Neuromodulation of Cerebral Blood Flow: A Physiological Mechanism and Methodological Review of Neurovascular Coupling
Abstract
:1. Introduction
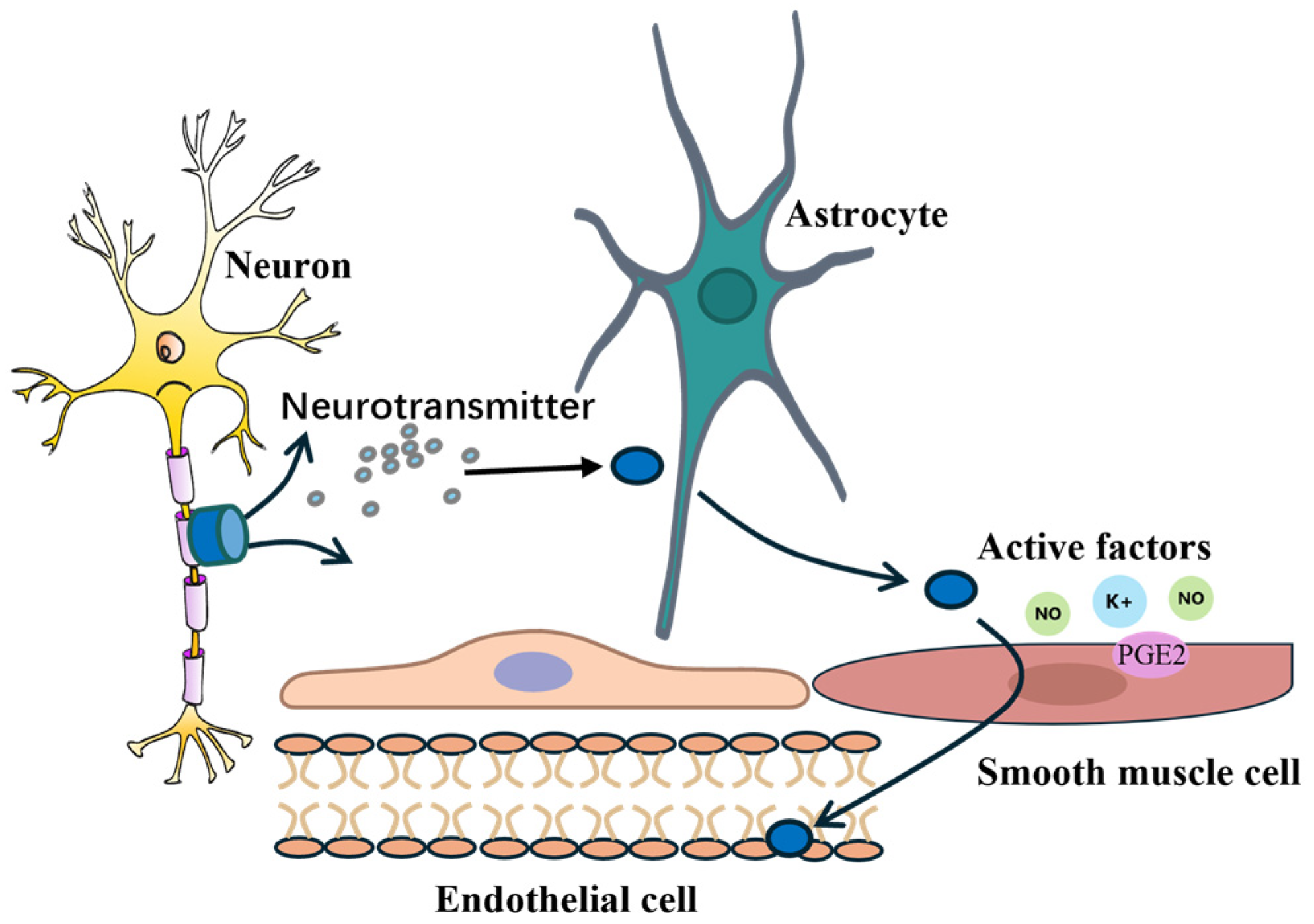
2. Physiological Mechanisms of Neurovascular Coupling
2.1. The Link Between Neuronal Activity and Blood Flow Supply
2.2. Intercellular Signal Transmission
2.3. Synergistic Action of Signaling Molecules
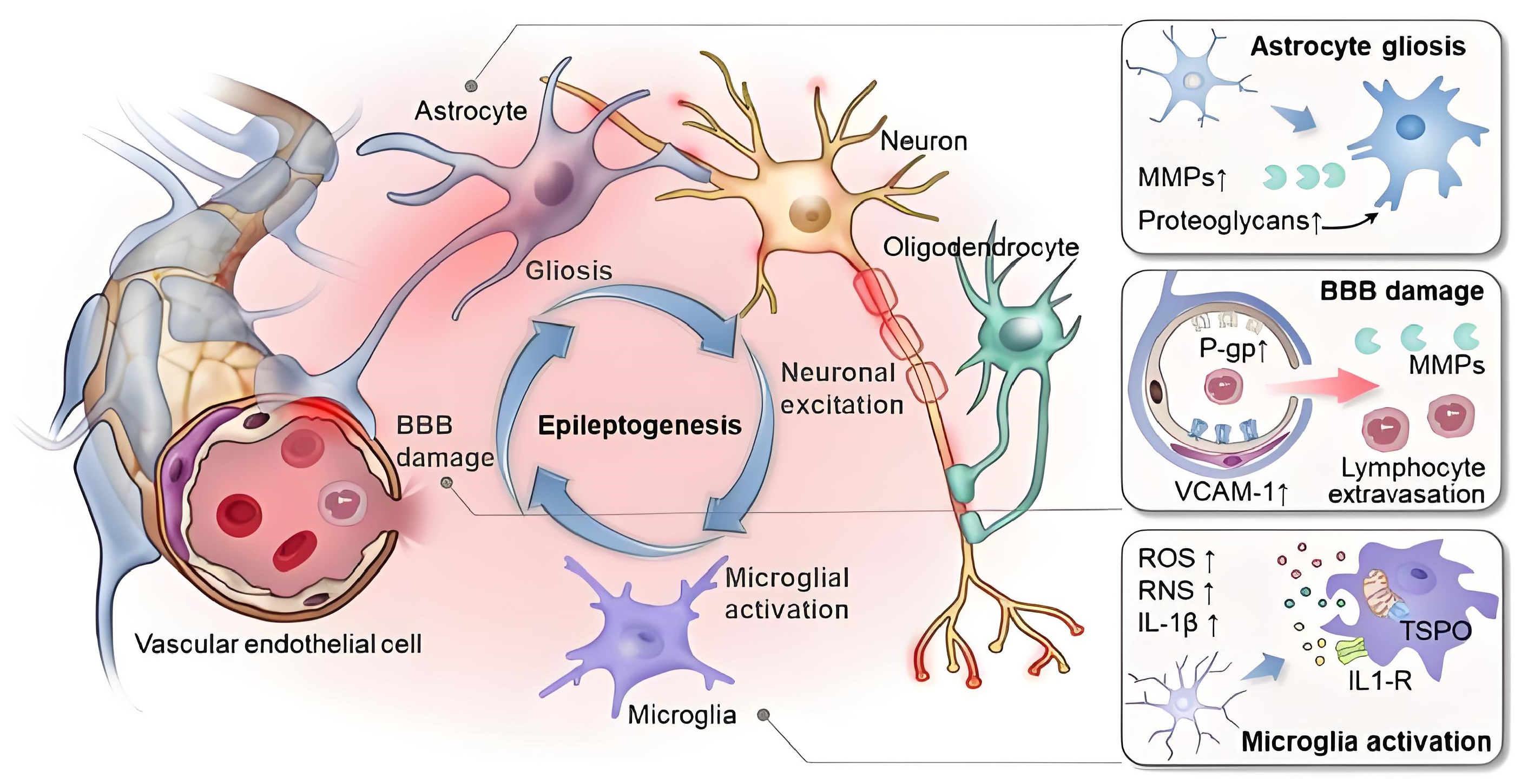
3. Neurovascular Coupling Impairment in Neurological and Cerebrovascular Diseases
3.1. Mechanisms of Neurovascular Coupling Impairment
3.2. Impairment of Neurovascular Coupling in Alzheimer’s Disease
3.3. Impairment of Neurovascular Coupling After Stroke
3.4. Modeling Strategies to Explore Impaired Neurovascular Coupling
3.5. Biomarkers Associated with Neurovascular Coupling Impairment
4. Neural Activity and Blood Flow Measurement Methods
4.1. Transcranial Doppler
4.2. Near-Infrared Spectroscopy
4.3. Functional Magnetic Resonance Imaging
4.4. Multimodal Techniques
5. Future Prospects
5.1. Basic Mechanisms
5.2. New Detection Methods
5.3. Clinical Applications
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- van Dijk, S.E.; Drenth, N.; Hafkemeijer, A.; Labadie, G.; Witjes-Ané, M.N.W.; Baas, F.; Vreijling, J.P.; Blauw, G.J.; Rombouts, S.A.; van der Grond, J. Neurovascular Decoupling Is Associated with Lobar Intracerebral Hemorrhages and White Matter Hyperintensities. J. Am. Heart Assoc. 2025, 14, e038819. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.S.; Sherrington, C.S. On the regulation of the blood-supply of the brain. J. Physiol. 1890, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Lueck, C.J.; Zeki, S.; Friston, K.J.; Deiber, M.-P.; Cope, P.; Cunningham, V.J.; Lammertsma, A.; Kennard, C.; Frackowiak, R. The colour centre in the cerebral cortex of man. Nature 1989, 340, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Webb, A.J.S. Reduced neurovascular coupling is associated with increased cardiovascular risk without established cerebrovascular disease: A cross-sectional analysis in UK biobank. J. Cereb. Blood Flow Metab. 2025, 45, 897–907. [Google Scholar] [CrossRef]
- Meng, L.; Rasmussen, M.; Meng, D.M.; White, F.A.; Wu, L.-J. Integrated Feedforward and Feedback Mechanisms in Neurovascular Coupling. Anesth. Analg. 2022, 10, 1213. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Iadecola, C. The pathobiology of neurovascular aging. Neuron 2025, 113, 49–70. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, R.; Chen, L.; Gong, J.; Luo, T.; Lv, F. Altered neurovascular coupling in subcortical ischemic vascular disease. Front. Aging. Neurosci. 2021, 13, 598365. [Google Scholar] [CrossRef]
- Owens, C.D.; Pinto, C.B.; Mukli, P.; Gulej, R.; Velez, F.S.; Detwiler, S.; Olay, L.; Hoffmeister, J.R.; Szarvas, Z.; Muranyi, M. Neurovascular coupling, functional connectivity, and cerebrovascular endothelial extracellular vesicles as biomarkers of mild cognitive impairment. Alzheimer’s Dement. 2024, 20, 5590–5606. [Google Scholar] [CrossRef]
- Larios, A.; Gu, C. Investigating the Role of Neurovascular Coupling on Brain Function and Health. Physiology 2023, 38, 5732872. [Google Scholar] [CrossRef]
- Kandimalla, M.; Lim, S.; Thakkar, J.; Dewan, S.; Kang, D.; In, M.-H.; Jo, H.J.; Jang, D.P.; Nedelska, Z.; Lapid, M.I. Cardiorespiratory Dynamics in the Brain: Review on the Significance of Cardiovascular and Respiratory Correlates in functional MRI signal. NeuroImage 2025, 306, 121000. [Google Scholar] [CrossRef]
- Al-Jaf, S.; Soliman, A.Y.; El-Yazbi, A.F.; Abd-Elrahman, K.S. Unveiling the Interplay: Neurovascular Coupling, Astrocytes and G Protein-Coupled Receptors in Alzheimer’s Disease. ACS Pharmacol. Transl. Sci. 2025, 8, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Bonin Pinto, C.; Detwiler, S.; Olay, L.; Pinaffi-Langley, A.C.d.C.; Mukli, P.; Peterfi, A.; Szarvas, Z.; James, J.A.; Galvan, V. Neurovascular coupling impairment as a mechanism for cognitive deficits in COVID-19. Brain Commun. 2024, 6, fcae080. [Google Scholar] [CrossRef]
- Gordon, G.R. Neurovascular coupling during hypercapnia in cerebral blood flow regulation. Nat. Commun. 2024, 15, 7636. [Google Scholar] [CrossRef]
- The Human Brain: Surface, Blood Supply, and Three-Dimensional Anatomy, 2nd ed; Sure, U., Duvernoy, H.M., Eds.; completely revised; Springer Wien New York: Wien, Austria, 1999; p. 2007. [Google Scholar]
- Kataeva, G.; Korotkov, A. The regional cerebral blood flow pattern of the normal human brain and its factor structure. Hum. Physiol. 2007, 33, 383–387. [Google Scholar] [CrossRef]
- Srisaikaew, P.; Vaniyapong, T.; Das, S.; Mahhakranukrauh, P. Clinical significance of blood supply of the fornix of brain: A cadaveric study. Sains Malays. 2020, 49, 399–404. [Google Scholar] [CrossRef]
- Chauvel, M.; Pascucci, M.; Uszynski, I.; Herlin, B.; Mangin, J.-F.; Hopkins, W.D.; Poupon, C. Comparative analysis of the chimpanzee and human brain superficial structural connectivities. Brain Struct. Funct. 2024, 229, 1943–1977. [Google Scholar] [CrossRef]
- Yang, H.; Cho, K.-C.; Hong, I.; Kim, Y.; Kim, Y.B.; Kim, J.-J.; Oh, J.H. Influence of circle of Willis modeling on hemodynamic parameters in anterior communicating artery aneurysms and recommendations for model selection. Sci. Rep. 2024, 14, 8476. [Google Scholar] [CrossRef]
- Greggio, J.; Malamateniou, C.; Baruteau, K.P.; Reyes-Aldasoro, C.C.; Huckstep, O.J.; Francis, J.M.; Williamson, W.; Leeson, P.; Lewandowski, A.J.; Lapidaire, W. Distinct Circle of Willis anatomical configurations in healthy preterm born adults: A 3D time-of-flight magnetic resonance angiography study. BMC Med. Imaging 2025, 25, 33. [Google Scholar] [CrossRef]
- Isaacs, D.; Xiang, L.; Hariharan, A.; Longden, T.A. KATP channel–dependent electrical signaling links capillary pericytes to arterioles during neurovascular coupling. Proc. Natl. Acad. Sci. USA 2024, 121, e2405965121. [Google Scholar] [CrossRef]
- Vestergaard, M.B.; Bakhtiari, A.; Osler, M.; Mortensen, E.L.; Lindberg, U.; Law, I.; Lauritzen, M.; Benedek, K.; Larsson, H.B.W. The cerebral blood flow response to neuroactivation is reduced in cognitively normal men with β-amyloid accumulation. Alzheimer’s Res. Ther. 2025, 17, 4. [Google Scholar] [CrossRef]
- Adriani, G.; Ma, D.; Pavesi, A.; Kamm, R.D.; Goh, E.L. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier. Lab. Chip 2017, 17, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fourriere, L.; Gleeson, P. Local Secretory Trafficking Pathways in Neurons and the Role of Dendritic Golgi Outposts in Different Cell Models. Front. Mol. Neurosci. 2020, 13, 597391. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Hong, M.; Lasser-Ross, N.; Ross, W.N. Modulation of calcium wave propagation in the dendrites and to the soma of rat hippocampal pyramidal neurons. J. Physiol. 2006, 575, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Puebla, M.D.; Muñoz, M.F.; Figueroa, X. Neurovascular Coupling is Coordinated by The Activation of Nitric Oxide Production in Astrocytes. FASEB J. 2016, 30, 989.983. [Google Scholar] [CrossRef]
- Gryglewski, R.; Chłopicki, S.; Uracz, W.; Marcinkiewicz, E. Significance of endothelial prostacyclin and nitric oxide in peripheral and pulmonary circulation. Med. Sci. Monit. 2001, 7, 1–16. [Google Scholar]
- Lourenço, C.F.; Laranjinha, J. Nitric oxide pathways in neurovascular coupling under normal and stress conditions in the brain: Strategies to rescue aberrant coupling and improve cerebral blood flow. Front. Physiol. 2021, 12, 729201. [Google Scholar] [CrossRef]
- Zhang, D.; Ruan, J.; Peng, S.; Li, J.; Hu, X.; Zhang, Y.; Zhang, T.; Ge, Y.; Zhu, Z.; Xiao, X. Synaptic-like transmission between neural axons and arteriolar smooth muscle cells drives cerebral neurovascular coupling. Nat. Neurosci. 2024, 27, 232–248. [Google Scholar] [CrossRef]
- Dormanns, K.; Brown, R.; David, T. The role of nitric oxide in neurovascular coupling. J. Theor. Biol. 2016, 394, 1–17. [Google Scholar] [CrossRef]
- Namkoong, S.; Lee, S.-J.; Kim, C.-K.; Kim, Y.-M.; Chung, H.-T.; Lee, H.; Han, J.-A.; Ha, K.-S.; Kwon, Y.-G.; Kim, Y.-M. Prostaglandin E2 stimulates angiogenesis by activating the nitric oxide/cGMP pathway in human umbilical vein endothelial cells. Exp. Mol. Med. 2005, 37, 588–600. [Google Scholar] [CrossRef]
- Davidson, J.M.; Wong, C.T.; Rai-Bhogal, R.; Li, H.; Crawford, D.A. Prostaglandin E2 elevates calcium in differentiated neuroectodermal stem cells. Mol. Cell. Neurosci. 2016, 74, 71–77. [Google Scholar] [CrossRef]
- Xu, Q.; Yin, W.; Zhou, X.; Wang, S.; Chen, S.; Yang, J.; Xi, C.; Sun, Z. Transcranial direct current stimulation for patients with walking difficulties caused by cerebral small vessel disease: A randomized controlled study. Front. Aging Neurosci. 2025, 16, 1511287. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.E.; Gant, J.C.; Lin, R.L.; Kraner, S.D.; Thibault, O.; Sompol, P.; Norris, C.M. Deciphering the Relationship Between Astrocyte Calcium Signaling, Neurovascular Coupling, and Cerebrovascular Function in Alzheimer’s Disease. Alzheimer’s Dement. 2024, 20, e095554. [Google Scholar] [CrossRef]
- Maggio, P.; Salinet, A.S.; Panerai, R.B.; Robinson, T.G. Does hypercapnia-induced impairment of cerebral autoregulation affect neurovascular coupling? A functional TCD study. J. Appl. Physiol. 2013, 115, 491–497. [Google Scholar] [PubMed]
- Salinet, A.S.; Robinson, T.G.; Panerai, R.B. Cerebral blood flow response to neural activation after acute ischemic stroke: A failure of myogenic regulation? J. Neurol. 2013, 260, 2588–2595. [Google Scholar] [CrossRef]
- Brouns, R.; De Deyn, P. The complexity of neurobiological processes in acute ischemic stroke. Clin. Neurol. Neurosurg. 2009, 111, 483–495. [Google Scholar] [CrossRef]
- Takeda, S.; Sato, N.; Takeuchi, D.; Kurinami, H.; Shinohara, M.; Niisato, K.; Kano, M.; Ogihara, T.; Rakugi, H.; Morishita, R. Angiotensin receptor blocker prevented β-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension 2009, 54, 1345–1352. [Google Scholar] [CrossRef]
- Janzarik, W.G.; Ehmann, R.; Ehlers, E.; Allignol, A.; Mayer, S.; Gabriel, B.; Weiller, C.; Prömpeler, H.; Reinhard, M. Neurovascular coupling in pregnancy and the risk of preeclampsia. Stroke 2014, 45, 2792–2794. [Google Scholar] [CrossRef]
- Østergaard, L.; Engedal, T.S.; Aamand, R.; Mikkelsen, R.; Iversen, N.K.; Anzabi, M.; Næss-Schmidt, E.T.; Drasbek, K.R.; Bay, V.; Blicher, J.U. Capillary transit time heterogeneity and flow-metabolism coupling after traumatic brain injury. J. Cereb. Blood Flow Metab. 2014, 34, 1585–1598. [Google Scholar] [CrossRef]
- Nicolakakis, N.; Hamel, E. Neurovascular function in Alzheimer’s disease patients and experimental models. J. Cereb. Blood Flow Metab. 2011, 31, 1354–1370. [Google Scholar] [CrossRef]
- Vetri, F.; Xu, H.; Paisansathan, C.; Pelligrino, D.A. Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is linked to PKC modulation of BKCa and Kir channels. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1274–H1284. [Google Scholar] [CrossRef]
- Promkan, M.; Phikulthong, K.; Kimseng, R.; Pauss, K.; Lee, T.; Weekman, E.M.; Nelson, P.T.; Wilcock, D.M.; Sompol, P. Cerebrovascular pathology and neurovascular coupling impairment in aged-mouse model of Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, e095727. [Google Scholar] [CrossRef]
- Liu, C.; Cardenas-Rivera, A.; Arnal, J.A.; Yaseen, A. Image the effect of systemic inflammation on neurovascular coupling in a mouse model of Alzheimer’s disease. In Proceedings of the Microscopy Histopathology and Analytics, Fort Lauderdale, FL, USA, 7–10 April 2024; p. JM4A. 38. [Google Scholar]
- Bjerkan, J.; Meglič, B.; Lancaster, G.; Kobal, J.; McClintock, P.V.; Crawford, T.J.; Stefanovska, A. Neurovascular phase coherence is altered in Alzheimer’s disease. Brain Commun. 2025, 7, fcaf007. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, C.; Sun, W.; Li, C. Tailoring materials for epilepsy imaging: From biomarkers to imaging probes. Adv. Mater. 2022, 34, 2203667. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Odum, J.; Shunnarah, J.G.; Austin, N.; Kaddoumi, A. Blood–brain barrier breakdown in Alzheimer’s disease: Mechanisms and targeted strategies. Int. J. Mol. Sci. 2023, 24, 16288. [Google Scholar] [CrossRef]
- Wang, D.; Chen, F.; Han, Z.; Yin, Z.; Ge, X.; Lei, P. Relationship between amyloid-β deposition and blood–brain barrier dysfunction in Alzheimer’s disease. Front. Cell. Neurosci. 2021, 15, 695479. [Google Scholar] [CrossRef]
- Yue, Q.; Hoi, M.; Pui, M. Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease. Neural Regen. Res. 2023, 18, 1890–1902. [Google Scholar]
- Wang, Q.; Huang, X.; Su, Y.; Yin, G.; Wang, S.; Yu, B.; Li, H.; Qi, J.; Chen, H.; Zeng, W. Activation of Wnt/β-catenin pathway mitigates blood–brain barrier dysfunction in Alzheimer’s disease. Brain 2022, 145, 4474–4488. [Google Scholar] [CrossRef]
- Carmignoto, G.; Gómez-Gonzalo, M. The contribution of astrocyte signalling to neurovascular coupling. Brain Res. Rev. 2010, 63, 138–148. [Google Scholar] [CrossRef]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: A longitudinal PET study. J. Neuroinflamm. 2020, 17, 151. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, C.; Zhang, W. Research progress on transport mechanism of amyloid beta-peptides across blood-brain barrier. Chin. Pharmacol. Bull. 2016, 12, 1348–1352. [Google Scholar]
- Siepen, B.M.; Forfang, E.; Branca, M.; Drop, B.; Mueller, M.; Goeldlin, M.B.; Katan, M.; Michel, P.; Cereda, C.; Medlin, F. Intracerebral haemorrhage in patients taking different types of oral anticoagulants: A pooled individual patient data analysis from two national stroke registries. Stroke Vasc. Neurol. 2024, 9, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hasan, S.; Sadan, O.; Rosenthal, E.S.; Pu, Y.; Wen, Z.; Fang, C.; Liu, X.; Duan, W.; Liu, L. Contralateral Neurovascular Coupling in Patients with Ischemic Stroke After Endovascular Thrombectomy. Neurocrit. Care 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Ding, Y.; Simo, L.; Li, F.; Geng, X. Mechanisms of Autophagy in Ineffective Reperfusion After Ischemic Stroke. J. Neurosci. Res. 2025, 103, e70017. [Google Scholar] [CrossRef]
- Taha, B.A.; Kadhim, A.C.; Addie, A.J.; Al-Jubouri, Q.; Azzahrani, A.S.; Haider, A.J.; Alkawaz, A.N.; Arsad, N. Optical Spectroscopy of Cerebral Blood Flow for Tissue Interrogation in Ischemic Stroke Diagnosis. ACS Chem. Neurosci. 2025, 16, 895–907. [Google Scholar] [CrossRef]
- Lu, W.; Wen, J. Crosstalk Among Glial Cells in the Blood–Brain Barrier Injury After Ischemic Stroke. Mol. Neurobiol. 2024, 61, 6161–6174. [Google Scholar] [CrossRef]
- Petautschnig, S.; Teo, E.; Sanders, L.; Jhamb, A.; Maingard, J.; Isik, F.; Lee, J.; Dixon, B. Optical brain pulse monitoring of microvascular blood flow during endovascular treatment for acute ischemic stroke. medRxiv 2025. [Google Scholar] [CrossRef]
- Hasanpour-Segherlou, Z.; Masheghati, F.; Shakeri-Darzehkanani, M.; Hosseini-Siyanaki, M.-R.; Lucke-Wold, B. Neurodegenerative Disorders in the Context of Vascular Changes after Traumatic Brain Injury. J. Vasc. Dis. 2024, 3, 319–332. [Google Scholar] [CrossRef]
- Zheng, Y.; Mayhew, J. A time-invariant visco-elastic windkessel model relating blood flow and blood volume. Neuroimage 2009, 47, 1371–1380. [Google Scholar] [CrossRef]
- Mut, F. Extensions to the Computational Hemodynamics Modeling of Cerebral Aneurysms; George Mason University: Fairfax, VA, USA, 2008. [Google Scholar]
- Saqr, K.M. Computational fluid dynamics simulations of cerebral aneurysm using Newtonian, power-law and quasi-mechanistic blood viscosity models. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2020, 234, 711–719. [Google Scholar] [CrossRef]
- Amponsah, J.; Lopes, B.S.; Cobbina, A. Computational modeling of predicting cerebrovascular injury in traumatic brain injury patients. J. Eng. Res. 2024. [Google Scholar] [CrossRef]
- Arslan, B.; Zetterberg, H. Neurofilament light chain as neuronal injury marker–what is needed to facilitate implementation in clinical laboratory practice? Clin. Chem. Lab. Med. 2023, 61, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Teunissen, C.E.; Lehmann, S.; Otto, M.; Piehl, F.; Ziemssen, T.; Bittner, S.; Sormani, M.P.; Gattringer, T.; Abu-Rumeileh, S. Neurofilaments as biomarkers in neurological disorders—Towards clinical application. Nat. Rev. Neurol. 2024, 20, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Baroni, S.; Rapisarda, A.; Gentili, V.; Burattini, B.; Moretti, G.; Sarlo, F.; Izzo, A.; D’Ercole, M.; Olivi, A.; Urbani, A. CSF neuron-specific enolase as a biomarker of neurovascular conflict severity in drug-resistant trigeminal neuralgia: A prospective study in patients submitted to microvascular decompression. Neurol. Sci. 2023, 44, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Schaller-Paule, M.A.; Maiworm, M.; Schäfer, J.H.; Friedauer, L.; Hattingen, E.; Wenger, K.J.; Weber, F.; Jakob, J.; Steffen, F.; Bittner, S. Matching proposed clinical and MRI criteria of aggressive multiple sclerosis to serum and cerebrospinal fluid markers of neuroaxonal and glial injury. J. Neurol. 2024, 271, 3512–3526. [Google Scholar] [CrossRef] [PubMed]
- Bruyns-Haylett, M.; Zheng, Y.; Berwick, J.; Jones, M. Temporal coupling between stimulus-evoked neural activity and hemodynamic responses from individual cortical columns. Phys. Med. Biol. 2010, 55, 2203. [Google Scholar] [CrossRef]
- Lu, L.; Li, G.; Song, Z.; Zhang, Z.; Tang, X. Hemodynamic response function (HRF) as a novel brain marker: Applications in subjective cognitive decline (SCD). Neurosci. Inform. 2022, 2, 100093. [Google Scholar] [CrossRef]
- Rosengarten, B.; Osthaus, S.; Kaps, M. Influence of stimulus duration on the neurovascular coupling response. Ultraschall Der Med. -Eur. J. Ultrasound 2004, 25, 116–119. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, X.; Xu, Q.; Huang, Y.; Liu, Y. TCD study of hemodynamic changes in PCA response to photic stimulation. J. Hejiang Univ. Sci. 2003, 4, 228–231. [Google Scholar] [CrossRef]
- Im, C.-H.; Jung, Y.-J.; Lee, S.; Koh, D.; Kim, D.-W.; Kim, B.-M. Estimation of directional coupling between cortical areas using Near-Infrared Spectroscopy (NIRS). Opt. Express 2010, 18, 5730–5739. [Google Scholar] [CrossRef]
- Shu, C.; Sanganahalli, B.; Coman, D.; Herman, P.; Hyder, F. New horizons in neurometabolic and neurovascular coupling from calibrated fMRI. Prog. Brain Res. 2016, 225, 99–122. [Google Scholar]
- Alam, M.; Ahmed, G.; Ling, Y.T.; Zheng, Y.-P. Measurement of neurovascular coupling in human motor cortex using simultaneous transcranial doppler and electroencephalography. Physiol. Meas. 2018, 39, 065005. [Google Scholar] [CrossRef] [PubMed]
- Edwards, O.; Ball, J.; Sensier, Y.; Panerai, R.; Beishon, L. 2632 Determining the feasibility of a TCD-NIRS protocol to measure cerebral haemodynamics in dementia, delirium, and depression. Age Ageing 2025, 54, afae277.075. [Google Scholar] [CrossRef]
- Rosa, M.J.; Kilner, J.M.; Penny, W.D. Bayesian comparison of neurovascular coupling models using EEG-fMRI. PLoS Comput. Biol. 2011, 7, e1002070. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, M.T.; Frost, H.R.; Diamond, S.G. Modeling Neurovascular Coupling from Clustered Parameter Sets for Multimodal EEG-NIRS. Comput. Math. Methods Med. 2015, 2015, 830849. [Google Scholar] [CrossRef]
- Deffieux, T.; Demené, C.; Tanter, M. Functional ultrasound imaging: A new imaging modality for neuroscience. Neuroscience 2021, 474, 110–121. [Google Scholar] [CrossRef]
- Pereira, V.M.; Lylyk, P.; Cancelliere, N.; Lylyk, P.N.; Lylyk, I.; Anagnostakou, V.; Bleise, C.; Nishi, H.; Epshtein, M.; King, R.M. Volumetric microscopy of cerebral arteries with a miniaturized optical coherence tomography imaging probe. Sci. Transl. Med. 2024, 16, eadl4497. [Google Scholar] [CrossRef]
- Li, Y.; Lin, R.; Liu, C.; Chen, J.; Liu, H.; Zheng, R.; Gong, X.; Song, L. In vivo photoacoustic/ultrasonic dual-modality endoscopy with a miniaturized full field-of-view catheter. J. Biophotonics 2018, 11, e201800034. [Google Scholar] [CrossRef]
- El Safty, H.G.; Youns, H.M.E. Transcranial Doppler in Non-Invasive Assessment of Increased Intracranial Pressure in Traumatic Brain Injury. Egypt. J. Hosp. Med. 2024, 97, 3554–3567. [Google Scholar]
- Gunda, S.T.; Ng, T.K.V.; Liu, T.-Y.; Chen, Z.; Han, X.; Chen, X.; Pang, M.Y.-C.; Ying, M.T.-C. A comparative study of transcranial color-coded Doppler (TCCD) and transcranial Doppler (TCD) ultrasonography techniques in assessing the intracranial cerebral arteries haemodynamics. Diagnostics 2024, 14, 387. [Google Scholar] [CrossRef]
- Bowers, D.C.; Johnson, M.D. Feasibility of transcranial Doppler to evaluate vasculopathy among survivors of childhood brain tumors exposed to cranial radiation therapy. Pediatr. Blood Cancer 2025, 72, e31392. [Google Scholar] [CrossRef]
- Çıtışlı, V.; Dalbastı, O.T. The effectiveness of Transcranial Doppler (TCD) in detection of vasospasm in patient with subarachnoid hemorrhage. Dokuz Eylül Üniversitesi Tıp Fakültesi Derg. 2024, 38, 83–96. [Google Scholar] [CrossRef]
- Boban, M.; Črnac, P.; Junaković, A.; Garami, Z.; Malojčić, B. Blood flow velocity changes in anterior cerebral arteries during cognitive tasks performance. Brain Cogn. 2014, 84, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-K.; Ryu, S.-J.; Hsu, P.-W. Interhemispheric comparisons of cerebral blood flow velocity changes during mental tasks with transcranial Doppler sonography. J. Ultrasound Med. 2009, 28, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Bögli, S.Y.; Cucciolini, G.; Cherchi, M.S.; Motroni, V.; Olakorede, I.; O’Leary, R.; Beqiri, E.; Smith, C.A.; Smielewski, P. Feasibility and Safety of Integrating Extended TCD Assessments in a Full Multimodal Neuromonitoring Protocol After Traumatic Brain Injury. Ultrasound Med. Biol. 2024, 50, 1704–1715. [Google Scholar] [CrossRef]
- Negadi, M.; Bouguetof, H.; El Halimi, K.; Boumendil, D.; Mentouri, Z. 979 Transcranial Doppler (TCD) in Severe Trauma Brain Injuries (TBI) in Pediatric Intensive Care Unit (PICU) in Algeria. Preliminary Results. Arch. Dis. Child. 2012, 97, A280. [Google Scholar] [CrossRef]
- Marrero-García, R.; Cruz-Tabares, Y.; Gonzalez-Cava, J.M.; Méndez-Pérez, J.A.; Reboso-Morales, J.A. Evaluation of a low-cost portable NIRS device for monitoring muscle ischemia. J. Clin. Monit. Comput. 2024, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Li, G. Non-Invasive Assessment of Dynamic Cerebral Blood Flow Using Near-Field Coupling and Synchronized Electrocardiography. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 15–19 July 2024. [Google Scholar]
- Gutierrez-Martinez, J.; Vega-Martinez, G.; Toledo-Peral, C.L.; Mercado-Gutierrez, J.A.; Quinzaños-Fresnedo, J. A NIRS-Based Technique for Monitoring Brain Tissue Oxygenation in Stroke Patients. Sensors 2024, 24, 8175. [Google Scholar] [CrossRef]
- Rodrigues, A.; Shingai, K.; Gómez, C.A.; Rassam, P.; Rozenberg, D.; Goligher, E.; Brochard, L.; Roblyer, D.; Reid, W.D. Continuous measurements of respiratory muscle blood flow and oxygen consumption using noninvasive frequency-domain near-infrared spectroscopy and diffuse correlation spectroscopy. J. Appl. Physiol. 2024, 137, 382–393. [Google Scholar] [CrossRef]
- Xing, C.; Feng, J.; Yao, J.; Xu, X.-M.; Wu, Y.; Yin, X.; Salvi, R.; Chen, Y.-C.; Fang, X. Neurovascular coupling dysfunction associated with cognitive impairment in presbycusis. Brain Commun. 2024, 6, fcae215. [Google Scholar] [CrossRef]
- Pjevalica Dragic, J.; Zecevic, T.; Divac, I.; Pavlovic, A.; Bisenic, D.; Stanisic, L.; Kalanj, J.; Stefanovic, I.; Nikolic, D.; Petrov, I. Correlation of near-infrared spectroscopy (NIRS) with invasive arterial pressure monitoring during aortic coarctation surgery in pediatric patients. Healthcare 2024, 12, 1884. [Google Scholar] [CrossRef]
- Fuster, J.; Guiou, M.; Ardestani, A.; Cannestra, A.; Sheth, S.; Zhou, Y.-D.; Toga, A.; Bodner, M. Near-infrared spectroscopy (NIRS) in cognitive neuroscience of the primate brain. Neuroimage 2005, 26, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Pinti, P.; Aichelburg, C.; Lind, F.; Power, S.; Swingler, E.; Merla, A.; Hamilton, A.; Gilbert, S.; Burgess, P.; Tachtsidis, I. Using fiberless, wearable fNIRS to monitor brain activity in real-world cognitive tasks. J. Vis. Exp. JoVE 2015, 106, 53336. [Google Scholar]
- de Almeida, L.O.M.P.; Variane, G.F.T.; Pietrobom, R.F.R.; Magalhães, M.; Rodrigues, D.P.; Gasperini, R.; Netto, A. Near infrared spectroscopy (NIRS) in neonatal intensive care unit: Experience of a Brazilian university hospital. Residência Pediátrica 2021. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, X.; Liu, H.; Wang, Y.; Zhang, Z.; Zhang, Y.; Liu, Y. Individualized post-operative prediction of cochlear implantation outcomes in children with prelingual deafness using functional near-infrared spectroscopy. Laryngoscope Investig. Otolaryngol. 2024, 9, e70035. [Google Scholar] [CrossRef]
- Jafarian, A.; Litvak, V.; Cagnan, H.; Friston, K.J.; Zeidman, P. Comparing dynamic causal models of neurovascular coupling with fMRI and EEG/MEG. NeuroImage 2020, 216, 116734. [Google Scholar] [CrossRef]
- Pak, R.W.; Hadjiabadi, D.H.; Senarathna, J.; Agarwal, S.; Thakor, N.V.; Pillai, J.J.; Pathak, A.P. Implications of neurovascular uncoupling in functional magnetic resonance imaging (fMRI) of brain tumors. J. Cereb. Blood Flow Metab. 2017, 37, 3475–3487. [Google Scholar] [CrossRef]
- Fukuda, M.; Poplawsky, A.J.; Kim, S.-G. Time-dependent spatial specificity of high-resolution fMRI: Insights into mesoscopic neurovascular coupling. Philos. Trans. R. Soc. B 2021, 376, 20190623. [Google Scholar] [CrossRef]
- Ruan, Z.; Sun, D.; Zhou, X.; Yu, M.; Li, S.; Sun, W.; Li, Y.; Gao, L.; Xu, H. Altered neurovascular coupling in patients with vascular cognitive impairment: A combined ASL-fMRI analysis. Front. Aging Neurosci. 2023, 15, 1224525. [Google Scholar] [CrossRef]
- Mariia, B.; Larisa, S.; Tatiana, P.; Sofya, M.; Anastasia, S.; Larisa, D. Personalized adjustment of near infrared spectroscopy studies using fMRI data for research in neuroscience. In Proceedings of the 2023 Systems and Technologies of the Digital HealthCare (STDH), Tashkent, Uzbekistan, 4–6 October 2023; pp. 39–42. [Google Scholar]
- Song, L.; Wang, H.; Yang, W.; Li, M.; Xu, B.; Li, M.; Ding, H.; Lv, H.; Zhao, P.; Yang, Z. Combination of rs-fMRI, QSM, and ASL Reveals the Cerebral Neurovascular Coupling Dysfunction Is Associated with Cognitive Decline in Patients with Chronic Kidney Disease. CNS Neurosci. Ther. 2024, 30, e70151. [Google Scholar] [CrossRef]
- Bondi, E.; Ding, Y.; Zhang, Y.; Maggioni, E.; He, B. EEG-Informed fMRI Analysis Reveals Neurovascular Coupling in Motor Execution and Imagery. bioRxiv 2025, bioRxiv:2025.2001. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Nyul-Toth, A.; Csiszar, A.; Gulej, R.; Saunders, D.; Towner, R.; Turner, M.; Zhao, Y.; Abdelkari, D.; Rypma, B. Age-related alterations in the cerebrovasculature affect neurovascular coupling and BOLD fMRI responses: Insights from animal models of aging. Psychophysiology 2021, 58, e13718. [Google Scholar] [CrossRef] [PubMed]
- Matthews, P.M.; Honey, G.D.; Bullmore, E.T. Applications of fMRI in translational medicine and clinical practice. Nat. Rev. Neurosci. 2006, 7, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Sabino, D.; Marvulli, R.G.; Maria, D.; Giulia Alessia, G.; Salvati, A.; Fiore, P.; Megna, M. Prognostic and diagnostic value of clinical examination and fMRI in the evaluation of patients in a vegetative state. J. Neurol. Neurophysiol. 2017, 8, 3. [Google Scholar] [CrossRef]
- Koppe, G.; Toutounji, H.; Kirsch, P.; Lis, S.; Durstewitz, D. Identifying nonlinear dynamical systems via generative recurrent neural networks with applications to fMRI. PLoS Comput. Biol. 2019, 15, e1007263. [Google Scholar] [CrossRef]
- He, F.; Sullender, C.T.; Zhu, H.; Williamson, M.R.; Li, X.; Zhao, Z.; Jones, T.A.; Xie, C.; Dunn, A.K.; Luan, L. Multimodal mapping of neural activity and cerebral blood flow reveals long-lasting neurovascular dissociations after small-scale strokes. Sci. Adv. 2020, 6, eaba1933. [Google Scholar] [CrossRef]
- Yeung, M.K.; Chu, V.W. Viewing neurovascular coupling through the lens of combined EEG–fNIRS: A systematic review of current methods. Psychophysiology 2022, 59, e14054. [Google Scholar] [CrossRef]
- Smith, Q.; Limvaree, I.A.; Farrand, J.; Edwards, S.; Stephens, T.; Dunn, I.F.; Conner, A.; Ding, L.; Yuan, H. Test-retest Reliability of Neurovascular Coupling and Impairment in Epilepsy Measured by fNIRS and EEG. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 15–19 July 2024. [Google Scholar]
- Chiarelli, A.M.; Perpetuini, D.; Croce, P.; Filippini, C.; Cardone, D.; Rotunno, L.; Anzoletti, N.; Zito, M.; Zappasodi, F.; Merla, A. Evidence of neurovascular un-coupling in mild Alzheimer’s disease through multimodal EEG-fNIRS and multivariate analysis of resting-state data. Biomedicines 2021, 9, 337. [Google Scholar] [CrossRef]
- Yang, R.; Brugniaux, J.; Dhaliwal, H.; Beaudin, A.E.; Eliasziw, M.; Poulin, M.J.; Dunn, J.F. Studying cerebral hemodynamics and metabolism using simultaneous near-infrared spectroscopy and transcranial Doppler ultrasound: A hyperventilation and caffeine study. Physiol. Rep. 2015, 3, e12378. [Google Scholar] [CrossRef]
- Han, D.; Li, H.; Pan, S.; Xie, S.; Deryck, Y.; Luo, Y.; Li, J.; Ou-Yang, C. Measuring cerebral carbon dioxide reactivity with transcranial doppler and near-infrared spectroscopy in children with ventricular septal defect. J. Cardiothorac. Vasc. Anesth. 2020, 34, 344–348. [Google Scholar] [CrossRef]
- Zama, T.; Shimada, S. Simultaneous measurement of electroencephalography and near-infrared spectroscopy during voluntary motor preparation. Sci. Rep. 2015, 5, 16438. [Google Scholar] [CrossRef]
- Chen, C.-W.; Sun, C.-W. Combination of electroencephalography and near-infrared spectroscopy in evaluation of mental concentration during the mental focus task for Wisconsin card sorting test. Sci. Rep. 2017, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.W.; Lin, Y.D. Brain Activity Monitoring System Based on EEG-NIRS Measurement System. Appl. Mech. Mater. 2017, 870, 351–356. [Google Scholar] [CrossRef]
- Lane, T.J. Indication of dynamic neurovascular coupling from inconsistency between EEG and fMRI indices across sleep–wake states. Sleep Biol. Rhythm. 2019, 17, 423–431. [Google Scholar]
- Wang, Z.J.; Lee, H.-C.; Chuang, C.-H.; Hsiao, F.-C.; Lee, S.-H.; Hsu, A.-L.; Wu, C.W. Traces of EEG-fMRI coupling reveals neurovascular dynamics on sleep inertia. Sci. Rep. 2024, 14, 1537. [Google Scholar] [CrossRef]
- Rojas-Pescio, H.; Beishon, L.; Panerai, R.; Chacón, M. Statistical Complexity Analysis of Neurovascular Coupling with Cognitive Stimulation in Healthy Participants. J. Cogn. Neurosci. 2024, 36, 1995–2010. [Google Scholar] [CrossRef]
- Chen, H.Y.; Elmer, J.; Zafar, S.F.; Ghanta, M.; Moura Junior, V.; Rosenthal, E.S.; Gilmore, E.J.; Hirsch, L.J.; Zaveri, H.P.; Sheth, K.N. Combining transcranial Doppler and EEG data to predict delayed cerebral ischemia after subarachnoid hemorrhage. Neurology 2022, 98, e459–e469. [Google Scholar] [CrossRef]
- Shuhang, H.; Shangchun, G.; Shicong, T. Advances in the study of neurovascular coupling in bone repair. J. Shanghai Jiao Tong Univ. (Med. Sci.) 2024, 44, 373–378. [Google Scholar]
- Teskey, G.C.; Tran, C.H.T. Neurovascular coupling in seizures. Neuroglia 2021, 2, 36–47. [Google Scholar] [CrossRef]
- Balodis, A.; Mikijanskis, R.; Saulkalne, L.H.; Valante, R. Use of high-resolution magnetic resonance imaging (MRI) for radiological diagnosis of neurovascular conflict: A case report. Am. J. Case Rep. 2021, 22, e933566-1–e933566-6. [Google Scholar] [CrossRef]
- Montaldo, G.; Urban, A.; Macé, E. Functional ultrasound neuroimaging. Annu. Rev. Neurosci. 2022, 45, 491–513. [Google Scholar] [CrossRef]
- Li, L.; Tong, X.-K.; Hosseini Kahnouei, M.; Vallerand, D.; Hamel, E.; Girouard, H. Impaired hippocampal neurovascular coupling in a mouse model of Alzheimer’s disease. Front. Physiol. 2021, 12, 715446. [Google Scholar] [CrossRef] [PubMed]
- Soloukey, S.; Vincent, A.J.; Satoer, D.D.; Mastik, F.; Smits, M.; Dirven, C.M.; Strydis, C.; Bosch, J.G.; van der Steen, A.F.; De Zeeuw, C.I. Functional ultrasound (fUS) during awake brain surgery: The clinical potential of intra-operative functional and vascular brain mapping. Front. Neurosci. 2020, 13, 1384. [Google Scholar] [CrossRef]
- Chen, M.-J.; Zhang, W.; Yang, C.; Zhang, Z.; Wang, Y. Endoscopic neurovascular perspective in microvascular decompression of trigeminal neuralgia. Int. J. Oral Maxillofac. Surg. 2007, 36, 1026–1027. [Google Scholar] [CrossRef]
- Neilson, L.; Zande, J.; Abboud, H. Deep brain stimulation surgery in Parkinson’s disease. In Diagnosis and Management in Parkinson’s Disease; Elsevier: New York, NY, USA, 2020; pp. 577–596. [Google Scholar]
- Cordes, D.; Gerloff, C.; Heise, K.-F.; Hummel, F.C.; Schulz, R.; Wolf, S.; Haevernick, K.; Krüger, H.; Krause, L.; Suling, A. Efficacy and safety of transcranial direct current stimulation to the ipsilesional motor cortex in subacute stroke (NETS): A multicenter, randomized, double-blind, placebo-controlled trial. Lancet Reg. Health–Eur. 2024, 38, 100825. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.-J.; Rezaei, M. Unilateral right and bilateral dorsolateral prefrontal cortex transcranial magnetic stimulation in treatment post-traumatic stress disorder: A randomized controlled study. Brain Res. Bull. 2018, 140, 334–340. [Google Scholar] [CrossRef]
- Saadi, M.; Karkhah, A.; Pourabdolhossein, F.; Ataie, A.; Monif, M.; Nouri, H.R. Involvement of NLRC4 inflammasome through caspase-1 and IL-1β augments neuroinflammation and contributes to memory impairment in an experimental model of Alzheimer’s like disease. Brain Res. Bull. 2020, 154, 81–90. [Google Scholar] [CrossRef]
- Sayyah, M.; Seydyousefi, M.; Moghanlou, A.E.; Metz, G.A.; Shamsaei, N.; Faghfoori, M.H.; Faghfoori, Z. Activation of BDNF-and VEGF-mediated neuroprotection by treadmill exercise training in experimental stroke. Metab. Brain Dis. 2022, 37, 1843–1853. [Google Scholar] [CrossRef]

| Pathology | Impact on Neurovascular Coupling | Mechanism |
|---|---|---|
| Stroke | Impaired—particularly in the hemisphere of the insult | Brain edema, inflammation, impaired neurotransmission, and neuronal death impair normal neuronal activation. Also, the activation of non-specific brain structures further complicates interpretation [34,35,36]. |
| Hypertension | Impaired | Elevated circulating angiotensin II leads to (1) activation of ATI receptors on the cerebral blood vessels, (2) increased oxidative stress which inhibits neuronal and astrocytic vascular dilators [37]. |
| Autonomic Dysfunction | Impaired | Impaired blood pressure responses and the altered neurogenic regulation of neurovascular coupling [38]. |
| High level | Impaired | Unknown persistent dysfunction of neurovascular coupling after restoring normal blood pressure [39]. |
| Traumatic Brain Injury | Impaired | Neuronal death and astrocytic scar formation preclude the normal neurovascular response [40]. |
| Alzheimer’s | Impaired | Hypercontractility (phenylephrine) of smooth muscle, increased basal, and the enhanced occurrence of spontaneous Ca2+ waves. Amyloid Beta also directly inhibits functional hyperemia by promoting oxidative stress, which inhibits neuronal and astrocytic vascular dilators [41]. |
| Method | Principle | Temporal Resolution | Spatial Resolution | Advantages | Limitations |
|---|---|---|---|---|---|
| Transcranial Doppler (TCD) | Based on the Doppler effect, it monitors cerebral artery blood flow changes to assess the cerebral blood flow changes. | High (approximately 1–10 ms) | Low (>10 mm; no anatomical localization capability) | High temporal resolution and real-time capture of blood flow velocity changes. | Only measures the blood flow velocity in large brain arteries, not directly assessing brain tissue perfusion; affected by the skull thickness [71]. |
| Near-Infrared Spectroscopy (NIRS) | Optical imaging techniques measure the changes in oxygenated (HbO) and deoxygenated hemoglobin (HbR) concentrations in the brain. | Moderate (approximately 100 ms–1 s) | Moderate to low (approximately 1–3 cm; limited to the cortex) | High temporal resolution and real-time monitoring of blood flow changes induced by neural activity provide information on the local oxygenation status. | Limited to the cortex, cannot measure deep brain regions; affected by skin and skull [72]. |
| Functional Magnetic Resonance Imaging (fMRI) | Relies on the BOLD signal to detect the changes in the ratio of oxygenated to deoxygenated hemoglobin in brain tissue. | Low (approximately 1–2 s) | High (approximately 1–3 mm) | High spatial resolution and full-brain coverage, ideal for studying neurovascular coupling and task-related cerebral blood flow changes. | At lower temporal resolution, the BOLD signal indirectly reflects neural activity and may be influenced by various factors [73]. |
| Multimodal Techniques (EEG combined with TCD/NIRS/fMRI) | EEG is combined with other brain blood flow methods (TCD, NIRS, fMRI) to record the neural activity and blood flow changes simultaneously. | Complementary | Complementary | Provides synchronized information on neural activity and blood flow regulation, enhancing the comprehensive assessment of neurovascular coupling. | Synchronization issues between different techniques and increased complexity in data processing and analysis [74,75,76,77]. |
| Functional Ultrasound (fUS) | Measures cerebral blood volume (CBV) changes via ultrafast Doppler imaging. | High (<100 ms) | High (approximately 100 µm) | High spatial and temporal resolution; sensitive to deep and microvascular flow. | Requires a cranial window or acoustic access; relatively new in humans [78]. |
| Miniaturized Endoscopy | Uses small optical probes to directly visualize fluorescence or hemodynamic signals in the deep brain. | High (<100 ms) | High (approximately 100 µm) | Allows deep-brain imaging in freely moving animals; high-resolution access to specific structures. | Invasive; limited field of view; primarily animal studies [79,80]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Li, G.; Lv, Z.; Chen, J.; Wang, C.; Shao, A.; Gong, Z.; Wang, J.; Liu, S.; Luo, J.; et al. Neuromodulation of Cerebral Blood Flow: A Physiological Mechanism and Methodological Review of Neurovascular Coupling. Bioengineering 2025, 12, 442. https://doi.org/10.3390/bioengineering12050442
Zhong J, Li G, Lv Z, Chen J, Wang C, Shao A, Gong Z, Wang J, Liu S, Luo J, et al. Neuromodulation of Cerebral Blood Flow: A Physiological Mechanism and Methodological Review of Neurovascular Coupling. Bioengineering. 2025; 12(5):442. https://doi.org/10.3390/bioengineering12050442
Chicago/Turabian StyleZhong, Jiawen, Gen Li, Zexiang Lv, Jingbo Chen, Chunyan Wang, Ansheng Shao, Zhiwei Gong, Junjie Wang, Siqiao Liu, Jun Luo, and et al. 2025. "Neuromodulation of Cerebral Blood Flow: A Physiological Mechanism and Methodological Review of Neurovascular Coupling" Bioengineering 12, no. 5: 442. https://doi.org/10.3390/bioengineering12050442
APA StyleZhong, J., Li, G., Lv, Z., Chen, J., Wang, C., Shao, A., Gong, Z., Wang, J., Liu, S., Luo, J., Yang, S., Wu, S., Ning, L., Wang, Z., Li, J., & Wu, Y. (2025). Neuromodulation of Cerebral Blood Flow: A Physiological Mechanism and Methodological Review of Neurovascular Coupling. Bioengineering, 12(5), 442. https://doi.org/10.3390/bioengineering12050442






