Digital Workflow with Open-Source CAD-CAM Software Aimed to Design a Customized 3D Laser-Printed Titanium Mesh for Guided Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
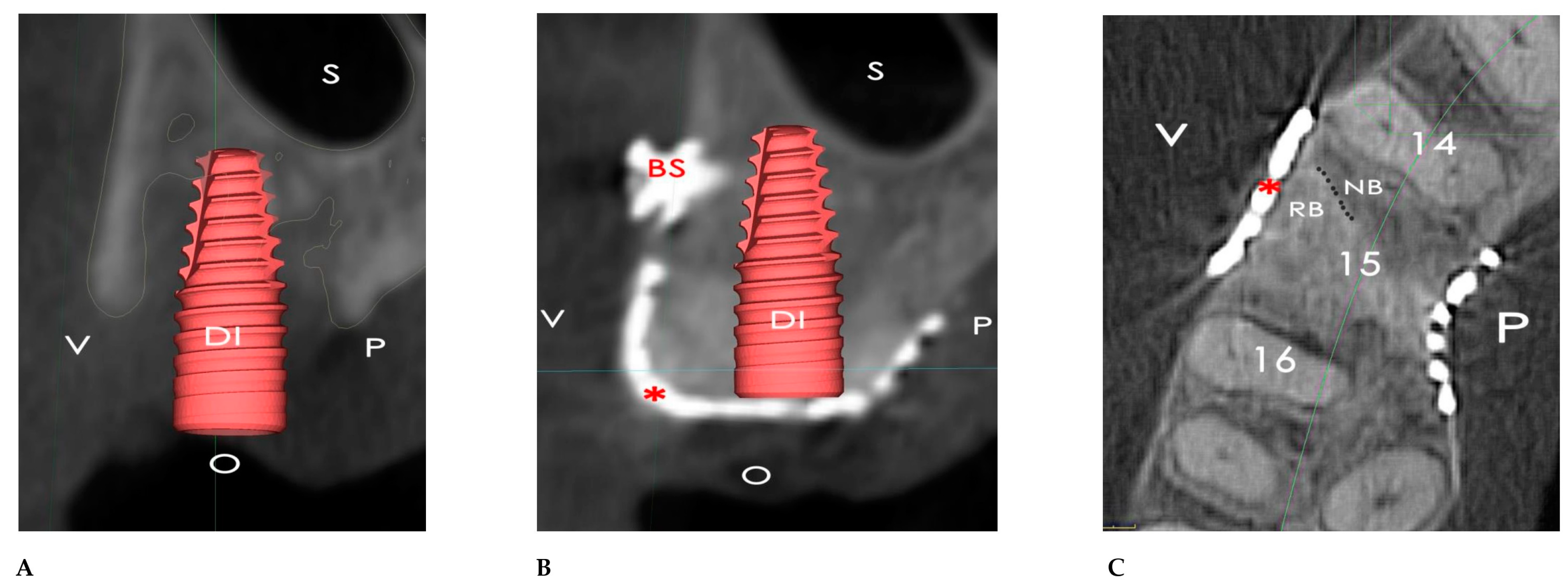
2.1. Surgical Simulation
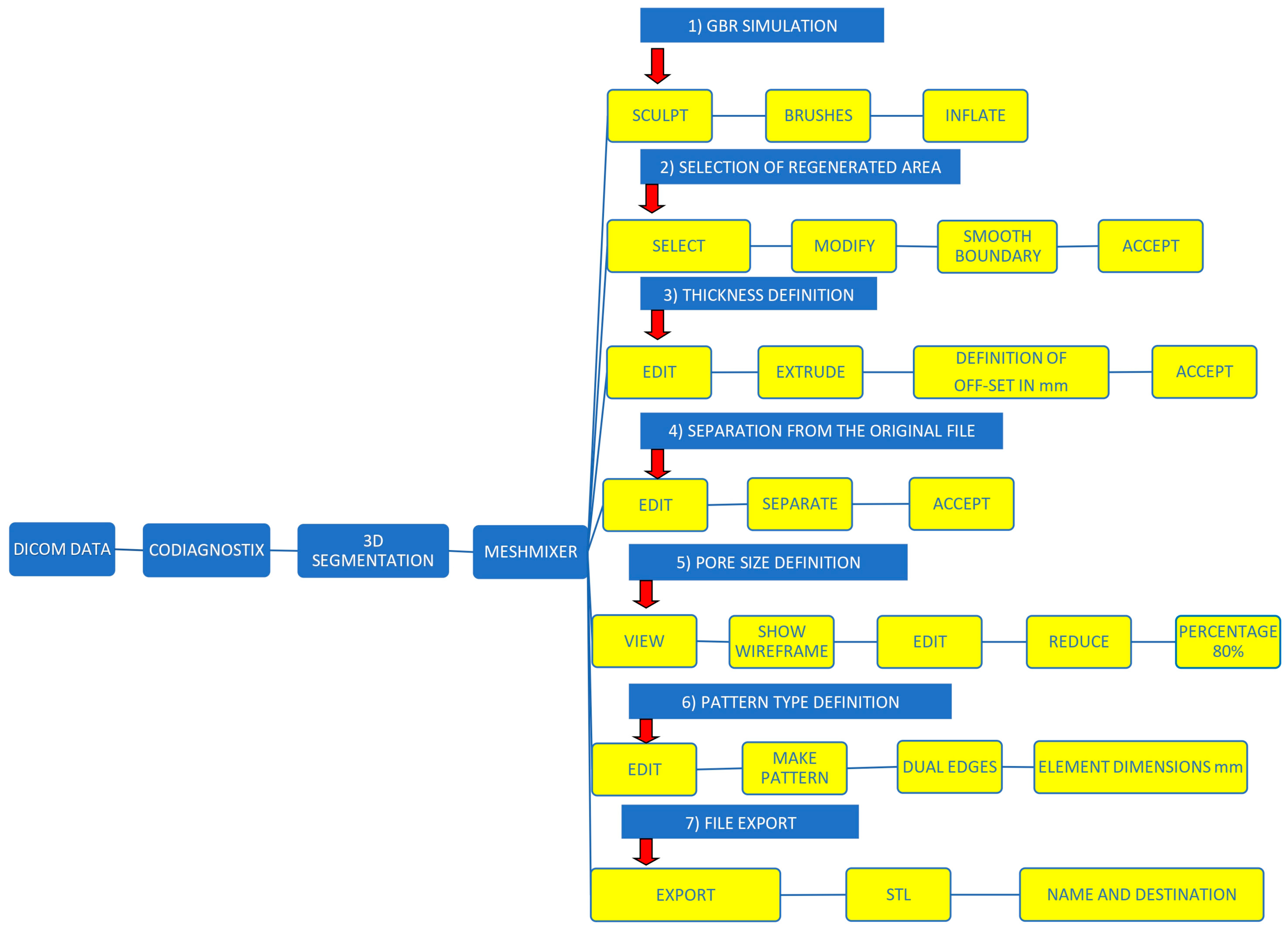
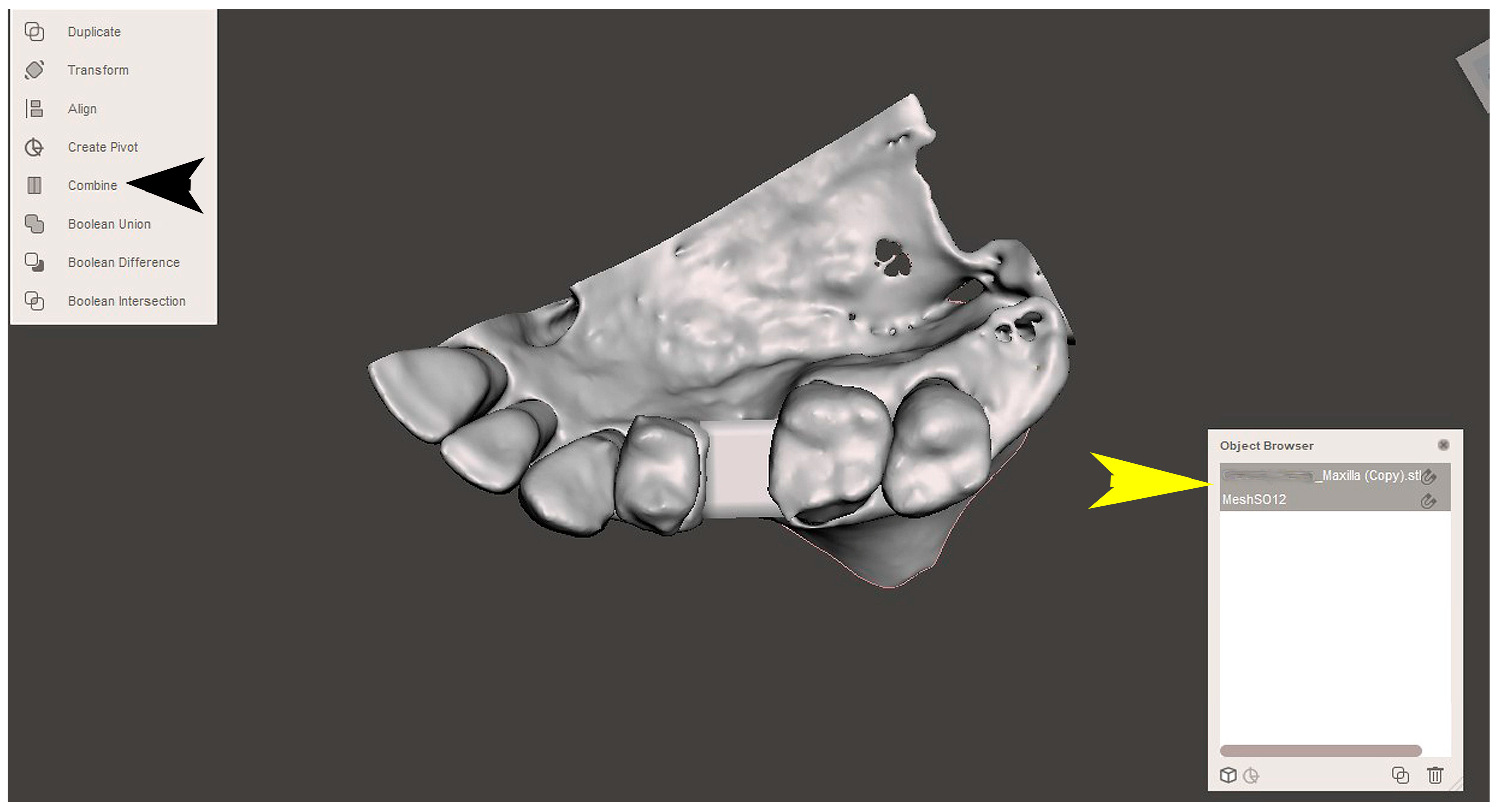
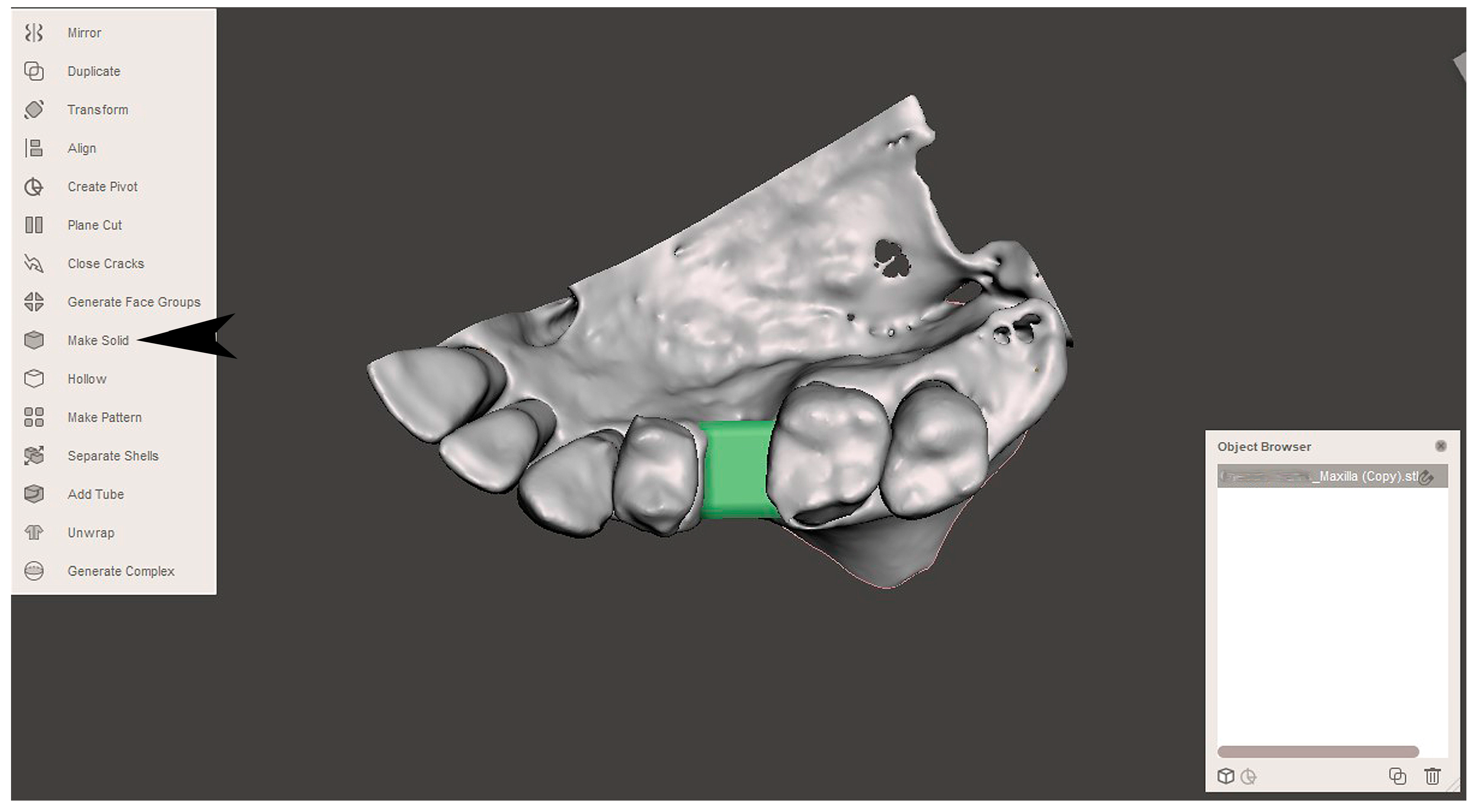
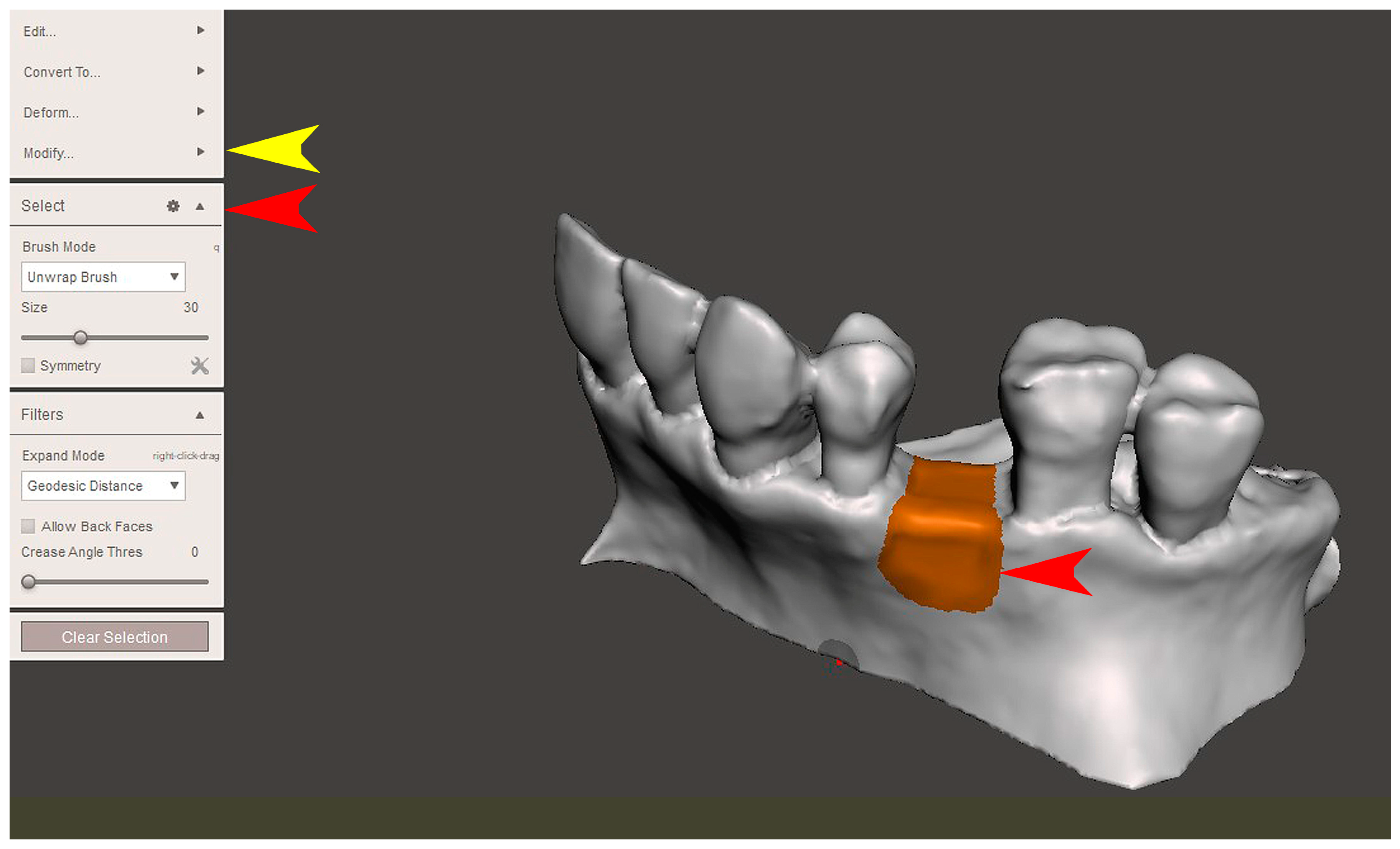
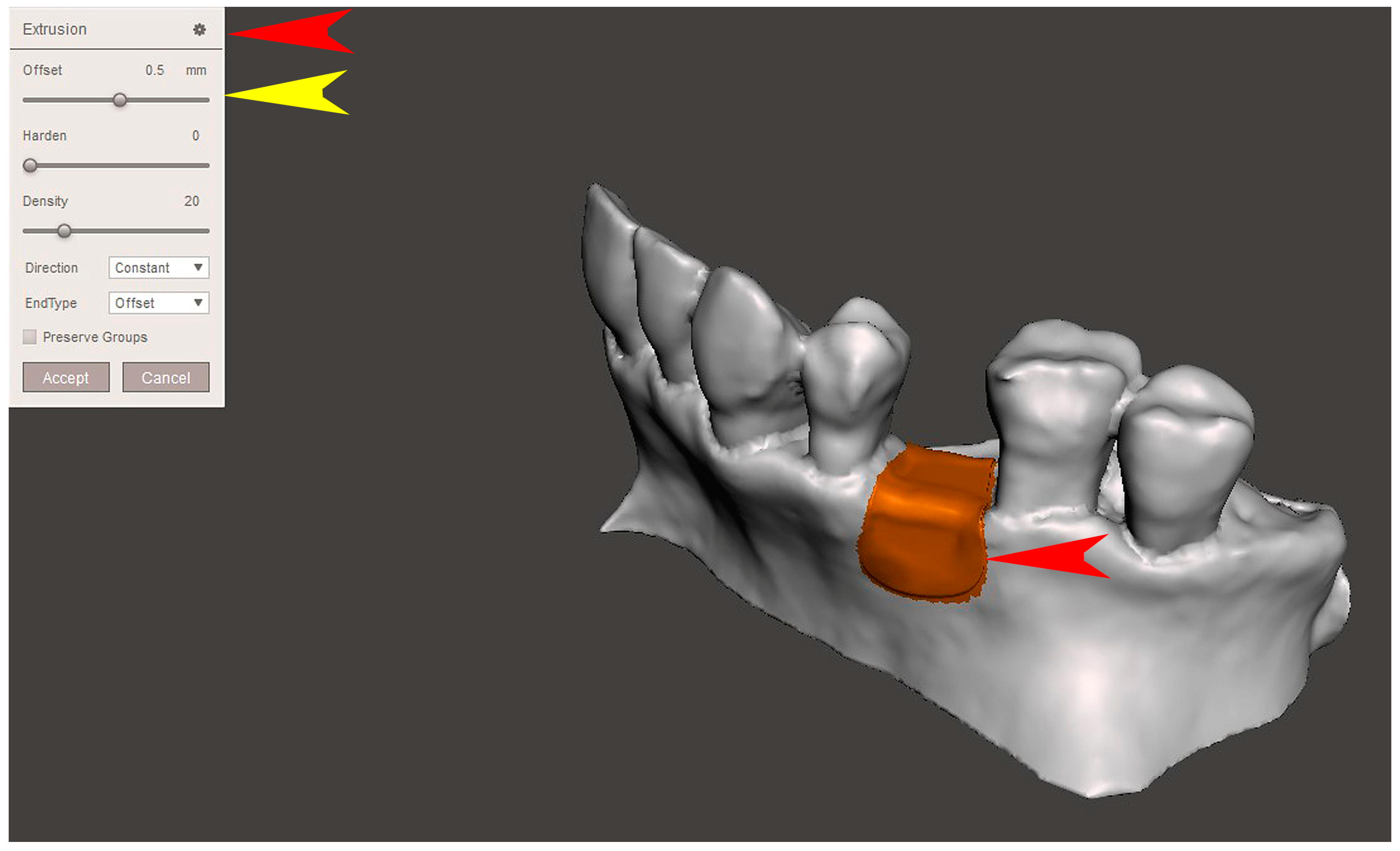
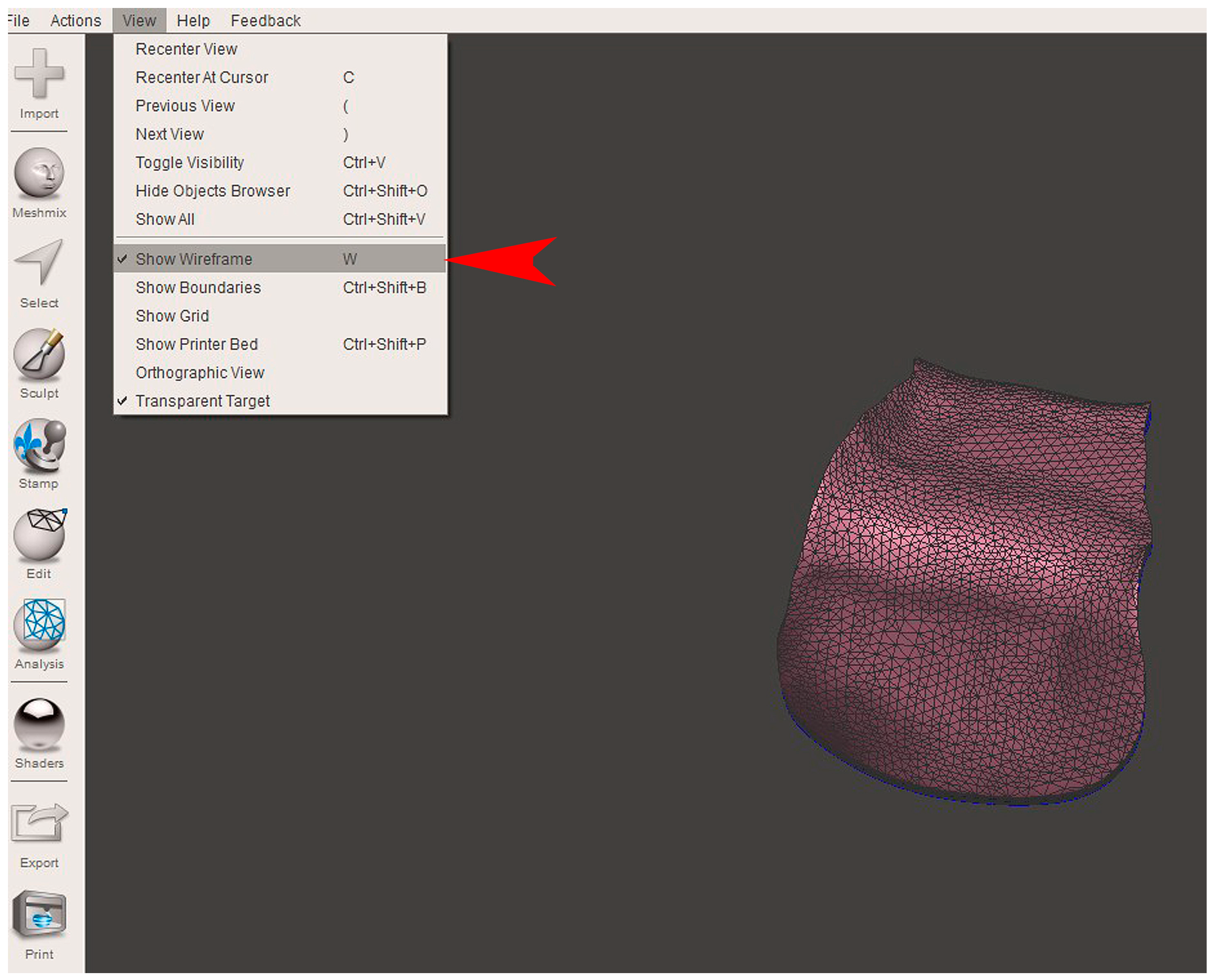
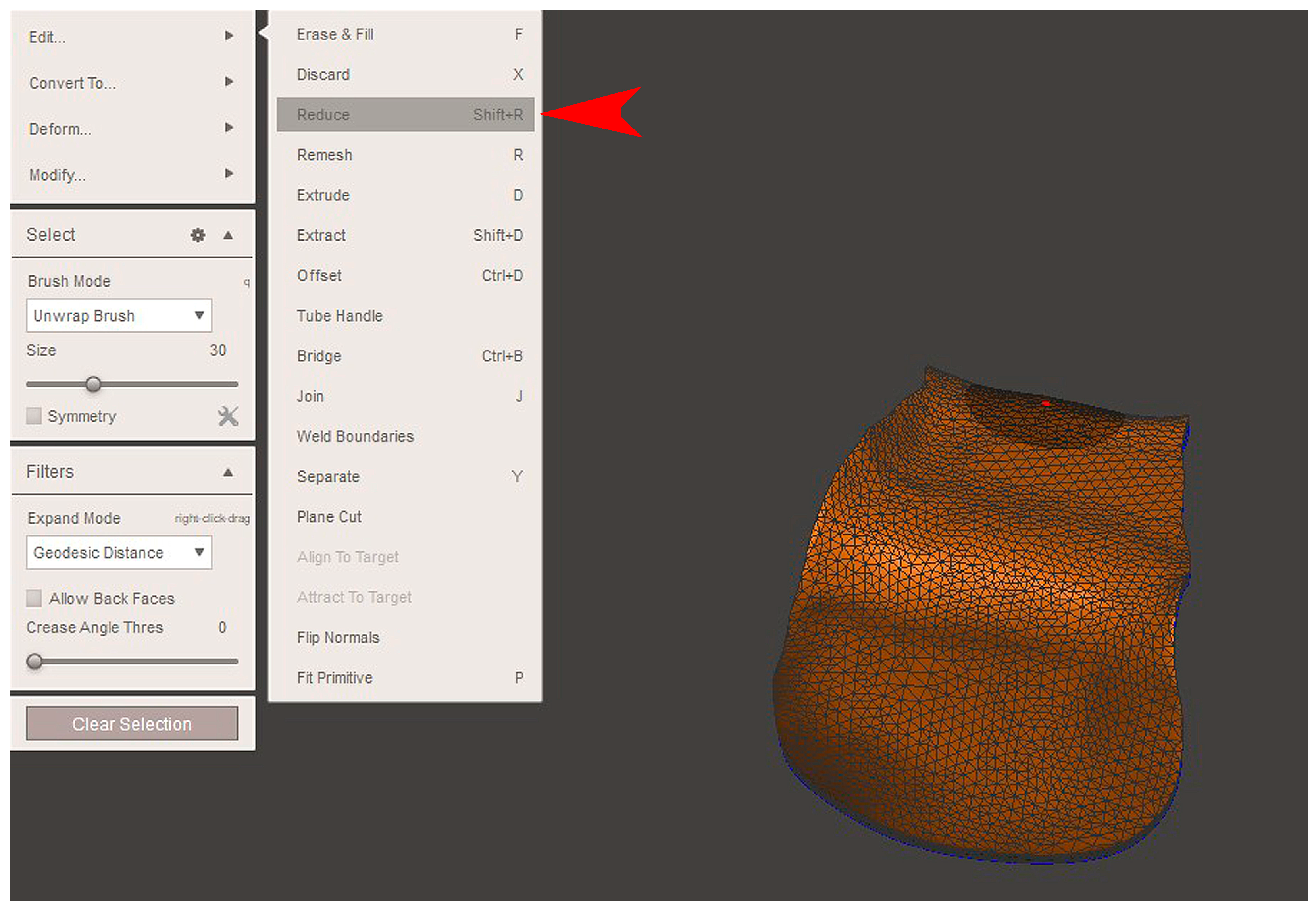
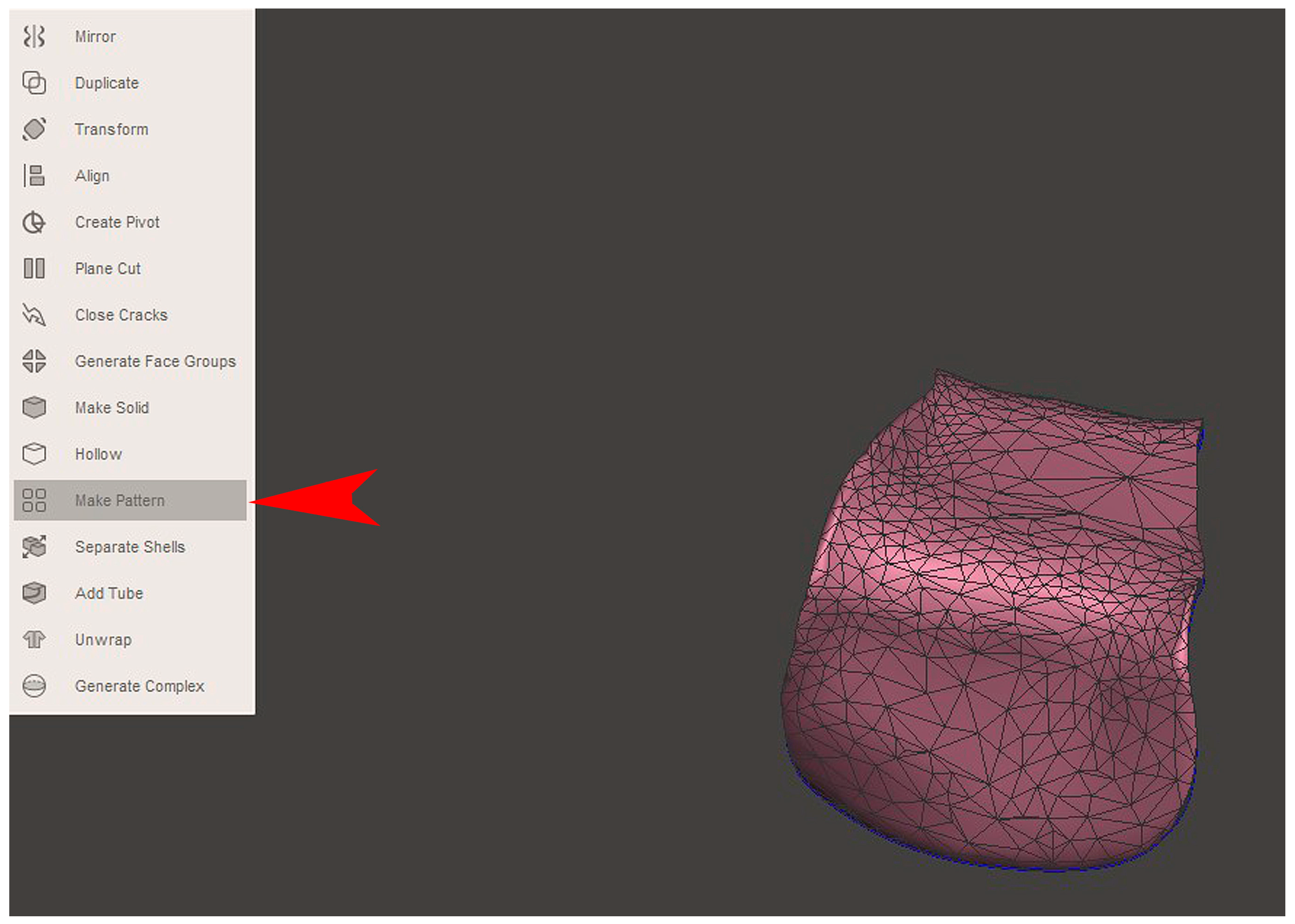
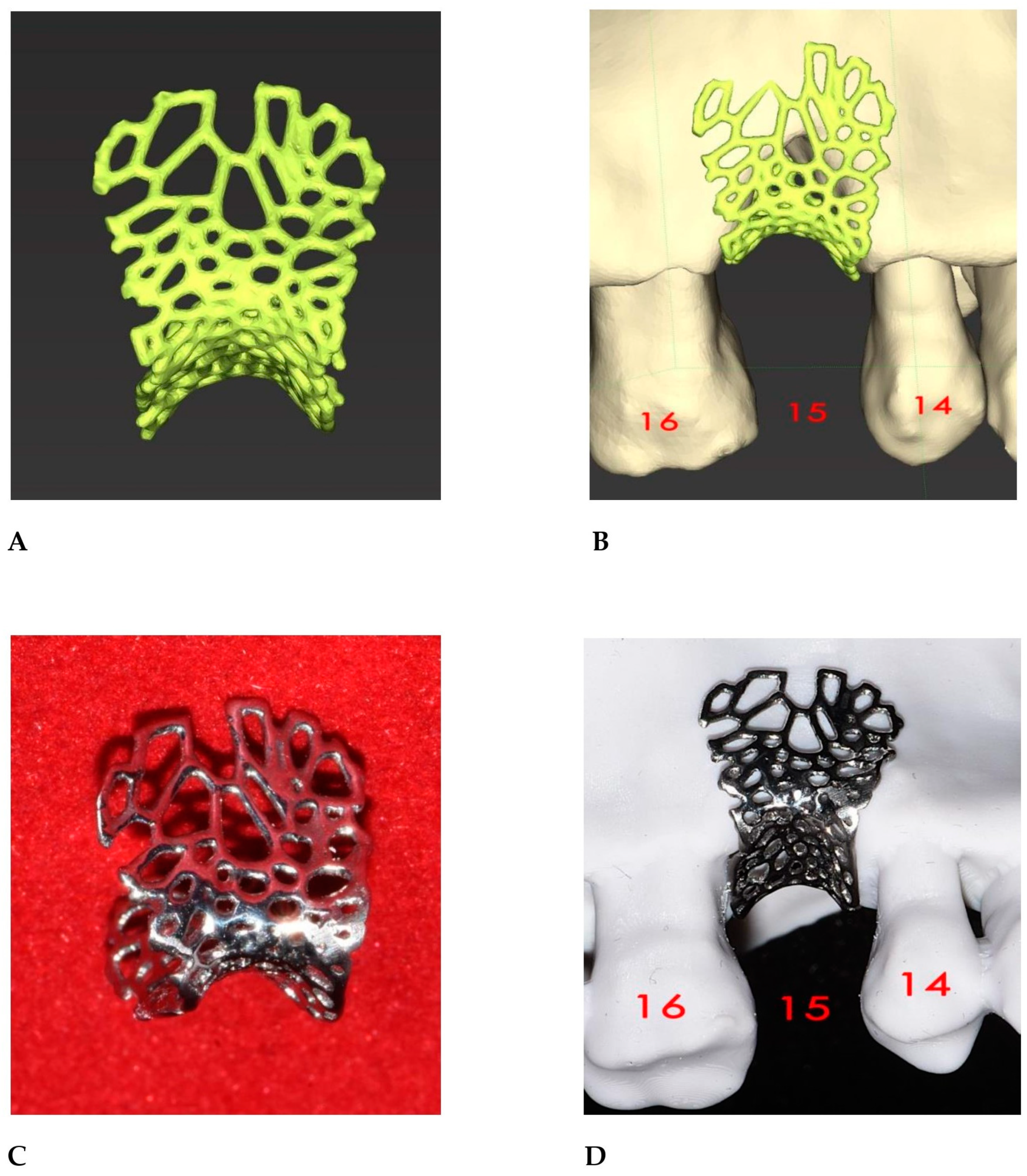
2.2. GBR Simulation and Mesh Design
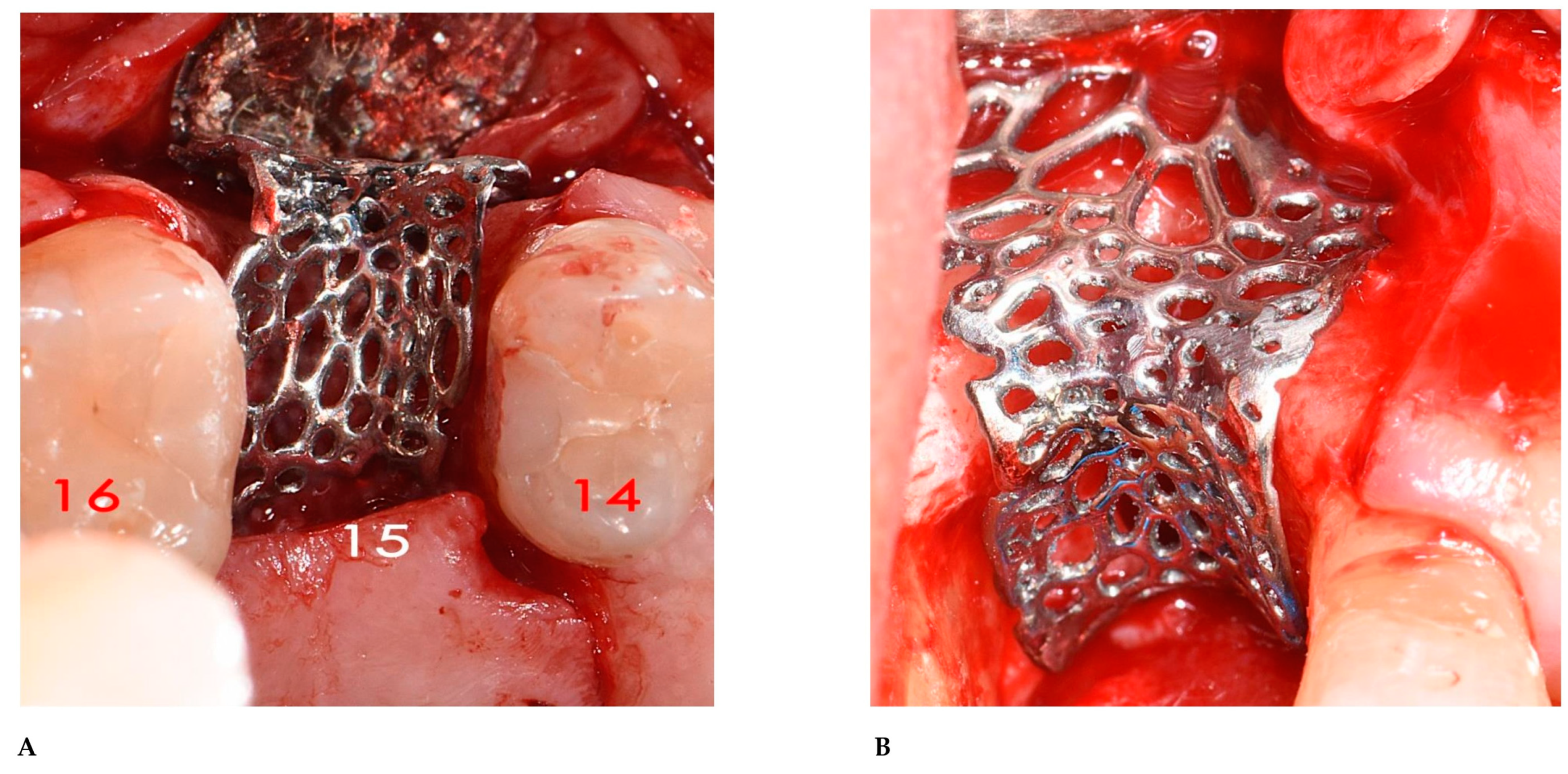
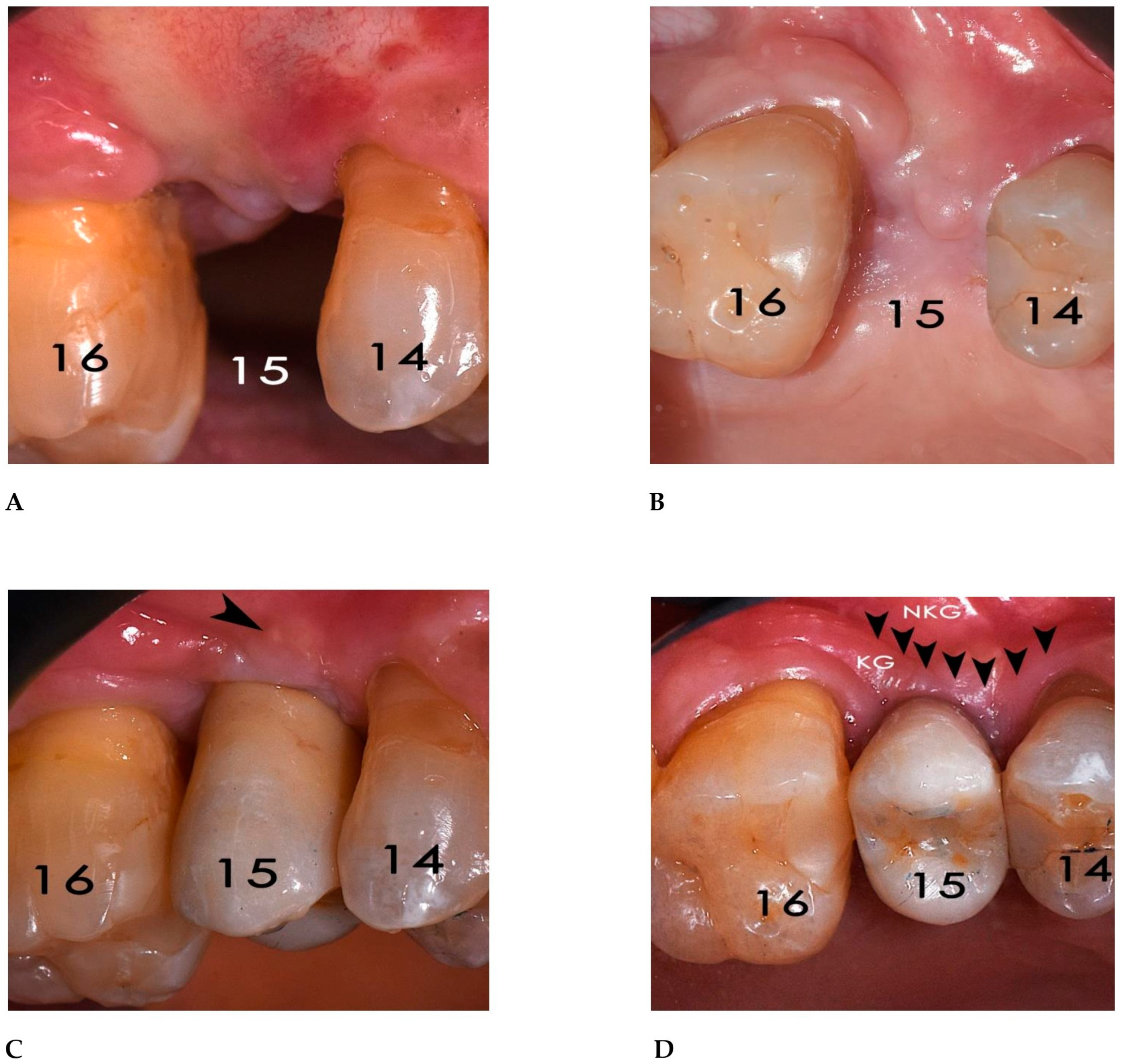
2.3. Surgical and Prosthetic Procedures
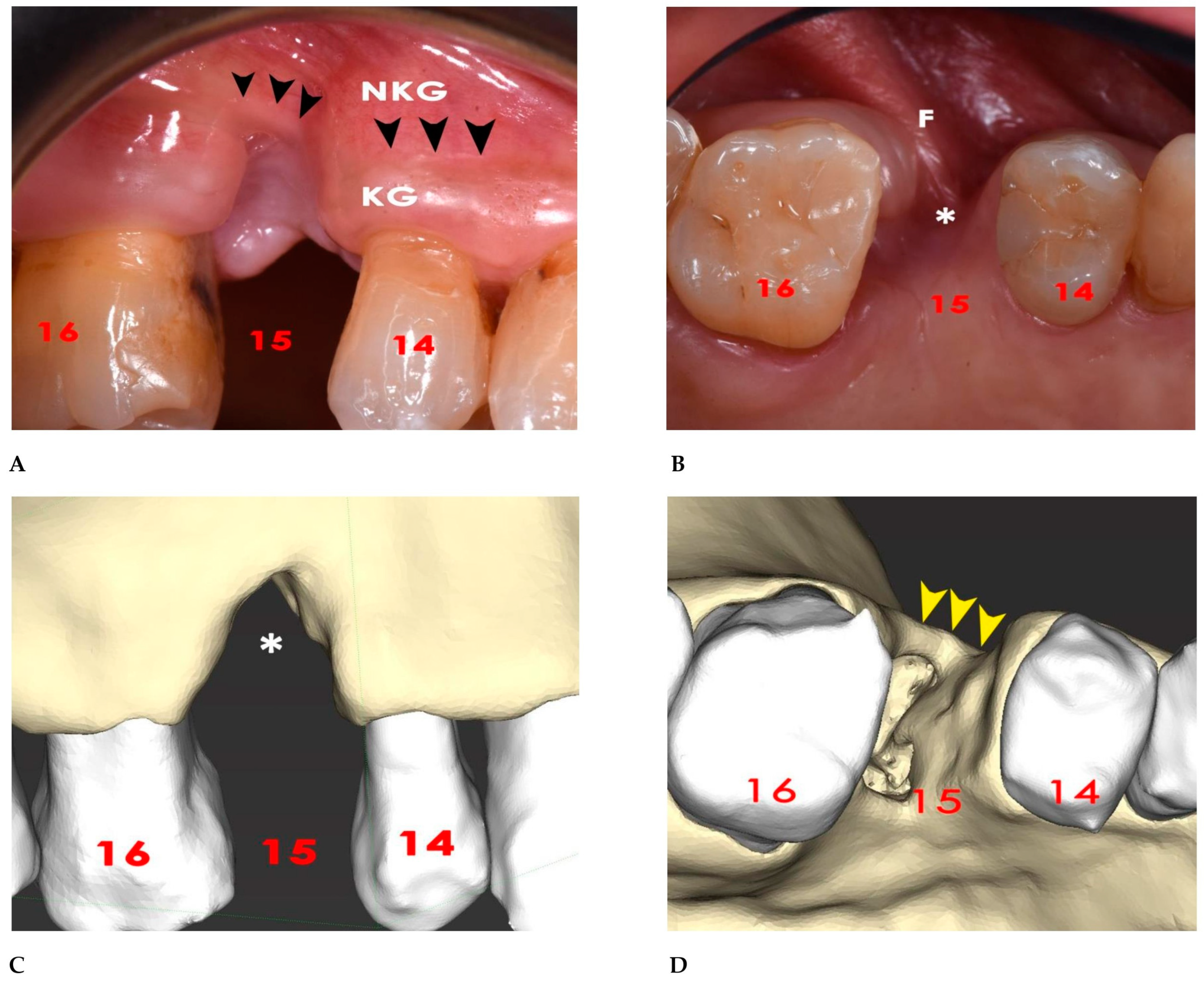
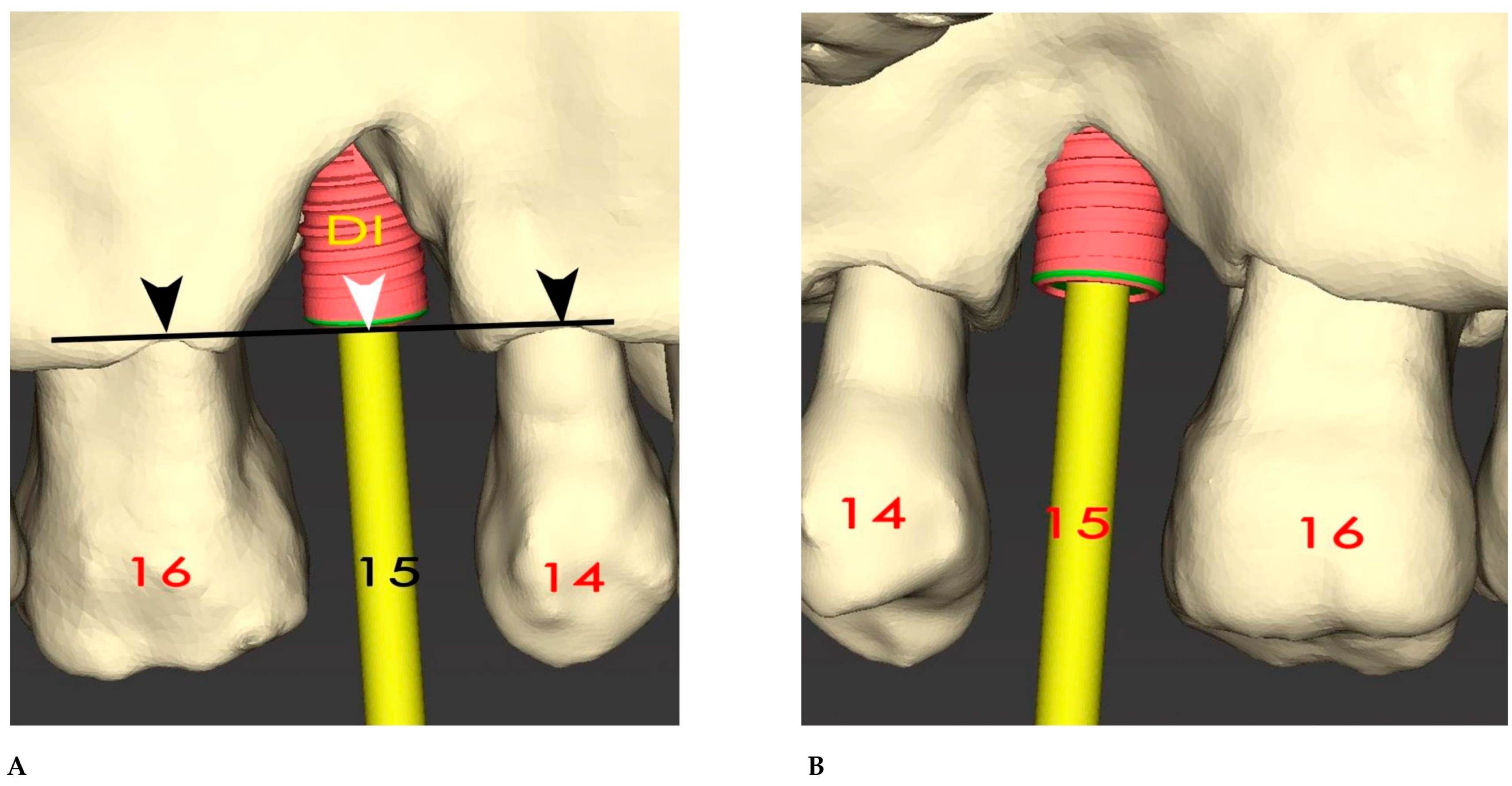
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GBR | Guided Bone Regeneration |
| TM | Titanium Mesh |
| CAD-CAM | Computer-Aided Design–Computer-Aided Manufacturing |
| STL | Standard Tesselation Language |
| DICOM | Digital Imaging and Communications in Medicine |
| CBCT | Cone Beam Computed Tomography |
| OSCS | Open-Source CAD-CAM Software |
References
- Mateo-Sidrón Antón, M.C.; Pérez-González, F.; Meniz-García, C. Titanium mesh for guided bone regeneration: A systematic review. Br. J. Oral Maxillofac. Surg. 2024, 62, 433–440. [Google Scholar] [CrossRef]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sánchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 319–339. [Google Scholar] [CrossRef]
- Cucchi, A.; Maiani, F.; Franceschi, D.; Sassano, M.; Fiorino, A.; Urban, I.A.; Corinaldesi, G. The influence of vertical ridge augmentation techniques on peri-implant bone loss: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2024, 26, 15–65. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Ramieri, G.; Spada, M.C.; Bianchi, S.D.; Berrone, S. Vertical alveolar ridge augmentation by means of a titanium mesh and autogenous bone grafts. Clin. Oral Implants Res. 2004, 15, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, F.F.; Rocchietta, I.; Buti, J.; D’Aiuto, F. Comparative evidence of different surgical techniques for the management of vertical alveolar ridge defects in terms of complications and efficacy: A systematic review and network meta-analysis. J. Clin. Periodontol. 2023, 50, 1487–1519. [Google Scholar] [CrossRef] [PubMed]
- Sabri, H.; Heck, T.; Manouchehri, N.; Alhachache, S.; Calatrava, J.; Misch, C.M.; Wang, H.L. Bone augmentation using titanium mesh: A systematic review and meta-analysis. Int. J. Oral Implantol. 2024, 17, 251–269. [Google Scholar] [PubMed]
- Lizio, G.; Pellegrino, G.; Corinaldesi, G.; Ferri, A.; Marchetti, C.; Felice, P. Guided bone regeneration using titanium mesh to augment 3-dimensional alveolar defects prior to implant placement. A pilot study. Clin. Oral Implants Res. 2022, 33, 607–621. [Google Scholar] [CrossRef]
- Cirrincione, C.; Ricci, M.; Guarnieri, G.; Morelli, A.; Ottanelli, G.; Piccarreta, E. Excessive stiffness of meshes for oral guided bone regeneration may cause mucosal dehiscence: An in-vitro comparative loading study between titanium alloy and polycaprolactone. Ital. J. Anat. Embryol. 2024, 128 (Suppl. S130). [Google Scholar]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef]
- Cucchi, A.; Bettini, S.; Ghensi, P.; Fiorino, A.; Corinaldesi, G. Vertical ridge augmentation with Ti-reinforced dense polytetrafluoroethylene (d-PTFE) membranes or Ti-meshes and collagen membranes: 3-year results of a randomized clinical trial. Clin. Implant Dent. Relat. Res. 2023, 25, 352–369. [Google Scholar] [CrossRef]
- Ciocca, L.; Ragazzini, S.; Fantini, M.; Corinaldesi, G.; Scotti, R. Work flow for the prosthetic rehabilitation of atrophic patients with a minimal-intervention CAD/CAM approach. J. Prosthet. Dent. 2015, 114, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Seiler, M.; Kämmerer, P.W.; Peetz, M.; Hartmann, A.G. Customized Titanium Lattice Structure in Three-Dimensional Alveolar Defect: An Initial Case Letter. J. Oral Implantol. 2018, 44, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Bianchi, A.; Calamai, P.; Rinaldi, L.; Mangano, F.; Vignudelli, E.; Corinaldesi, G. Clinical and volumetric outcomes after vertical ridge augmentation using computer-aided-design/computer-aided manufacturing (CAD/CAM) customized titanium meshes: A pilot study. BMC Oral Health 2020, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Casentini, P.; Tommasato, G.; Dellavia, C.; Del Fabbro, M. Customized CAD/CAM titanium meshes for the guided bone regeneration of severe alveolar ridge defects: Preliminary results of a retrospective clinical study in humans. Clin. Oral Implants Res. 2021, 32, 498–510. [Google Scholar] [CrossRef]
- Tommasato, G.; Piano, S.; Casentini, P.; De Stavola, L.; Chiapasco, M. Digital planning and bone regenerative technologies: A narrative review. Clin. Oral Implants Res. 2024, 35, 906–921. [Google Scholar] [CrossRef]
- Cucchi, A.; Bettini, S.; Tedeschi, L.; Urban, I.; Franceschi, D.; Fiorino, A.; Corinaldesi, G. Complication, vertical bone gain, volumetric changes after vertical ridge augmentation using customized reinforced PTFE mesh or Ti-mesh. A non-inferiority randomized clinical trial. Clin. Oral Implants Res. 2024, 35, 1616–1639. [Google Scholar] [CrossRef]
- Cirrincione, C. Finite Element Analysis comparison between a polycaprolactone and a Ti6Al4V alloy meshs for bone reconstruction, designed with open-source CAD software: Importance of the mesh thickness and pore width. In Proceedings of the ITI World Symposium Singapore, Singapore, 9–11 May 2024. [Google Scholar]
- Cucchi, A.; Vignudelli, E.; Franceschi, D.; Randellini, E.; Lizio, G.; Fiorino, A.; Corinaldesi, G. Vertical and horizontal ridge augmentation using customized CAD/CAM titanium mesh with versus without resorbable membranes. A randomized clinical trial. Clin. Oral Implants Res. 2021, 32, 1411–1424. [Google Scholar] [CrossRef]
- Cucchi, A.; Sartori, M.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. A Proposal of Pseudo-periosteum Classification After GBR by Means of Titanium-Reinforced d-PTFE Membranes or Titanium Meshes Plus Cross-Linked Collagen Membranes. Int. J. Periodontics Restor. Dent. 2019, 39, e157–e165. [Google Scholar] [CrossRef]
- Uehara, S.; Kurita, H.; Shimane, T.; Sakai, H.; Kamata, T.; Teramoto, Y.; Yamada, S. Predictability of staged localized alveolar ridge augmentation using a micro titanium mesh. Oral Maxillofac. Surg. 2015, 19, 411–416. [Google Scholar] [CrossRef]
- Sumida, T.; Otawa, N.; Kamata, Y.U.; Kamakura, S.; Mtsushita, T.; Kitagaki, H.; Mori, S.; Sasaki, K.; Fujibayashi, S.; Takemoto, M.; et al. Custom-made titanium devices as membranes for bone augmentation in implant treatment: Clinical application and the comparison with conventional titanium mesh. J. Craniomaxillofac. Surg. 2015, 43, 2183–2188. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, S.H.; Lee, D.W. Vertical Ridge Augmentation with Customized Titanium Mesh Using a 3D-Printing Model: A Prospective Study in Humans. Int. J. Oral Maxillofac. Implants 2024, 39, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Gehrke, S.A.; Alla, I.; Tari, S.R.; Scarano, A. The Early Exposure Rate and Vertical Bone Gain of Titanium Mesh for Maxillary Bone Regeneration: A Systematic Review and Meta-Analysis. Dent. J. 2025, 13, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cirrincione, C.; Ottanelli, G. Three-dimensionally printed polycaprolactone shows more physiological stiffness compared with titanium alloy. In Proceedings of the 1st International Online Conference on Functional Biomaterials, Online, 10–12 July 2024; MDPI: Basel, Switzerland, 2024. [Google Scholar]
- Park, S.H.; Wang, H.L. Clinical significance of incision location on guided bone regeneration: Human study. J. Periodontol. 2007, 78, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Rong, Q.G.; Zhu, N.; Ma, T.; Zhang, Y.; Lin, Y. Finite element analysis of stress in oral mucosa and titanium mesh interface. BMC Oral Health 2023, 23, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.; Li, J.; Zhang, S.; Xiao, H.; Wang, Y.; Zhang, J. Assessment of the application of a novel three-dimension printing individualized titanium mesh in alveolar bone augmentation: A retrospective study. Clin. Implant Dent. Relat. Res. 2024, 26, 1111–1125. [Google Scholar] [CrossRef]
- Lizio, G.; Mazzone, N.; Corinaldesi, G.; Marchetti, C. Reconstruction of Extended and Morphologically Varied Alveolar Ridge Defects with the Titanium Mesh Technique: Clinical and Dental Implants Outcomes. Int. J. Periodontics Restor. Dent. 2016, 36, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Ronda, M.; Desantis, V.; Bruno, D.; Veneriano, L.; Elli, C.; Pispero, A. New Generation Customized Titanium Meshes for the Guided Bone Regeneration of Severe Alveolar Ridge Defects: Preliminary Results of a Retrospective Case Series. Int. J. Periodontics Restor. Dent. 2024, 6, 1–25. [Google Scholar] [PubMed]
- Felice, P.; Pistilli, R.; Pellegrino, G.; Bonifazi, L.; Tayeb, S.; Simion, M.; Barausse, C. A randomised controlled trial comparing the effectiveness of guided bone regeneration with polytetrafluoroethylene titanium-reinforced membranes, CAD/CAM semi-occlusive titanium meshes and CAD/CAM occlusive titanium foils in partially atrophic arches. Int. J. Oral Implantol. 2024, 17, 285–296. [Google Scholar] [PubMed]
- Vilanova-Corrales, P.; Demiquels-Punzano, E.; Caballé-Serrano, J.; Hernández-Alfaro, F.; Delgado, J.Á.; Pérez, R.A.; Gil, J.; Delgado, L.M. Biodegradable and reinforced membranes based on polycaprolactone and collagen for guided bone regeneration. Mater. Today Commun. 2024, 41, 111039. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Vaquette, C.; Ivanovski, S. Workflow for highly porous resorbable custom 3D printed scaffolds using medical grade polymer for large volume alveolar bone regeneration. Clin. Oral Implants Res. 2020, 31, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Staples, R.; Arora, H.; Vaquette, C.; Alayan, J. Alveolar bone regeneration using a 3D-printed patient-specific resorbable scaffold for dental implant placement: A case report. Clin. Oral Implants Res. 2024, 35, 1655–1668. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanaka, K.; Sailer, I.; Iwama, R.; Yamauchi, K.; Nogami, S.; Yoda, N.; Takahashi, T. Relationship between cortical bone thickness and implant stability at the time of surgery and secondary stability after osseointegration measured using resonance frequency analysis. J. Periodontal Implants Sci. 2018, 48, 360–372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brouwers, J.E.I.G.; Buis, S.; de Groot, P.G.; de Laat, B.; Remijn, J.A. Resonance frequency analysis with two different devices after conventional implant placement with ridge preservation: A prospective pilot cohort study. Clin. Implant Dent. Relat. Res. 2021, 23, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zheng, L.; Wang, C.; Huang, Y.; Huang, H.; Apicella, A.; Hu, G.; Wang, L.; Fan, Y. Evaluation of surgical placement accuracy of customized CAD/CAM titanium mesh using screws-position-guided template: A retrospective comparative study. Clin. Implant Dent. Relat. Res. 2023, 25, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, C.; Li, X.; Fu, G.; Chen, D.; Huang, Y. Research on the dimensional accuracy of customized bone augmentation combined with 3D-printing individualized titanium mesh: A retrospective case series study. Clin. Implant Dent. Relat. Res. 2021, 23, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.; Montero, E.; Sanz-Sánchez, I.; Palombo, D.; Monje, A.; Tommasato, G.; Chiapasco, M. Minimal invasiveness in vertical ridge augmentation. Periodontology 2000 2023, 91, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Simion, M.; Pistilli, R.; Vignudelli, E.; Pellegrino, G.; Barausse, C.; Bonifazi, L.; Roccoli, L.; Iezzi, G.; Felice, P. Semi-occlusive CAD/CAM titanium mesh for guided bone regeneration: Preliminary clinical and histological results. Int. J. Oral Implantol. 2023, 16, 327–336. [Google Scholar] [PubMed]
- Hartmann, A.; Hildebrandt, H.; Younan, Z.; Al-Nawas, B.; Kämmerer, P.W. Long-term results in three-dimensional, complex bone augmentation procedures with customized titanium meshes. Clin. Oral Implants Res. 2022, 33, 1171–1181. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirrincione, C.; Guarnieri, G.; Morelli, A. Digital Workflow with Open-Source CAD-CAM Software Aimed to Design a Customized 3D Laser-Printed Titanium Mesh for Guided Bone Regeneration. Bioengineering 2025, 12, 436. https://doi.org/10.3390/bioengineering12050436
Cirrincione C, Guarnieri G, Morelli A. Digital Workflow with Open-Source CAD-CAM Software Aimed to Design a Customized 3D Laser-Printed Titanium Mesh for Guided Bone Regeneration. Bioengineering. 2025; 12(5):436. https://doi.org/10.3390/bioengineering12050436
Chicago/Turabian StyleCirrincione, Claudio, Giulia Guarnieri, and Annamaria Morelli. 2025. "Digital Workflow with Open-Source CAD-CAM Software Aimed to Design a Customized 3D Laser-Printed Titanium Mesh for Guided Bone Regeneration" Bioengineering 12, no. 5: 436. https://doi.org/10.3390/bioengineering12050436
APA StyleCirrincione, C., Guarnieri, G., & Morelli, A. (2025). Digital Workflow with Open-Source CAD-CAM Software Aimed to Design a Customized 3D Laser-Printed Titanium Mesh for Guided Bone Regeneration. Bioengineering, 12(5), 436. https://doi.org/10.3390/bioengineering12050436






