The Role of Neck Imaging Reporting and Data System (NI-RADS) in the Management of Head and Neck Cancers
Abstract
1. Introduction
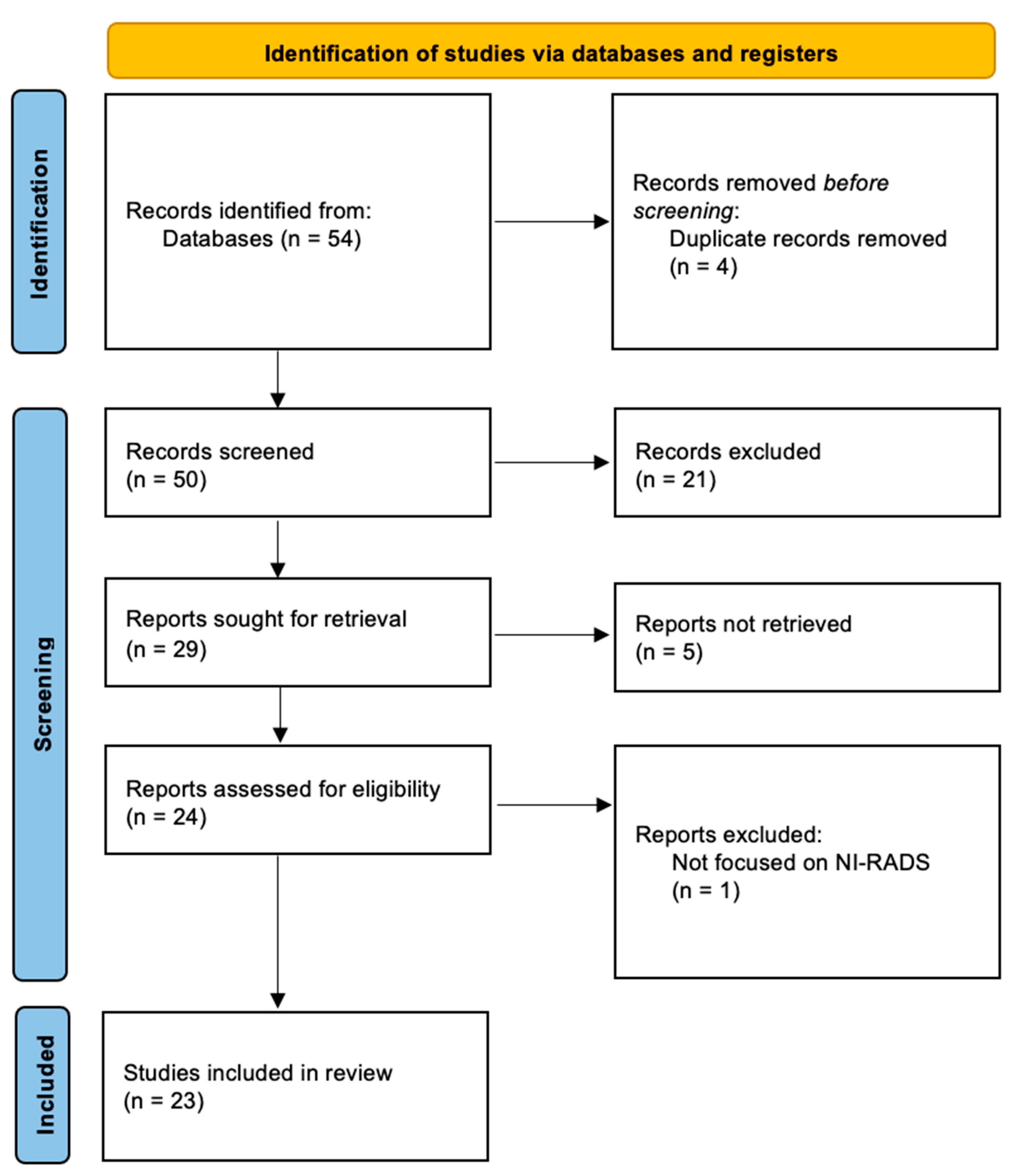
2. Methods
3. NI-RADS Score
4. The NI-RADS’ Diagnostic and Prognostic Value
5. The NI-RADS’s Reliability
6. The NI-RADS’ Report Quality and Acceptance by Physicians
7. Future Perspectives
8. A Summary of the NI-RADS’ Pros and Cons
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| AUC | Area under the curve |
| BI-RADS | Breast Imaging Reporting and Data System |
| CAD-RADS | Coronary Artery Disease Reporting and Data System |
| CECT | Contrast-Enhanced Computed Tomography |
| CT | Computed Tomography |
| DWI | Diffusion-Weighted Imaging |
| FDG-PET | Fluorodeoxyglucose Positron Emission Tomography |
| HR | hazard ratio |
| HIS | hyperspectral imaging |
| K | kappa test parameter |
| LI-RADS | Liver Imaging Reporting and Data System |
| MRI | Magnetic Resonance Imaging |
| NI-RADS | Neck Imaging Reporting and Data System |
| NPV | negative predictive value |
| OND | occult nodal disease |
| OSCC | oral squamous cell carcinoma |
| PPV | positive predictive value |
| RADS | Reporting and Data System |
| RT/CT | radio-chemotherapy |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-H.; Wu, C.-C.; Yuan, K.S.-P.; Wu, A.T.H.; Wu, S.-Y. Locoregionally Recurrent Head and Neck Squamous Cell Carcinoma: Incidence, Survival, Prognostic Factors, and Treatment Outcomes. Oncotarget 2017, 8, 55600–55612. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Nadgir, R.N.; Nakahira, M.; Takahashi, M.; Uchino, A.; Kimura, F.; Truong, M.T.; Sakai, O. Posttreatment CT and MR Imaging in Head and Neck Cancer: What the Radiologist Needs to Know. RadioGraphics 2012, 32, 1261–1282. [Google Scholar] [CrossRef]
- Cheung, P.K.F.; Chin, R.Y.; Eslick, G.D. Detecting Residual/Recurrent Head Neck Squamous Cell Carci-nomas Using PET or PET/CT: Systematic Review and Meta-analysis. Otolaryngol. Neck Surg. 2016, 154, 421–432. [Google Scholar] [CrossRef]
- Parillo, M.; Quattrocchi, C.C. Brain Tumor Reporting and Data System (BT-RADS) for the Surveillance of Adult-Type Diffuse Gliomas after Surgery. Surgeries 2024, 5, 764–773. [Google Scholar] [CrossRef]
- Parillo, M.; Vaccarino, F.; Vertulli, D.; Perillo, G.; Montanari, E.; Mallio, C.A.; Quattrocchi, C.C. Assessment of Reason for Exam Imaging Reporting and Data System (RI-RADS) in Inpatient Diagnostic Imaging Referrals. Insights Imaging 2024, 15, 268. [Google Scholar] [CrossRef]
- Parillo, M.; Quattrocchi, C.C. Node Reporting and Data System 1.0 (Node-RADS) for the Assessment of Oncological Patients’ Lymph Nodes in Clinical Imaging. J. Clin. Med. 2025, 14, 263. [Google Scholar] [CrossRef]
- Parillo, M.; Mallio, C.A. The Whole-Body MRI Reporting and Data System Guidelines for Prostate Cancer (MET-RADS-P), Multiple Myeloma (MY-RADS), and Cancer Screening (ONCO-RADS). Cancers 2025, 17, 275. [Google Scholar] [CrossRef] [PubMed]
- Aiken, A.H.; Rath, T.J.; Anzai, Y.; Branstetter, B.F.; Hoang, J.K.; Wiggins, R.H.; Juliano, A.F.; Glas-tonbury, C.; Phillips, C.D.; Brown, R.; et al. ACR Neck Imaging Reporting and Data Systems (NI-RADS): A White Paper of the ACR NI-RADS Committee. J. Am. Coll. Radiol. 2018, 15, 1097–1108. [Google Scholar] [CrossRef]
- Aiken, A.H.; Farley, A.; Baugnon, K.L.; Corey, A.; El-Deiry, M.; Duszak, R.; Beitler, J.; Hudgins, P.A. Implementation of a Novel Surveillance Template for Head and Neck Cancer: Neck Imaging Reporting and Data Sys-tem (NI-RADS). J. Am. Coll. Radiol. 2016, 13, 743–746.e1. [Google Scholar] [CrossRef]
- Parillo, M.; Van Der Molen, A.J.; Asbach, P.; Elsholtz, F.H.J.; Laghi, A.; Ronot, M.; Wu, J.S.; Mallio, C.A.; Quattrocchi, C.C. The Role of Iodinated Contrast Media in Computed Tomography Structured Reporting and Data Systems (RADS): A Narrative Review. Quant. Imaging Med. Surg. 2023, 13, 7621–7631. [Google Scholar] [CrossRef] [PubMed]
- Parillo, M.; Mallio, C.A.; Van Der Molen, A.J.; Rovira, À.; Dekkers, I.A.; Karst, U.; Stroomberg, G.; Clement, O.; Gianolio, E.; Nederveen, A.J.; et al. The Role of Gadolinium-Based Contrast Agents in Magnetic Resonance Imaging Structured Reporting and Data Sys-tems (RADS). Magn. Reson. Mater. Phys. Biol. Med. 2023, 37, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Dinkelborg, P.; Ro, S.-R.; Shnayien, S.; Schaafs, L.-A.; Koerdt, S.; Kreutzer, K.; Heiland, M.; Hamm, B.; Elsholtz, F.H.J. Retrospective Evaluation of NI-RADS for Detecting Postsurgical Recurrence of Oral Squamous Cell Carcinoma on Surveillance CT or MRI. Am. J. Roentgenol. 2021, 217, 198–206. [Google Scholar] [CrossRef]
- Krieger, D.A.; Hudgins, P.A.; Nayak, G.K.; Baugnon, K.L.; Corey, A.S.; Patel, M.R.; Beitler, J.J.; Saba, N.F.; Liu, Y.; Aiken, A.H. Initial Performance of NI-RADS to Predict Residual or Recurrent Head and Neck Squamous Cell Carcinoma. Am. J. Neuroradiol. 2017, 38, 1193–1199. [Google Scholar] [CrossRef]
- Parillo, M.; Vaccarino, F.; Zobel, B.B.; Mallio, C.A. ChatGPT and radiology report: Potential applications and limitations. Radiol. Med. 2024, 129, 1849–1863. [Google Scholar] [CrossRef]
- Wangaryattawanich, P.; Branstetter, B.F.; Hughes, M.; Clump, D.A.; Heron, D.E.; Rath, T.J. Negative Predictive Value of NI-RADS Category 2 in the First Posttreatment FDG-PET/CT in Head and Neck Squamous Cell Carcinoma. Am. J. Neuroradiol. 2018, 39, 1884–1888. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.; Chokshi, F.H.; Hudgins, P.A.; Kundu, S.; Beitler, J.J.; Patel, M.R.; Aiken, A.H. Predictive Value of First Posttreatment Imaging Using Standardized Reporting in Head and Neck Cancer. Otolaryngol. Neck Surg. 2019, 161, 978–985. [Google Scholar] [CrossRef]
- Zhong, J.; Sundersingh, M.; Dyker, K.; Currie, S.; Vaidyanathan, S.; Prestwich, R.; Scarsbrook, A. Post-Treatment FDG PET-CT in Head and Neck Carcinoma: Comparative Analysis of 4 Qualitative Interpretative Criteria in a Large Patient Cohort. Sci. Rep. 2020, 10, 4086. [Google Scholar] [CrossRef]
- Abdelaziz, T.T.; Abdel Razk, A.A.K.; Ashour, M.M.M.; Abdelrahman, A.S. Interreader Reproducibility of the Neck Imaging Reporting and Data System (NI-RADS) Lexicon for the Detection of Residual/Recurrent Disease in Treated Head and Neck Squamous Cell Carcinoma (HNSCC). Cancer Imaging 2020, 20, 61. [Google Scholar] [CrossRef]
- Elsholtz, F.H.J.; Ro, S.-R.; Shnayien, S.; Erxleben, C.; Bauknecht, H.-C.; Lenk, J.; Schaafs, L.-A.; Hamm, B.; Niehues, S.M. Inter- and Intrareader Agreement of NI-RADS in the Interpretation of Surveillance Contrast-Enhanced CT after Treatment of Oral Cavity and Oropharyngeal Squamous Cell Carcinoma. Am. J. Neuroradiol. 2020, 41, 859–865. [Google Scholar] [CrossRef]
- Wangaryattawanich, P.; Branstetter, B.F.; Ly, J.D.; Duvvuri, U.; Heron, D.E.; Rath, T.J. Positive Predic-tive Value of Neck Imaging Reporting and Data System Categories 3 and 4 Posttreatment FDG-PET/CT in Head and Neck Squamous Cell Carcinoma. Am. J. Neuroradiol. 2020, 41, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.C.; Magliocca, K.R.; Aiken, A.H.; Baugnon, K.L.; Brandon, D.C.; Stokes, W.A.; McDonald, M.W.; Patel, M.R.; Baddour, H.M.; Kaka, A.S.; et al. Outcomes and Predictive Value of Post-adjuvant Therapy PET/CT for Locally Advanced Oral Squamous Cell Carcinoma. Laryngoscope 2020, 130, E850–E857. [Google Scholar] [CrossRef]
- Elsholtz, F.H.J.; Erxleben, C.; Bauknecht, H.-C.; Dinkelborg, P.; Kreutzer, K.; Hamm, B.; Niehues, S.M. Reliability of NI-RADS Criteria in the Interpretation of Contrast-Enhanced Magnetic Resonance Imaging Considering the Potential Role of Diffusion-Weighted Imaging. Eur. Radiol. 2021, 31, 6295–6304. [Google Scholar] [CrossRef]
- Hsu, D.; Rath, T.J.; Branstetter, B.F.; Anzai, Y.; Phillips, C.D.; Juliano, A.F.; Mosier, K.M.; Bazylew-icz, M.P.; Poliashenko, S.M.; Kulzer, M.H.; et al. Interrater Re-liability of NI-RADS on Posttreatment PET/Contrast-Enhanced CT Scans in Head and Neck Squamous Cell Carcino-ma. Radiol. Imaging Cancer 2021, 3, e200131. [Google Scholar] [CrossRef]
- Ashour, M.M.; Darwish, E.A.F.; Fahiem, R.M.; Abdelaziz, T.T. MRI Posttreatment Surveillance for Head and Neck Squamous Cell Carcinoma: Proposed MR NI-RADS Criteria. Am. J. Neuroradiol. 2021, 42, 1123–1129. [Google Scholar] [CrossRef]
- Patel, L.D.; Bridgham, K.; Ciriello, J.; Almardawi, R.; Leon, J.; Hostetter, J.; Yazbek, S.; Raghavan, P. PET/MR Imaging in Evaluating Treatment Failure of Head and Neck Malignancies: A Neck Imaging Reporting and Data System–Based Study. Am. J. Neuroradiol. 2022, 43, 435–441. [Google Scholar] [CrossRef]
- Elsholtz, F.H.J.; Ro, S.-R.; Shnayien, S.; Dinkelborg, P.; Hamm, B.; Schaafs, L.-A. Impact of Double Reading on NI-RADS Diagnostic Accuracy in Reporting Oral Squamous Cell Carcinoma Surveillance Imaging—A Single-Center Study. Dentomaxillofac. Radiol. 2022, 51, 20210168. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kaht, D.; Ali, S.; Johnson, S.; Bullen, J.; Karakasis, C.; Lamarre, E.; Geiger, J.; Koyfman, S.; Stock, S. Performance of the Neck Imaging Reporting and Data System as Applied by General Neuroradiologists to Predict Recurrence of Head and Neck Cancers. Head Neck 2022, 44, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.; Reza, S.O.; Choudhary, S.; Shukla, R.C.; Mani, N.; Verma, A. Performance of NI-RADS on CECT Alone to Predict Recurrent Head and Neck Squamous Cell Carcinoma after Chemoradiotherapy: Added Value of RECIST 1.1. Indian J. Radiol. Imaging 2022, 32, 151–158. [Google Scholar] [CrossRef]
- Bunch, P.M.; Meegalla, N.T.; Abualruz, A.; Frizzell, B.A.; Patwa, H.S.; Porosnicu, M.; Williams, D.W.; Aiken, A.H.; Hughes, R.T. Initial Referring Physician and Radiologist Experience with Neck Imaging Reporting and Data System. Laryngoscope 2022, 132, 349–355. [Google Scholar] [CrossRef]
- Johansson, E.D.; Hughes, R.T.; Meegalla, N.T.; Porosnicu, M.; Patwa, H.S.; Lack, C.M.; Bunch, P.M. NECK IMAGING REPORTING AND DATA SYSTEM Category 3 on Surveillance COMPUTED TOMOGRAPHY: Incidence, Bi-opsy Rate, and Predictive Performance in Head and Neck Squamous Cell Carcinoma. Laryngoscope 2022, 132, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Unde, H.; Sable, N.P.; Shukla, S.; Vaish, R.; Patil, V.; Agarwal, U.; Agrawal, A.; Noronha, V.; Joshi, A.; et al. Response Assessment of Post-Treatment Head and Neck Cancers to Determine Further Management Using NI-RADS (Neck Imaging Reporting and Data System): A Subgroup Analysis of a Ran-domized Controlled Trial. Front. Oncol. 2023, 13, 1200366. [Google Scholar] [CrossRef]
- Paul, S.; Gupta, T.; Purandare, N.; Joshi, K.; Ghosh-Laskar, S.; Budrukkar, A.; Swain, M.; Sinha, S.; Ku-mar, A.; Joshi, A.; et al. Diagnostic Performance of Response As-sessment FDG-PET/CECT in HNSCC Treated With Definitive Radio(Chemo)Therapy Using NI-RADS. Otolaryngol. Neck Surg. 2023, 169, 938–947. [Google Scholar] [CrossRef]
- Chan, T.G.; Wicks, J.; Sethi, I.; Becker, J.; Brandon, D.; Schmitt, N.C.; Kaka, A.; Boyce, B.; Baddour, H.M.; El-Deiry, M.W.; et al. Radiologic Findings of Occult Nodal Metastasis during Clinically-N0 Salvage Total Laryngectomy. Head Neck 2025, 47, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Jajodia, A.; Mandal, G.; Yadav, V.; Khoda, J.; Goyal, J.; Pasricha, S.; Puri, S.; Dewan, A. Adding MR Dif-fusion Imaging and T2 Signal Intensity to Neck Imaging Reporting and Data System Categories 2 and 3 in Primary Sites of Postsurgical Oral Cavity Carcinoma Provides Incremental Diagnostic Value. Am. J. Neuroradiol. 2022, 43, 1018–1023. [Google Scholar] [CrossRef]
- Hiyama, T.; Miyasaka, Y.; Kuno, H.; Sekiya, K.; Sakashita, S.; Shinozaki, T.; Kobayashi, T. Posttreatment Head and Neck Cancer Imaging: Anatomic Considerations Based on Cancer Subsites. RadioGraphics 2024, 44, e230099. [Google Scholar] [CrossRef]
- Mohan, S.K.; Hudgins, P.A.; Patel, M.R.; Stapleton, J.; Duszak, R.; Aiken, A.H. Making Time for Pa-tients: Positive Impact of Direct Patient Reporting. Am. J. Roentgenol. 2018, 210, W12–W17. [Google Scholar] [CrossRef]
- Baba, A.; Kurokawa, R.; Kurokawa, M.; Yanagisawa, T.; Srinivasan, A. Performance of Neck Imaging Reporting and Data System (NI-RADS) for Diagnosis of Recurrence of Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-analysis. Am. J. Neuroradiol. 2023, 44, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.-C.; Chen, Y.-C.; Karmakar, R.; Mukundan, A.; Gabriel, G.; Wang, C.-C.; Wang, H.-C. Advancements in Hyperspectral Imaging and Computer-Aided Diagnostic Methods for the Enhanced Detection and Diagnosis of Head and Neck Cancer. Biomedicines 2024, 12, 2315. [Google Scholar] [CrossRef]
| Score | Imaging Findings | Management | |
|---|---|---|---|
| Primary Site | Neck Site | ||
| 0 | Incomplete study (prior imaging unavailable) | Obtain prior imaging and reassess. | |
| 1 | Expected anatomical changes following treatment | Hypoenhancing residual nodal tissue without FDG uptake (if PET available) | Continue routine surveillance. |
| 2a for Primary Site | Superficial mucosal abnormality with mild enhancement | - | Direct visual inspection. |
| 2b for Primary Site and 2 for Neck Site | Deep non-nodular, ill-defined soft tissue abnormality | Potential residual disease, including heterogeneous enhancement or mild/moderate FDG uptake in residual nodal tissue (if PET is available), new or enlarging lymph nodes without definitively abnormal morphology, and any PET/CT/MRI discordance | Close follow-up (3 months) with MRI/CT or PET to evaluate suspicious nodes or deep submucosal abnormalities. |
| 3 | Discrete primary site nodule/mass with intense focal FDG uptake | Concerning nodal findings, including intense FDG uptake or enlargement/increased enhancement in residual tissue, and necrosis, irregular borders, or focal intense FDG uptake in new/enlarging nodes. | Biopsy of the concerning area. |
| 4 | Proven pathological or clear radiologic/clinical disease progression. | Clinical management | |
| Investigators | Study Design | Clinical Setting | Number of MRIs/CTs | Main Findings |
|---|---|---|---|---|
| Krieger et al. (2017) [14] | Retrospective, quality-improvement study | Post-treatment | 318 PET-CTs | Strong performance of NI-RADS for predicting disease recurrence, significant discrimination between categories 1–3 (p < 0.001) |
| Mohan et al. (2018) [15] | Prospective | Post-surgery and post RT/CT | 27 FDG PET-CTs | Direct patient reporting improved understanding of imaging findings and radiologist′s role (70–93%). Patients preferred radiologist consultation (44%) or combined with the physician (33%). |
| Wangaryattawanich et al. (2018) [16] | Retrospective | Post RT/CT | 110 FDG-PET/CTs | A total of 85% NPV in NI-RADS 2; treatment failure mainly in cervical lymph nodes (15% within 2 years). |
| Hsu et al. (2019) [17] | Retrospective | Post-surgery and post RT/CT | 199 PET/CECTs | NI-RADS categories strongly correlated with treatment failure risk; higher categories show higher failure rates. |
| Zhong et al. (2020) [18] | Retrospective | Post RT/CT | 562 FDG PET/CTs | Compared NI-RADS, Porceddu, Hopkins, and Deauville for predicting loco-regional control and PFS. Porceddu and Deauville minimized indeterminate outcomes. |
| Abdelaziz et al. (2020) [19] | Retrospective | Post-surgery or Post RT/CT | 97 CECT/MRIs (58 patients) | High inter-reader agreement for primary lesions and lymph nodes (K = 0.808, K = 0.806). Better agreement for CT (K = 0.843) than MRI (K = 0.77). Substantial agreement for tissue and mucosal enhancement. Variable confidence in individual features, but overall NI-RADS category is unaffected. |
| Elsholtz et al. (2020) [20] | Retrospective | Post-treatment | 101 CTs | Good inter-reader reproducibility; higher agreement in patients with proven recurrence (W = 0.96, kF = 0.65).Effective intra-reader agreement across primary site and neck (tB = 0.67–0.82, kw = 0.85–0.96). |
| Wangaryattawanich et al. (2020) [21] | Retrospective | Post-surgery | 128 PET/CTs | PPV of NI-RADS 3: 56%; NI-RADS 4: 100%; confirmation needed for NI-RADS 3. |
| Qian et al. (2020) [22] | Retrospective | Post surgery and post RT/CT | 220 PET/CTs | Suspicious scans (30%) predict locoregional failure (HR 14.0), distant failure (HR 18.4), and poorer survival (HR 9.5). Overall PPV: 85%, Sensitivity: 58%, Specificity: 92%. Salvage success rate: 11%. |
| Elsholtz et al. (2021) [23] | Retrospective | Post-treatment | 104 MRIs | NI-RADS inter-reader agreement was moderate (κFleiss = 0.53) for primary site, substantial for neck (κFleiss = 0.67), and high for DWI (κFleiss = 0.83). DWI may improve agreement reliability. |
| Dinkelborg et al. (2021) [13] | Retrospective | Post-surgery | 503 CECT/MRIs | NI-RADS effectively detects OSCC recurrence at the primary site and neck with high AUC values (0.934/0.959)Recurrence rates: 100% in NI-RADS 4, 66.7% in NI-RADS 3. Inter-reader agreement: 0.67–0.81. |
| Hsu et al. (2021) [24] | Retrospective | Post-treatment | 80 PET/CTs | Moderate inter-reader agreement with Light k = 0.55 (primary site) and 0.60 (neck site) using NI-RADS categories. |
| Ashour et al. (2021) [25] | Retrospective | Post-surgery | 69 MRIs | Adding T2 signal and DWI enhances NI-RADS accuracy and specificity. |
| Patel et al. (2022) [26] | Retrospective | Post RT/CT | 46 PET/MRIs | PET/MRI showed substantial inter-reader agreement (κ = 0.634). High diagnostic accuracy for treatment failure (AUC 0.864–0.987).Potential role in surveillance imaging. |
| Elsholtz et al. (2022) [27] | Prospective | Post-surgery/RT/CT | 150 CT/MRIs | A total of 26% of reports were modified after supervision by subspecialized radiologists.Higher ROC AUC with supervision: 0.89 vs. 0.86 (primary site), 0.94 vs. 0.91 (neck).Statistically significant improvement in specificity and PPV after supervision. |
| Lee et al. (2022) [28] | Retrospective | Post-surgery or post-RT/CT | 608 CT/MRIs | NI-RADS categories predict recurrence: primary site (AUC 0.765), lymph nodes (AUC 0.820). Recurrence rates: NI-RADS 1 (5%), 2 (29%), 3 (65%). |
| Kumar et al. (2022) [29] | Prospective | Post-RT/CT | 30 CECTs | NI-RADS 1: 4% persistence; NI-RADS 2: 24%; NI-RADS 3: 80% persistence rates. Nodal recurrence rates: NI-RADS 1: 5.3%, NI-RADS 2: 25%, NI-RADS 3: 66.7%. |
| Bunch et al. (2022) [30] | Quality improvement | Post-surgery | 22 CTs | NI-RADS reports were clear, understandable, and guided clinical management. Radiologists’ reporting consistency improved significantly. |
| Johansson et al. (2022) [31] | Retrospective | Post-surgery/RT | 580 CTs | PPV of NI-RADS 3: 71% |
| Jajodia et al. (2022) [25] | Retrospective | Post-surgery | 61 MRIs | Evaluated NI-RADS categories 2 and 3 with T2WI, DWI, and ADC imaging, showing improved diagnostic accuracy. |
| Mahajan et al. (2023) [32] | Retrospective | Post RT/CT | 462 PET/CTs | NI-RADS accuracy: Nodes (NI-RADS 1–4): 92%, 97%, 90%, 67%. Primary: PET/CT better at NI-RADS2 (91% vs. 55% CECT), CECT better at NI-RADS3 (57% vs. 41% PET/CT). |
| Paul et al. (2023) [33] | Retrospective | Post RT/CT | 190 PET/CECTs | NI-RADS showed high diagnostic accuracy; 2-year locoregional control 94.2% for NI-RADS 1. |
| Chan et al. (2025) [34] | Retrospective | Post-surgery | 81 PET/CTs | A total of 16% had OND; occult nodes were subtle on imaging; most had SUVmax below blood pool on PET; NI-RADS 1 or 2 in all cases. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vertulli, D.; Parillo, M.; Mallio, C.A. The Role of Neck Imaging Reporting and Data System (NI-RADS) in the Management of Head and Neck Cancers. Bioengineering 2025, 12, 398. https://doi.org/10.3390/bioengineering12040398
Vertulli D, Parillo M, Mallio CA. The Role of Neck Imaging Reporting and Data System (NI-RADS) in the Management of Head and Neck Cancers. Bioengineering. 2025; 12(4):398. https://doi.org/10.3390/bioengineering12040398
Chicago/Turabian StyleVertulli, Daniele, Marco Parillo, and Carlo Augusto Mallio. 2025. "The Role of Neck Imaging Reporting and Data System (NI-RADS) in the Management of Head and Neck Cancers" Bioengineering 12, no. 4: 398. https://doi.org/10.3390/bioengineering12040398
APA StyleVertulli, D., Parillo, M., & Mallio, C. A. (2025). The Role of Neck Imaging Reporting and Data System (NI-RADS) in the Management of Head and Neck Cancers. Bioengineering, 12(4), 398. https://doi.org/10.3390/bioengineering12040398








