, L.G.; Bower, K.J.; Pua, Y.H.; McGaw, R.; Heywood, S.; Clark, R.A. Gait assessment using the Microsoft Xbox One Kinect: Concurrent validity and inter-day reliability of spatiotemporal and kinematic variables. J. Biomech. 2015, 48, 2166–2170. [Google Scholar] [CrossRef]
- Barthuly, A.M.; Bohannon, R.W.; Gorack, W. Gait speed is a responsive measure of physical performance for patients undergoing short-term rehabilitation. Gait Posture 2012, 36, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, B.; Artemiadis, P. A Systematic Method for Outlier Detection in Human Gait Data. In Proceedings of the 2022 International Conference on Rehabilitation Robotics (ICORR), Rotterdam, The Netherlands, 25–29 July 2022. [Google Scholar] [CrossRef]
- Reberšek, P.; Novak, D.; Podobnik, J.; Munih, M. Intention detection during gait initiation using supervised learning. In Proceedings of the 2011 11th IEEE-RAS International Conference on Humanoid Robots, Bled, Slovenia, 26–28 October 2011; pp. 34–39. [Google Scholar] [CrossRef]
- Vera, M.J.; Dubravka, B.; Nikola, J.; Vojin, I.; Bojana, P.B. Detecting and removing outlier(s) in electromyographic gait-related patterns. J. Appl. Stat. 2013, 40, 1319–1332. [Google Scholar] [CrossRef]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W.; Schuler, T.C. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Guzik, A.; Drużbicki, M.; Wolan-Nieroda, A.; Turolla, A.; Kiper, P. Estimating minimal clinically important differences for knee range of motion after stroke. J. Clin. Med. 2020, 9, 3305. [Google Scholar] [CrossRef]
- Guzik, A.; Drużbicki, M.; Perenc, L.; Wolan-Nieroda, A.; Turolla, A.; Kiper, P. Establishing the Minimal Clinically Important Differences for Sagittal Hip Range of Motion in Chronic Stroke Patients. Front. Neurol. 2021, 12, 700190. [Google Scholar] [CrossRef]
- Dolatabadi, E.; Taati, B.; Mihailidis, A. Concurrent validity of the Microsoft Kinect for Windows v2 for measuring spatiotemporal gait parameters. Med. Eng. Phys. 2016, 38, 952–958. [Google Scholar] [CrossRef]
- Eltoukhy, M.; Oh, J.; Kuenze, C.; Signorile, J. Improved kinect-based spatiotemporal and kinematic treadmill gait assessment. Gait Posture 2017, 51, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Zago, M.; Luzzago, M.; Marangoni, T.; De Cecco, M.; Tarabini, M.; Galli, M. 3D Tracking of Human Motion Using Visual Skeletonization and Stereoscopic Vision. Front. Bioeng. Biotechnol. 2020, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Baldinger, M.; Reimer, L.M.; Senner, V. Influence of the Camera Viewing Angle on OpenPose Validity in Motion Analysis. Sensors 2025, 25, 799. [Google Scholar] [CrossRef] [PubMed]
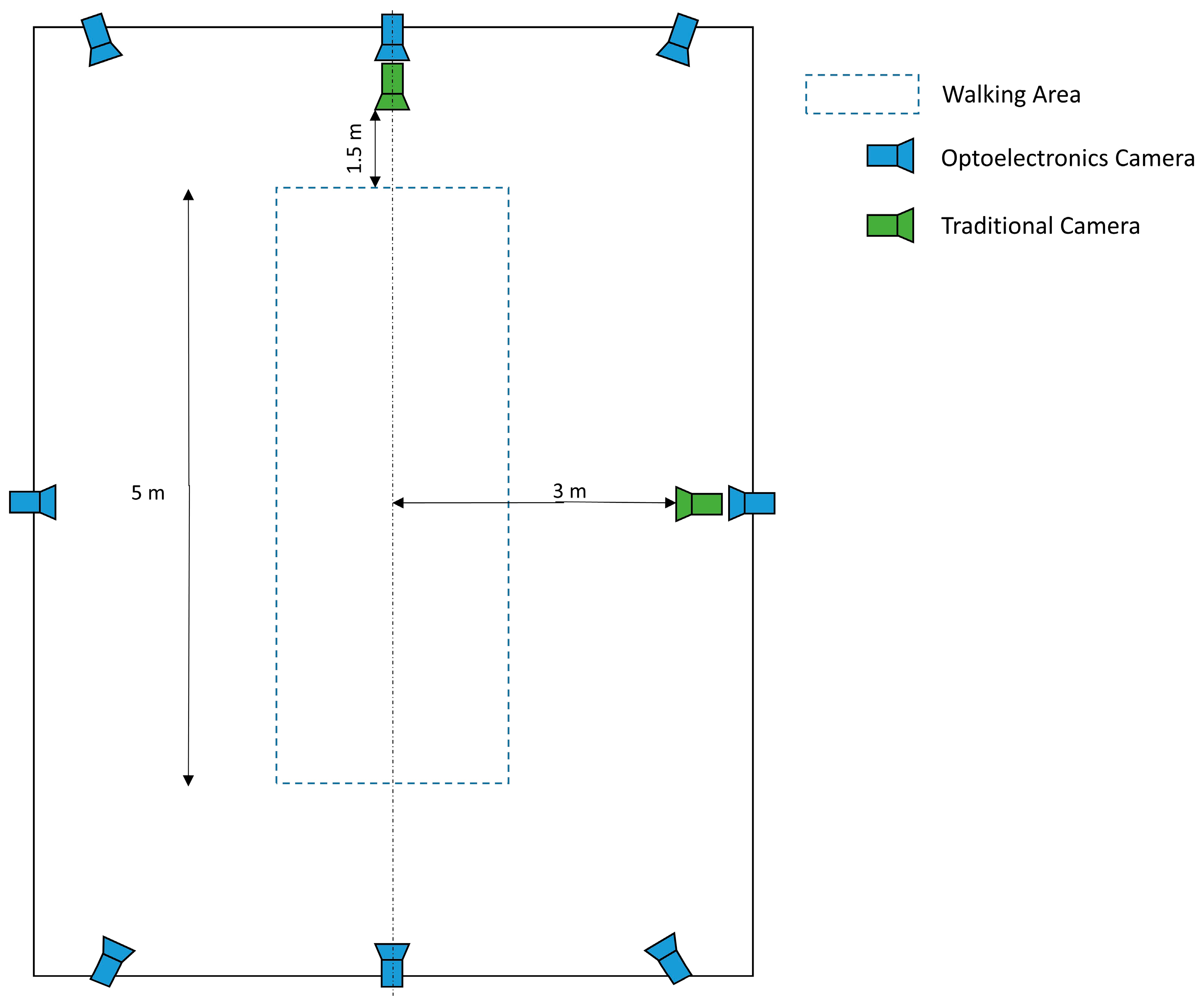
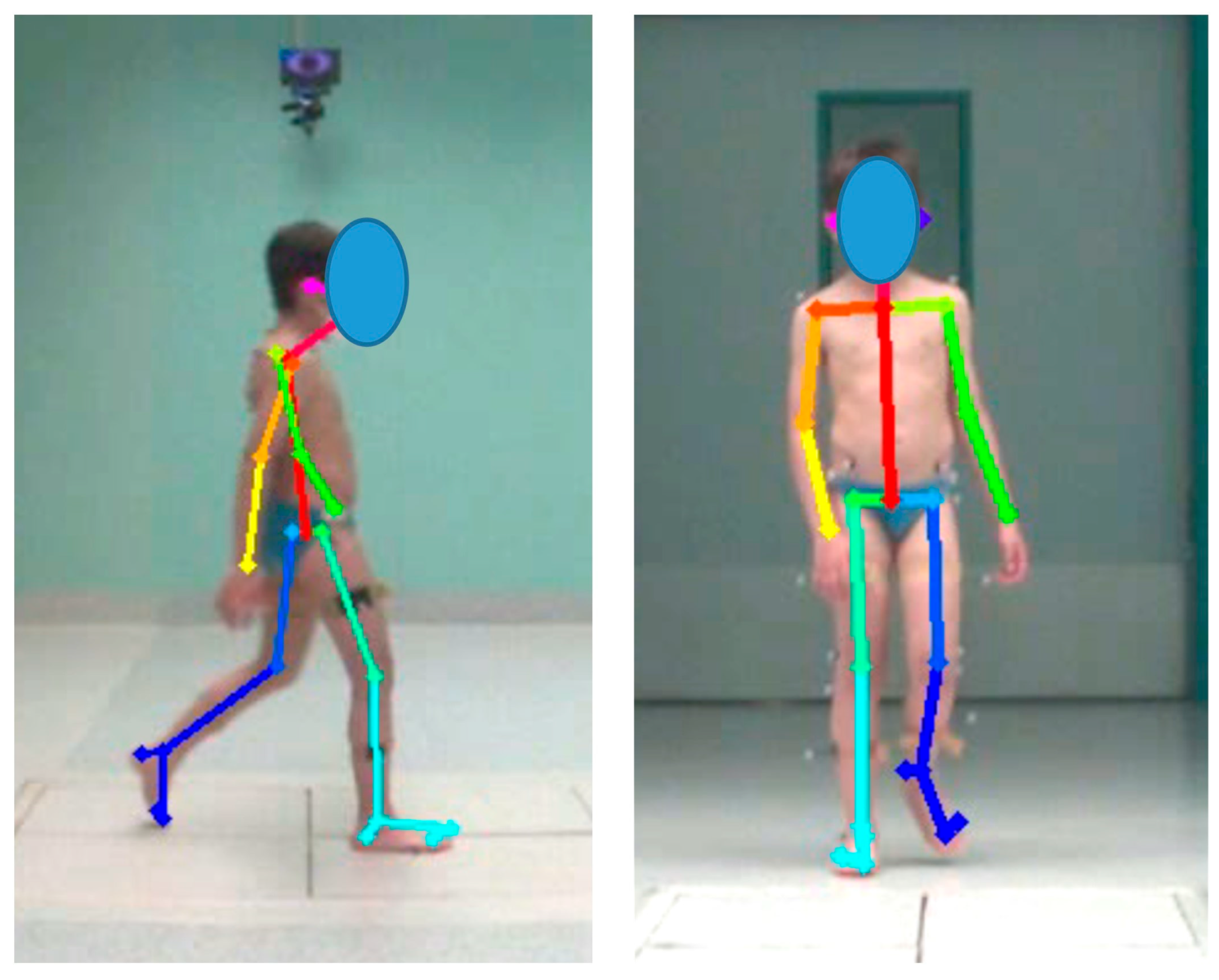

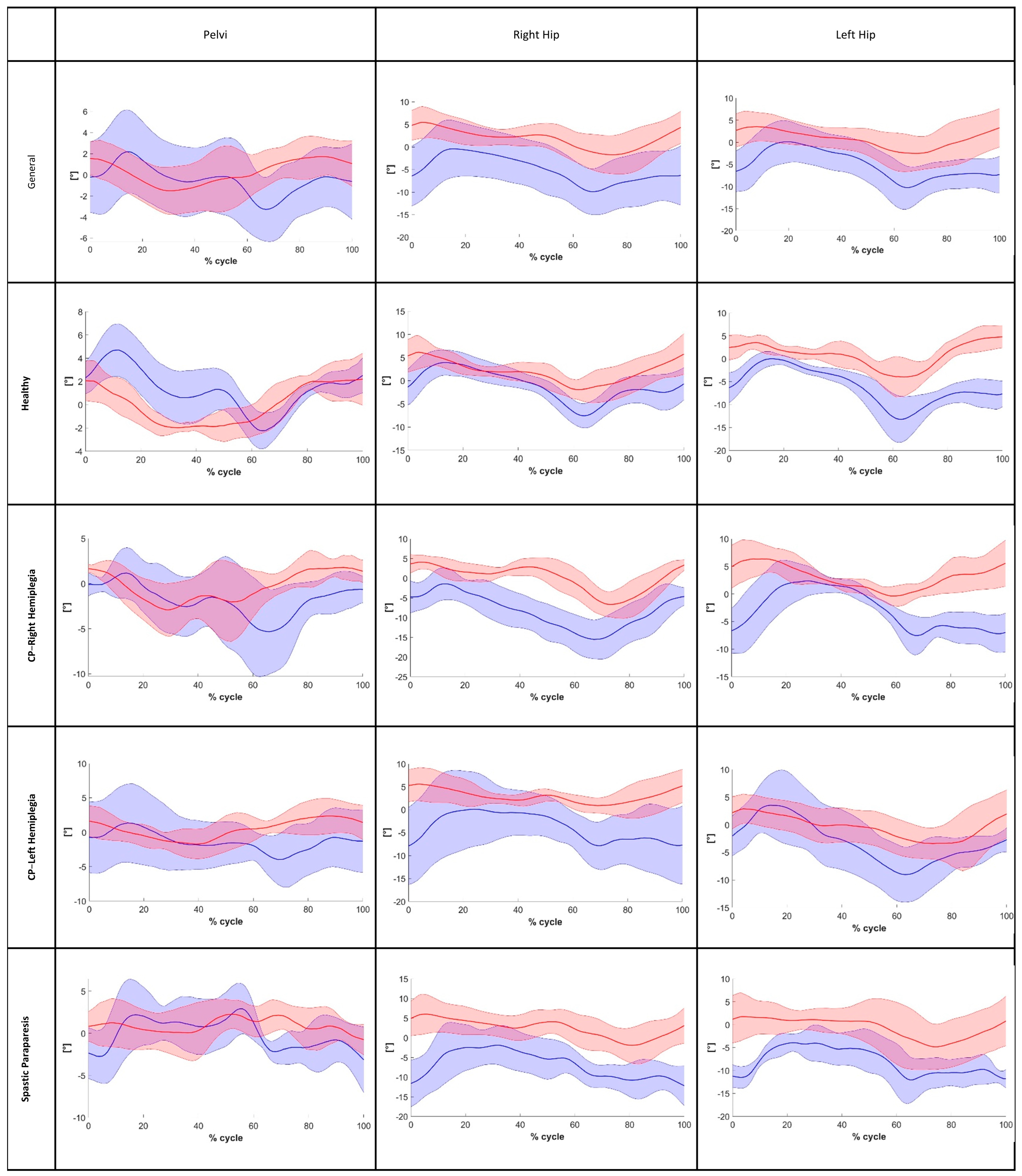


| Baseline Features (Mean—SD; Range) | |||||
|---|---|---|---|---|---|
| Sample | Healthy | CP Right Hemiplegia | CP Left Hemiplegia | Spastic Paraparesis | |
| Numbers of Subjects | 20 | 5 | 5 | 5 | 5 |
| Age [y] | 11.11–SD 3.43; 5.4–17.7 | 9.24–SD 3.98; 5.4–15.0 | 11.44 –SD 3.55; 7.6–15.9 | 10.88–SD 3.16; 6.80–14.50 | 12.44–SD 3.46; 9.40–17.70 |
| Weight [Kg] | 38.64–SD 15.40; 69–17 | 27.40–SD 13.15; 170–49 | 45.50–SD 20.86; 21–69 | 37.40–SD 10.60; 24–49 | 41.60–SD 13.83; 29–59 |
| Height [cm] | 142.19–SD 21.14; 180–105 | 129.70–SD 24.76; 105–167. | 149–SD 24.99; 113–180 | 142.00–SD15.03; 120–156 | 142.00–SD 16.63; 125–168 |
| BMI) | 18.31–SD 3.36; 27.3–13.4 | 15.52–SD 1.51; 13.5–17.5 | 19.40–SD 3.79; 15.0−24.5 | 18.28–SD 2.82; 13.4–20.1 | 20.24–SD 3.97; 17.8–27.3 |
| Segments and Joints Angles | |
|---|---|
| Shoulder Elevation/Depression | The angle of a straight line connecting “RShoulder” and “LShoulder” relative to the horizontal |
| Pelvis Elevation/Depression | The angle of a straight line connecting “RHip” and “LHip” relative to the horizontal |
| Hip Flexion/Extension | The angle of a straight line connecting “RHip” and “RKnee” relative to a straight line connecting “Neck” and “MidHip |
| Hip Abduction/Adduction | The angle of the straight line connecting “RHip” and “RKnee” relative to the perpendicular line connecting “RHip” and “LHip” |
| Knee Flexion/Extension | The angle of a straight line connecting “RHip”, “RKnee” and relative to a straight line connecting “, “RKnee” and “Rankle” |
| Ankle Dorsiflexion/Plantar flexion | The angle of a straight line connecting “RHeel” and “RSmallToe” relative to a straight line connecting “RKnee” and “RAnkle” |
| Metrics | OP | OS | MAE | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD; Max | ||
| Sample | Cadence [step/min] | 56.21 ± 7.01 | 56.43 ± 7.08 | 1.63 ± 1.57; 5.9 |
| Gait Speed [m/s] | 0.76 ± 0.16 | 0.92 ± 0.18 | 0.16 ± 0.04; 0.24 | |
| Right Stride Length [m] | 0.80 ± 0.13 | 0.99 ± 0.16 | 0.19 ± 0.04; 0.26 | |
| Left Stride Length [m] | 0.80 ± 0.15 | 0.98 ± 0.17 | 0.18 ± 0.05; 0.28 | |
| Step Width [m] | 0.13 ± 0.06 | 0.12 ± 0.05 | 0.03 ± 0.03; 0.1 | |
| Healthy | Cadence [step/min] | 55.91 ± 7.20 | 56.80 ± 6.48 | 1.28 ± 2.29; 5.32 |
| Gait Speed [m/s] | 0.83 ± 0.13 | 0.97 ± 0.13 | 0.16 ± 0.24; 0.18 | |
| Right Stride Length [m] | 0.89 ± 0.14 | 1.04 ± 0.16 | 0.15 ± 0.04; 0.19 | |
| Left Stride Length [m] | 0.88 ± 0.16 | 1.05 ± 0.17 | 0.18 ± 0.04; 0.23 | |
| Step Width [m] | 0.09 ± 0.04 | 0.10 ± 0.04 | 0.01 ± 0.01; 0.03 | |
| CP Right Hemiplegia | Cadence [step/min] | 57.13 ± 7.10 | 56.04 ± 8.94 | 1.96 ± 0.81; 2.62 |
| Gait Speed [m/s] | 0.80 ± 0.12 | 0.96 ± 0.14 | 0.16 ± 0.03; 0.19 | |
| Right Stride Length [m] | 0.83 ± 0.09 | 1.03 ± 0.12 | 0.21 ± 0.04; 0.25 | |
| Left Stride Length [m] | 0.83 ± 0.11 | 1.03 ± 0.13 | 0.20 ± 0.05; 0.28 | |
| Step Width [m] | 0.13 ± 0.03 | 0.12 ± 0.01 | 0.02 ± 0.01; 0.04 | |
| CP Left Hemiplegia | Cadence [step/min] | 56.54 ± 7.18 | 58.80 ± 7.82 | 2.35 ± 2.28; 5.90 |
| Gait Speed [m/s] | 0.74 ± 0.22 | 0.92 ± 0.26 | 0.18 ± 0.04; 0.22 | |
| Right Stride Length [m] | 0.75 ± 0.16 | 0.95 ± 0.21 | 0.20 ± 0.06; 0.26 | |
| Left Stride Length [m] | 0.76 ± 0.19 | 0.94 ± 0.20 | 0.17 ± 0.06; 0.24 | |
| Step Width [m] | 0.14 ± 0.08 | 0.13 ± 0.07 | 0.03 ± 0.03; 0.09 | |
| Spastic Paraparesis | Cadence [step/min] | 55.26 ± 8.79 | 55.08 ± 6.45 | 1.91 ± 1.59; 3.98 |
| Gait Speed [m/s] | 0.70 ± 0.18 | 0.85 ± 0.21 | 0.15 ± 0.04; 0.19 | |
| Right Stride Length [m] | 0.12 ± 0.05 | 0.14 ± 0.06 | 0.03 ± 0.02; 0.21 | |
| Left Stride Length [m] | 0.73 ± 0.12 | 0.91 ± 0.18 | 0.18 ± 0.06; 0.23 | |
| Step Width [m] | 0.12 ± 0.05 | 0.14 ± 0.06 | 0.03 ± 0.02; 0.05 | |
| Sample | Healthy | CP—Right Hemiplegia | CP—Left Hemiplegia | Spastic Paraparesis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICC | ICC level | ICC | ICC level | ICC | ICC level | ICC | ICC level | ICC | ICC level | |
| Cadence [step/min] | 0.974 | +++ | 0.999 | +++ | 0.983 | +++ | 0.987 | +++ | 0.975 | +++ |
| Gait Speed [m/s] | 0.811 | ++ | 0.784 | ++ | 0.707 | + | 0.868 | ++ | 0.859 | ++ |
| Right Stride Length [m] | 0.687 | + | 0.781 | ++ | 0.514 | + | 0.753 | ++ | 0.658 | + |
| Left Stride Length [m] | 0.734 | + | 0.769 | ++ | 0.566 | + | 0.813 | ++ | 0.713 | + |
| Step Width [m] | 0.846 | ++ | 0.936 | +++ | 0.500 | + | 0.909 | +++ | 0.893 | ++ |
| Metrics | OP | OS | MAE | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD; MAX | ||
| Sample | ROM_ShoulderObliquity [°] | 5.99 ± 4.43 | 7.02 ± 4.25 | 1.59 ± 2.02; 8.60 |
| ROM_PelviObliquity [°] | 6.40 ± 1.83 | 8.08 ± 3.11 | 2.34 ± 1.87; 8.30 | |
| ROM r_hip_AA [°] | 9.50 ± 3.36 | 13.53 ± 3.92 | 4.70 ± 3.71; 12.66 | |
| ROM l_hip_AA [°] | 9.18 ± 3.13 | 13.74 ± 3.47 | 4.56 ± 2.53; 10.08 | |
| ROM r_hip_FE [°] | 47.89 ± 7.79 | 50.38 ± 7.40 | 3.19 ± 2.85; 11.68 | |
| ROM l_hip_FE [°] | 43.39 ± 7.61 | 47.64 ± 8.78 | 5.41 ± 3.30; 10.61 | |
| ROM r_knee_FE [°] | 55.28 ± 9.66 | 59.23 ± 8.94 | 5.81 ± 2.47; 11.89 | |
| ROM l_knee_FE [°] | 50.09 ± 11.19 | 54.27 ± 11.54 | 4.98 ± 2.81; 12.18 | |
| ROM r_ankle_FE [°] | 27.05 ± 7.97 | 27.84 ± 8.67 | 3.61 ± 2.55; 9.23 | |
| ROM l_ankle_FE [°] | 29.90 ± 14.20 | 29.01 ± 13.85 | 4.97 ± 7.67; 27.29 | |
| Healthy | ROM_ShoulderObliquity [°] | 3.06 ± 1.10 | 3.36 ± 1.79 | 0.77 ± 0.45; 1.20 |
| ROM_PelviObliquity [°] | 5.98 ± 1.26 | 7.10 ± 2.81 | 2.12 ± 1.32; 4.27 | |
| ROM r_hip_AA [°] | 10.19 ± 3.29 | 11.64 ± 1.60 | 2.58 ± 0.67; 3.11 | |
| ROM l_hip_AA [°] | 9.88 ± 4.17 | 14.26 ± 5.34 | 4.38 ± 2.48; 7.82 | |
| ROM r_hip_FE [°] | 48.36 ± 4.81 | 48.54 ± 4.04 | 1.62 ± 1.03;2.93 | |
| ROM l_hip_FE [°] | 45.32 ± 6.30 | 45.24 ± 3.93 | 3.99 ± 2.98; 8.08 | |
| ROM r_knee_FE [°] | 63.03 ± 3.39 | 62.34 ± 6.16 | 4.95 ± 1.03; 6.28 | |
| ROM l_knee_FE [°] | 55.27 ± 8.52 | 60.42 ± 5.39 | 5.77 ± 2.82; 8.07 | |
| ROM r_ankle_FE [°] | 31.20 ± 8.33 | 29.92 ± 9.98 | 3.23 ± 2.03; 5.92 | |
| ROM l_ankle_FE [°] | 38.50 ± 12.40 | 32.70 ± 12.13 | 7.11 ± 11.30; 27.29 | |
| CP—Right Hemiplegia Healthy | ROM_ShoulderObliquity [°] | 4.90 ± 4.91 | 7.22 ± 3.65 | 2.32 ± 3.60; 8.60 |
| ROM_PelviObliquity [°] | 7.17 ± 2.78 | 8.84 ± 2.07 | 1.74 ± 1.21; 3.26 | |
| ROM r_hip_AA [°] | 11.93 ± 2.45 | 15.34 ± 4.59 | 4.29 ± 3.54; 10.19 | |
| ROM l_hip_AA [°] | 8.77 ± 3.15 | 13.78 ± 1.29 | 5.01 ± 2.08; 7.78 | |
| ROM r_hip_FE [°] | 50.34 ± 10.42 | 51.74 ± 8.06 | 2.74 ± 2.39; 4.98 | |
| ROM l_hip_FE [°] | 46.51 ± 3.97 | 51.62 ± 4.62 | 5.11 ± 3.56; 9.95 | |
| ROM r_knee_FE [°] | 55.33 ± 10.45 | 62.40 ± 8.33 | 7.07 ± 3.26; 11.89 | |
| ROM l_knee_FE [°] | 53.04 ± 3.17 | 56.30 ± 4.11 | 3.72 ± 2.28; 6.78 | |
| ROM r_ankle_FE [°] | 23.38 ± 2.83 | 24.76 ± 7.16 | 3.77 ± 3.66; 9.23 | |
| ROM l_ankle_FE [°] | 23.59 ± 12.49 | 23.82 ± 11.32 | 1.40 ± 1.19; 3.17 | |
| CP—Left Hemiplegia Healthy | ROM_ShoulderObliquity [°] | 4.92 ± 1.76 | 6.04 ± 1.69 | 1.12 ± 0.40; 1.59 |
| ROM_PelviObliquity [°] | 5.93 ± 1.57 | 6.82 ± 2.48 | 2.10 ± 1.22; 3.51 | |
| ROM r_hip_AA [°] | 6.24 ± 3.11 | 13.46 ± 3.17 | 7.22 ± 3.19; 12.25 | |
| ROM l_hip_AA [°] | 9.47 ± 3.11 | 14.64 ± 4.15 | 5.17 ± 3.73; 10.08 | |
| ROM r_hip_FE [°] | 44.88 ± 5.40 | 49.32 ± 8.26 | 4.44 ± 4.19; 11.68 | |
| ROM l_hip_FE [°] | 37.88 ± 10.32 | 42.06 ± 13.18 | 4.78 ± 4.31; 10.61 | |
| ROM r_knee_FE [°] | 54.70 ± 7.41 | 57.32 ± 9.24 | 4.43 ± 2.61; 7.91 | |
| ROM l_knee_FE [°] | 43.93 ± 17.12 | 46.54 ± 19.28 | 4.73 ± 2.34; 7.69 | |
| ROM r_ankle_FE [°] | 26.66 ± 10.78 | 28.92 ± 9.06 | 4.56 ± 2.53; 7.31 | |
| ROM l_ankle_FE [°] | 29.42 ± 17.97 | 27.92 ± 13.06 | 4.58 ± 3.68; 10.50 | |
| Spastic Paraparesis Healthy | ROM_ShoulderObliquity [°] | 11.07 ± 4.34 | 11.46 ± 4.89 | 2.14 ± 1.99; 4.80 |
| ROM_PelviObliquity [°] | 6.53 ± 1.69 | 9.56 ± 4.57 | 3.40 ± 3.14; 8.30 | |
| ROM r_hip_AA [°] | 9.64 ± 2.44 | 13.66 ± 5.56 | 4.70 ± 5.32; 12.66 | |
| ROM l_hip_AA [°] | 8.58 ± 2.87 | 12.26 ± 2.40 | 3.68 ± 2.09; 5.74 | |
| ROM r_hip_FE [°] | 47.97 ± 10.41 | 51.92 ± 9.97 | 3.95 ± 2.87; 7.83 | |
| ROM l_hip_FE [°] | 43.87 ± 7.64 | 51.62 ± 8.36 | 7.75 ± 1.25; 8.91 | |
| ROM r_knee_FE [°] | 48.08 ± 11.29 | 54.86 ± 11.55 | 6.78 ± 2.03; 8.52 | |
| ROM l_knee_FE [°] | 48.10 ± 11.07 | 53.82 ± 9.35 | 5.72 ± 3.93; 12.18 | |
| ROM r_ankle_FE [°] | 26.94 ± 8.32 | 27.74 ± 10.28 | 2.88 ± 2.24; 5.74 | |
| ROM l_ankle_FE [°] | 28.08 ± 13.35 | 31.60 ± 20.12 | 6.78 ± 10.53; 25.32 | |
| Sample | Healthy | CP—Right Hemiplegia | CP—Left Hemiplegia | Spastic Paraparesis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICC | ICC Level | ICC | ICC Level | ICC | ICC Level | ICC | ICC Level | ICC | ICC Level | |
| ROM Shoulder Obliquity [°] | 0.909 | +++ | 0.904 | +++ | 0.757 | ++ | 0.893 | ++ | 0.896 | ++ |
| ROM Pelvis Obliquity [°] | 0.605 | + | 0.557 | + | 0.830 | ++ | 0.482 | − | 0.530 | + |
| ROM right hip AA [°] | 0.280 | − | 0.674 | + | 0.298 | − | 0.241 | − | 0.510 | + |
| ROM left hip AA [°] | 0.535 | + | 0.767 | ++ | 0.338 | − | 0.407 | − | 0.532 | + |
| ROM right hip FE [°] | 0.920 | +++ | 0.953 | +++ | 0.964 | +++ | 0.852 | ++ | 0.947 | +++ |
| ROM left hip FE [°] | 0.850 | ++ | 0.696 | + | 0.574 | + | 0.931 | +++ | 0.805 | ++ |
| ROM right knee FE [°] | 0.882 | ++ | 0.591 | + | 0.851 | ++ | 0.904 | +++ | 0.912 | +++ |
| ROM left knee FE [°] | 0.938 | +++ | 0.812 | ++ | 0.665 | + | 0.980 | +++ | 0.896 | ++ |
| ROM right ankle FE [°] | 0.925 | +++ | 0.957 | +++ | 0.709 | + | 0.930 | +++ | 0.963 | +++ |
| ROM left ankle FE [°] | 0.885 | ++ | 0.666 | + | 0.995 | +++ | 0.966 | +++ | 0.865 | ++ |
| Sample | Healthy | CP—Right Hemiplegia | CP—Left Hemiplegia | Spastic Paraparesis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CCC | CCC Level | CCC | CCC Level | CCC | CCC Level | CCC | CCC Level | CCC | CCC Level | |
| Shoulder obliquity [°] | 0.971 | +++ | 0.791 | +++ | 0.921 | +++ | 0.859 | +++ | 0.979 | +++ |
| Pelvic obliquity [°] | 0.187 | + | 0.389 | + | 0.221 | + | 0.194 | + | 0.093 | + |
| Right hip AA [°] | 0.713 | +++ | 0.749 | +++ | 0.748 | +++ | 0.116 | + | 0.543 | ++ |
| Left hip AA [°] | 0.667 | ++ | 0.611 | ++ | 0.084 | + | 0.836 | +++ | 0.627 | ++ |
| Right hip FE [°] | 0.958 | +++ | 0.993 | +++ | 0.977 | +++ | 0.930 | +++ | 0.887 | +++ |
| Left hip FE [°] | 0.957 | +++ | 0.982 | +++ | 0.924 | +++ | 0.956 | +++ | 0.930 | +++ |
| Right knee FE [°] | 0.887 | +++ | 0.979 | +++ | 0.924 | +++ | 0.773 | +++ | 0.761 | +++ |
| Left knee FE [°] | 0.896 | +++ | 0.956 | +++ | 0.801 | +++ | 0.934 | +++ | 0.825 | +++ |
| Right ankle FE [°] | 0.813 | +++ | 0.933 | +++ | 0.776 | +++ | 0.633 | ++ | 0.656 | ++ |
| Left ankle FE [°] | 0.726 | +++ | 0.854 | +++ | 0.627 | ++ | 0.713 | +++ | 0.745 | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molteni, L.E.; Andreoni, G. Comparing the Accuracy of Markerless Motion Analysis and Optoelectronic System for Measuring Gait Kinematics of Lower Limb. Bioengineering 2025, 12, 424. https://doi.org/10.3390/bioengineering12040424
Molteni LE, Andreoni G. Comparing the Accuracy of Markerless Motion Analysis and Optoelectronic System for Measuring Gait Kinematics of Lower Limb. Bioengineering. 2025; 12(4):424. https://doi.org/10.3390/bioengineering12040424
Chicago/Turabian StyleMolteni, Luca Emanuele, and Giuseppe Andreoni. 2025. "Comparing the Accuracy of Markerless Motion Analysis and Optoelectronic System for Measuring Gait Kinematics of Lower Limb" Bioengineering 12, no. 4: 424. https://doi.org/10.3390/bioengineering12040424
APA StyleMolteni, L. E., & Andreoni, G. (2025). Comparing the Accuracy of Markerless Motion Analysis and Optoelectronic System for Measuring Gait Kinematics of Lower Limb. Bioengineering, 12(4), 424. https://doi.org/10.3390/bioengineering12040424









