Finite Element Driven Design Domain Identification of a Beating Left Ventricular Simulator
Abstract
:1. Introduction
2. Materials and Methods
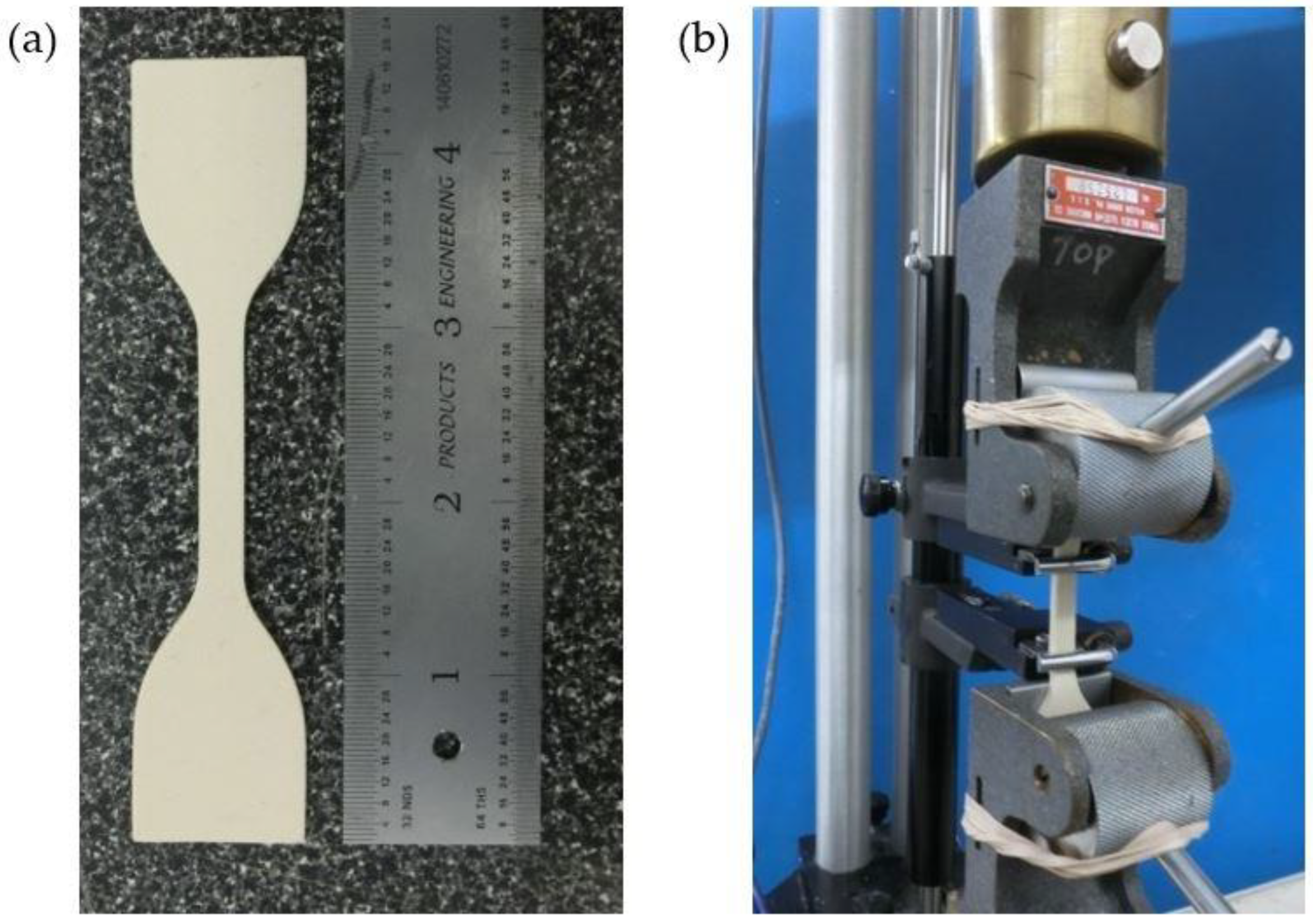
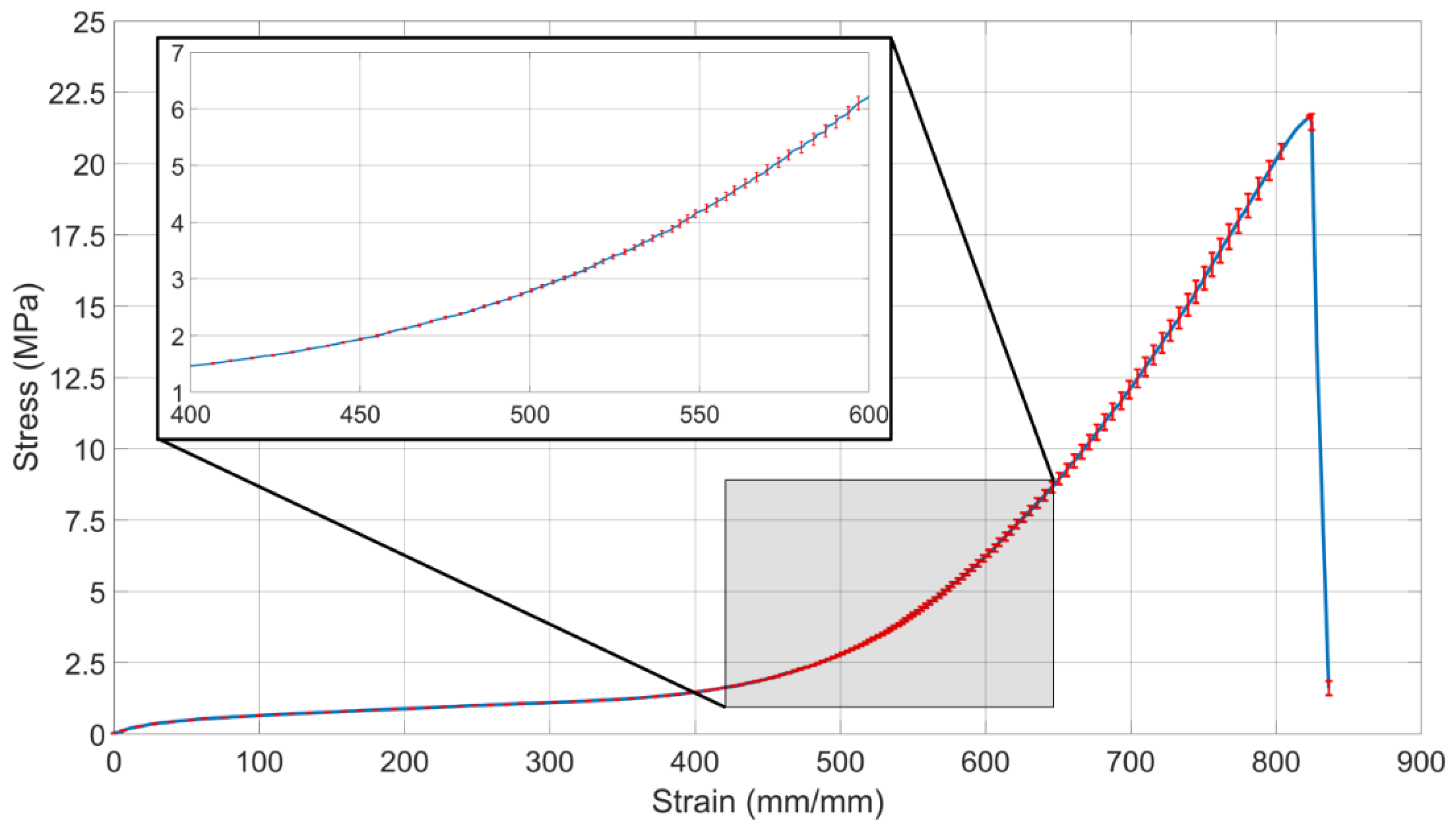
2.1. Tensile Testing of Latex Rubber
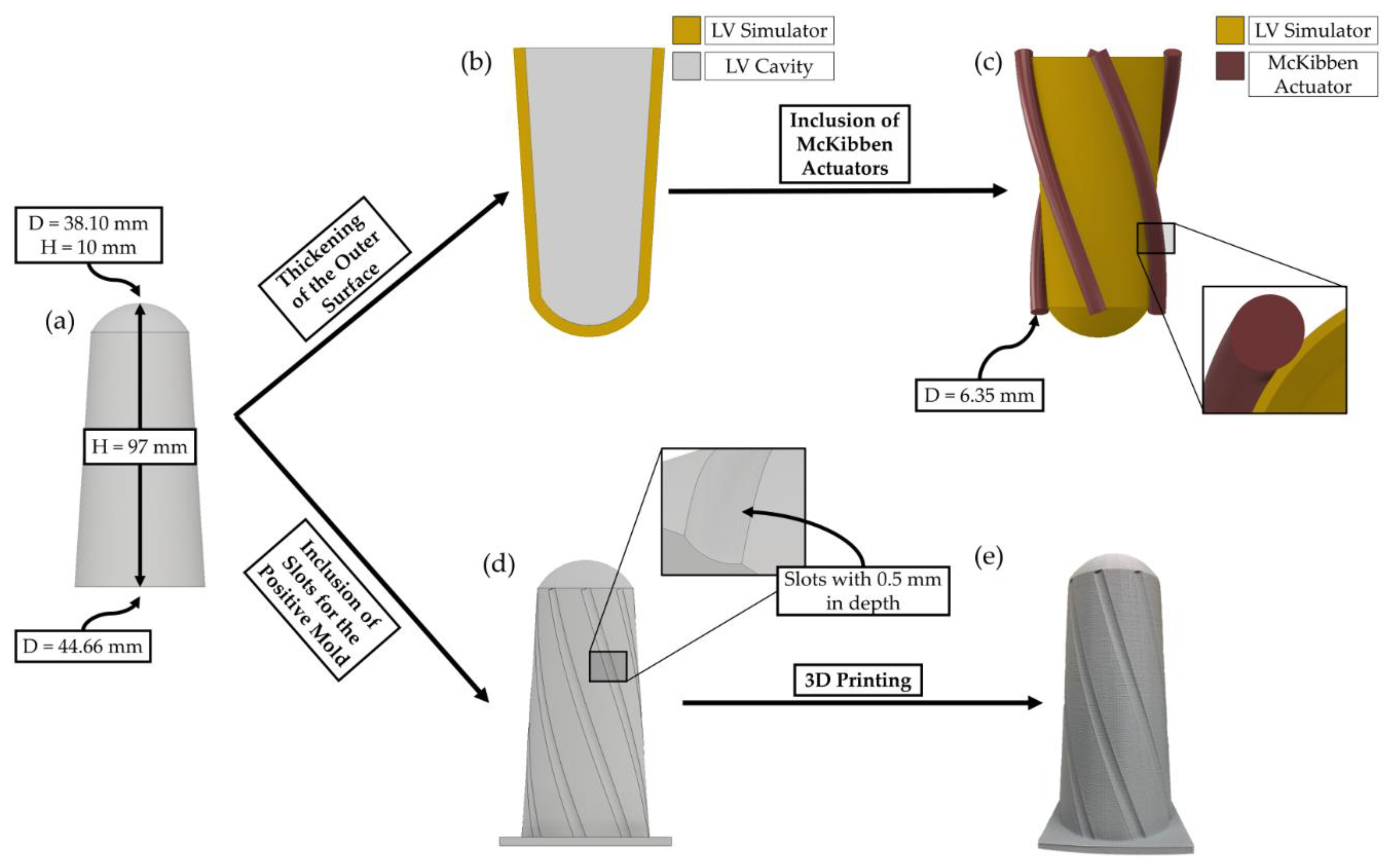
2.2. Beating LV Simulator Geometry and Mold Generation
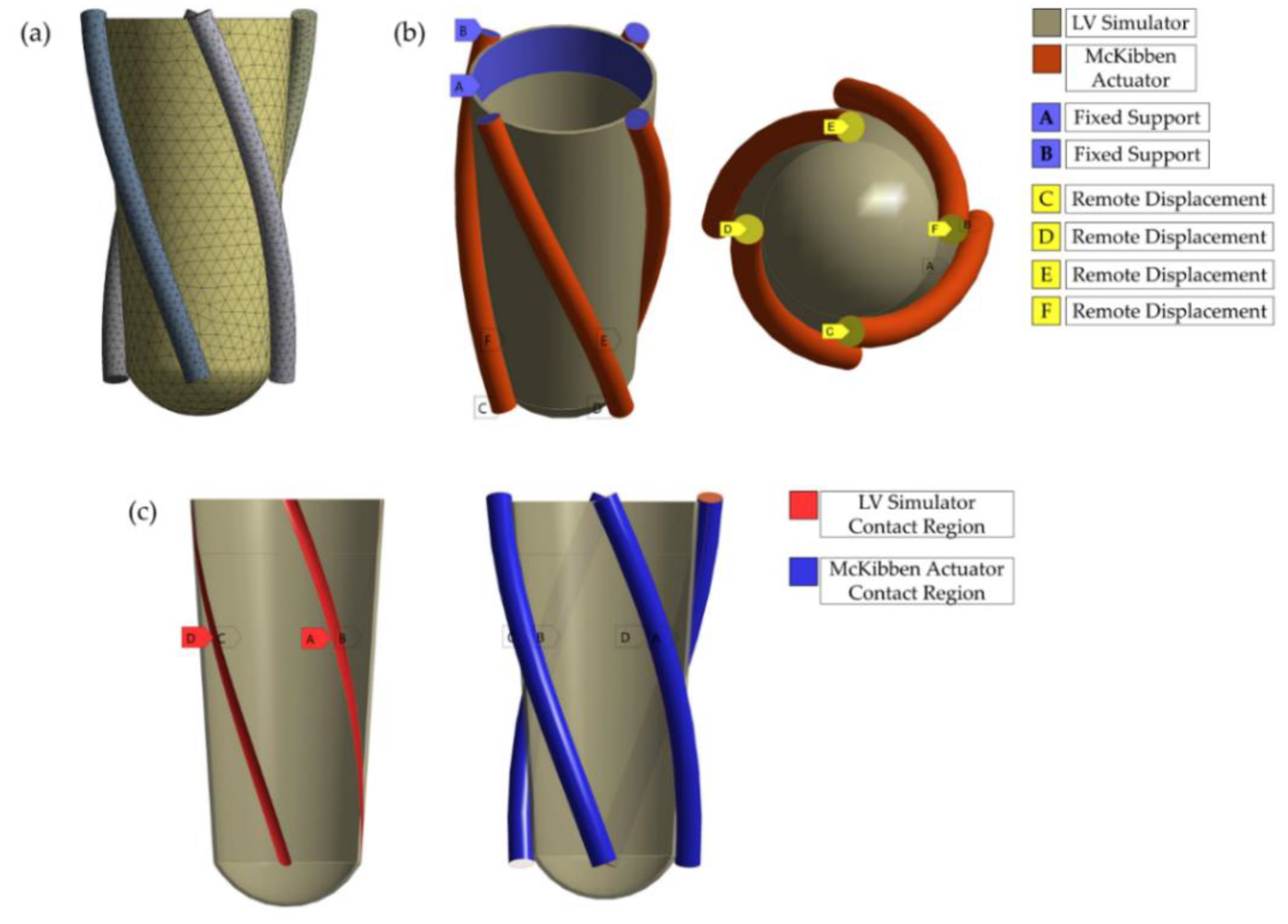
2.3. FE Models
2.4. Validation of the FE Model
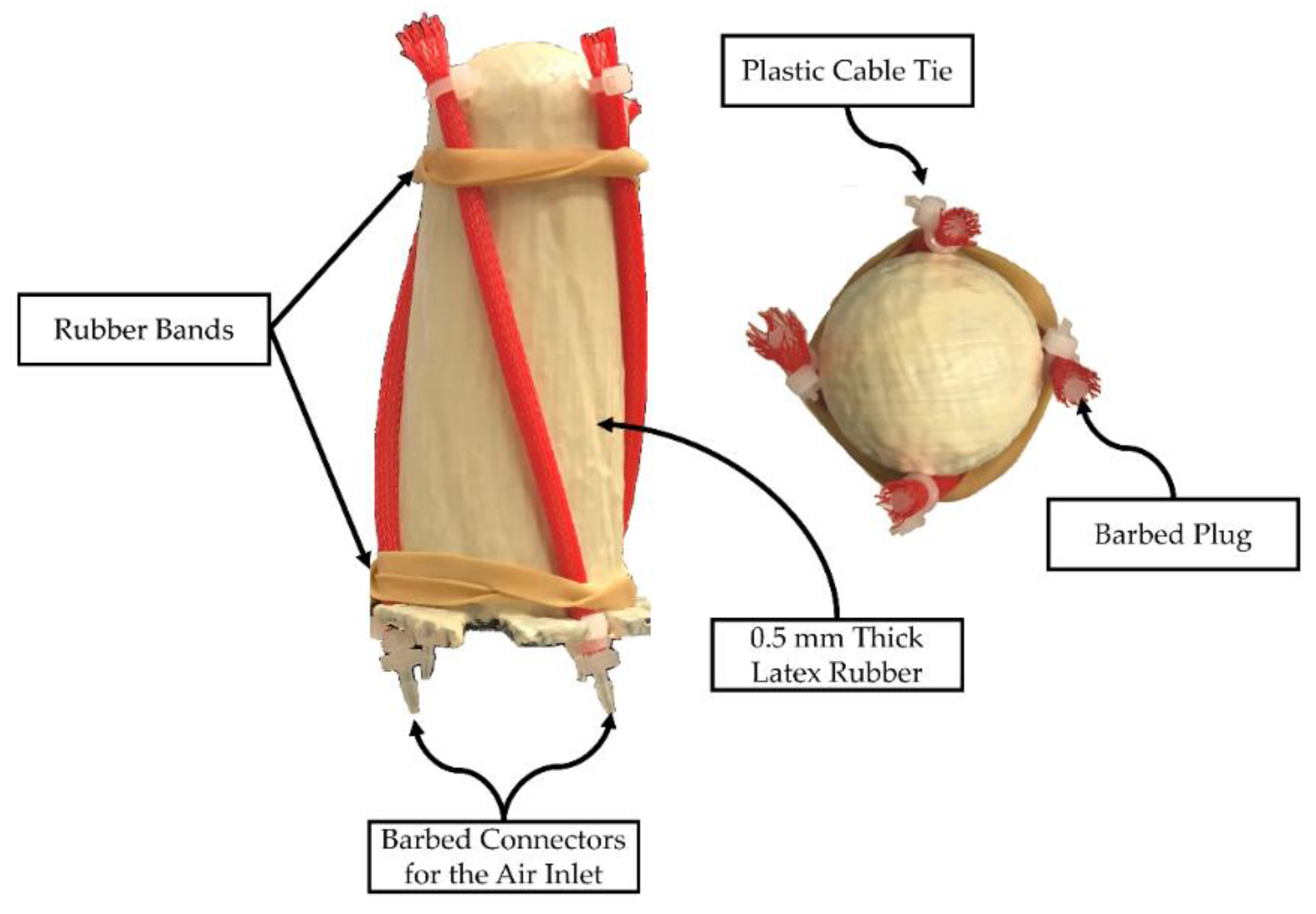
2.4.1. Prototype Manufacturing
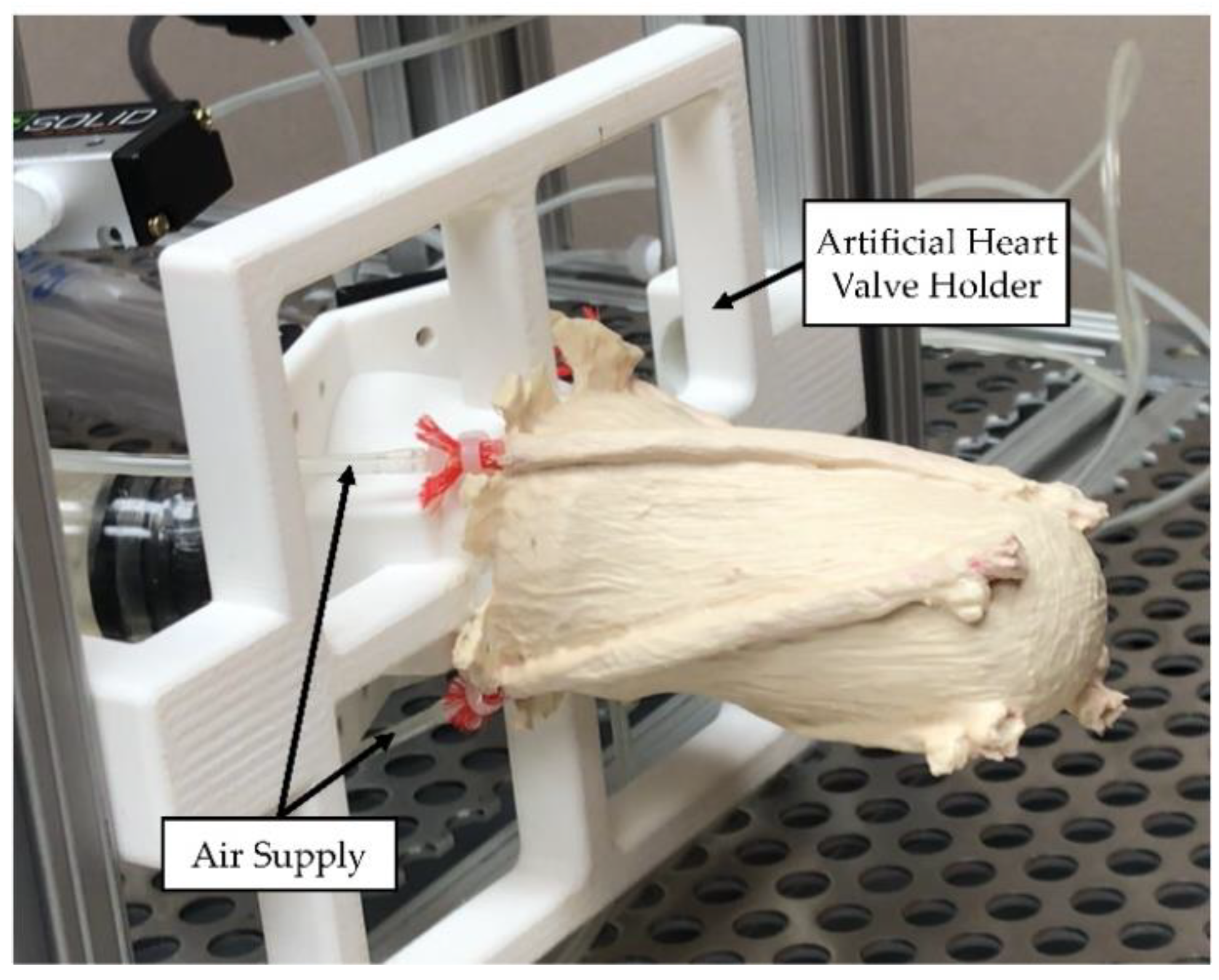
2.4.2. Validation Experiment Setup
3. Results
3.1. Tensile Testing of Latex Rubber
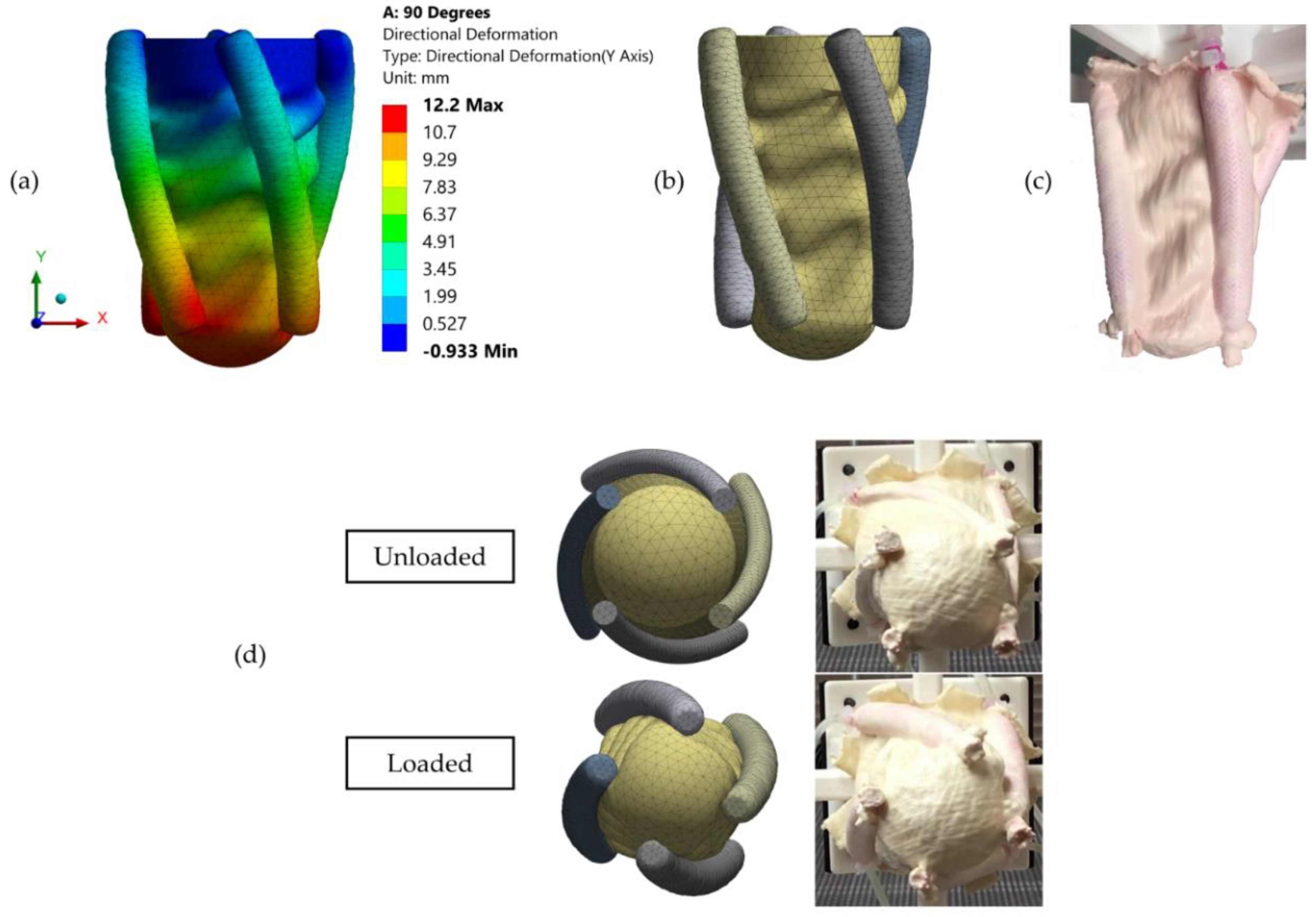
3.2. Validation of the FE Model
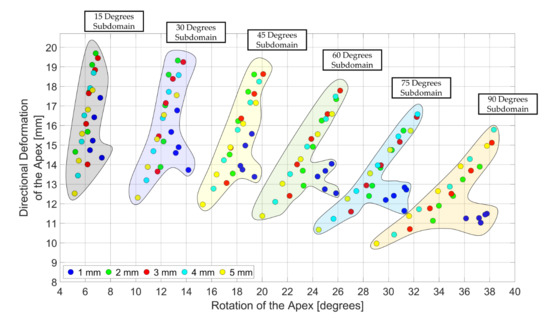
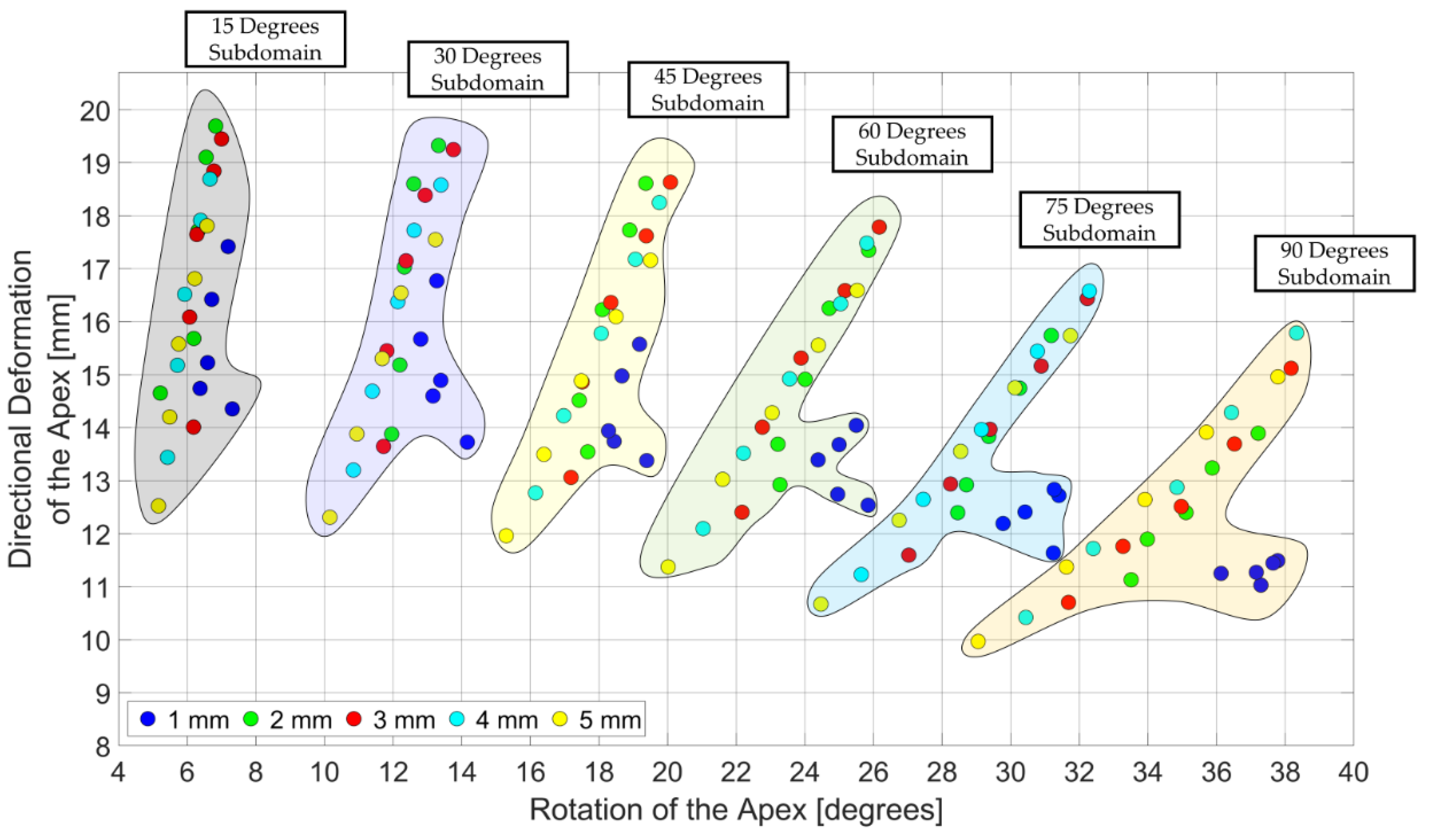
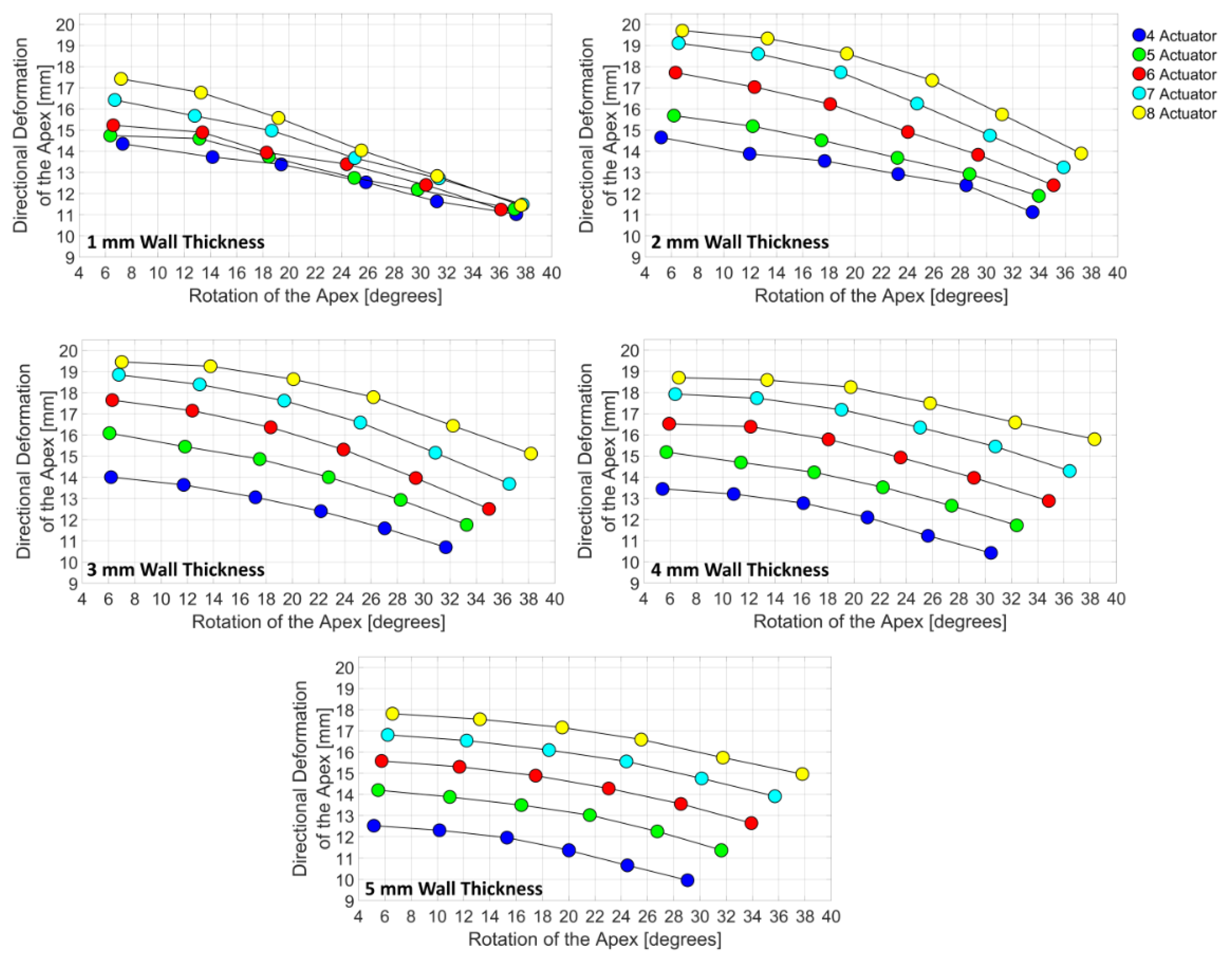
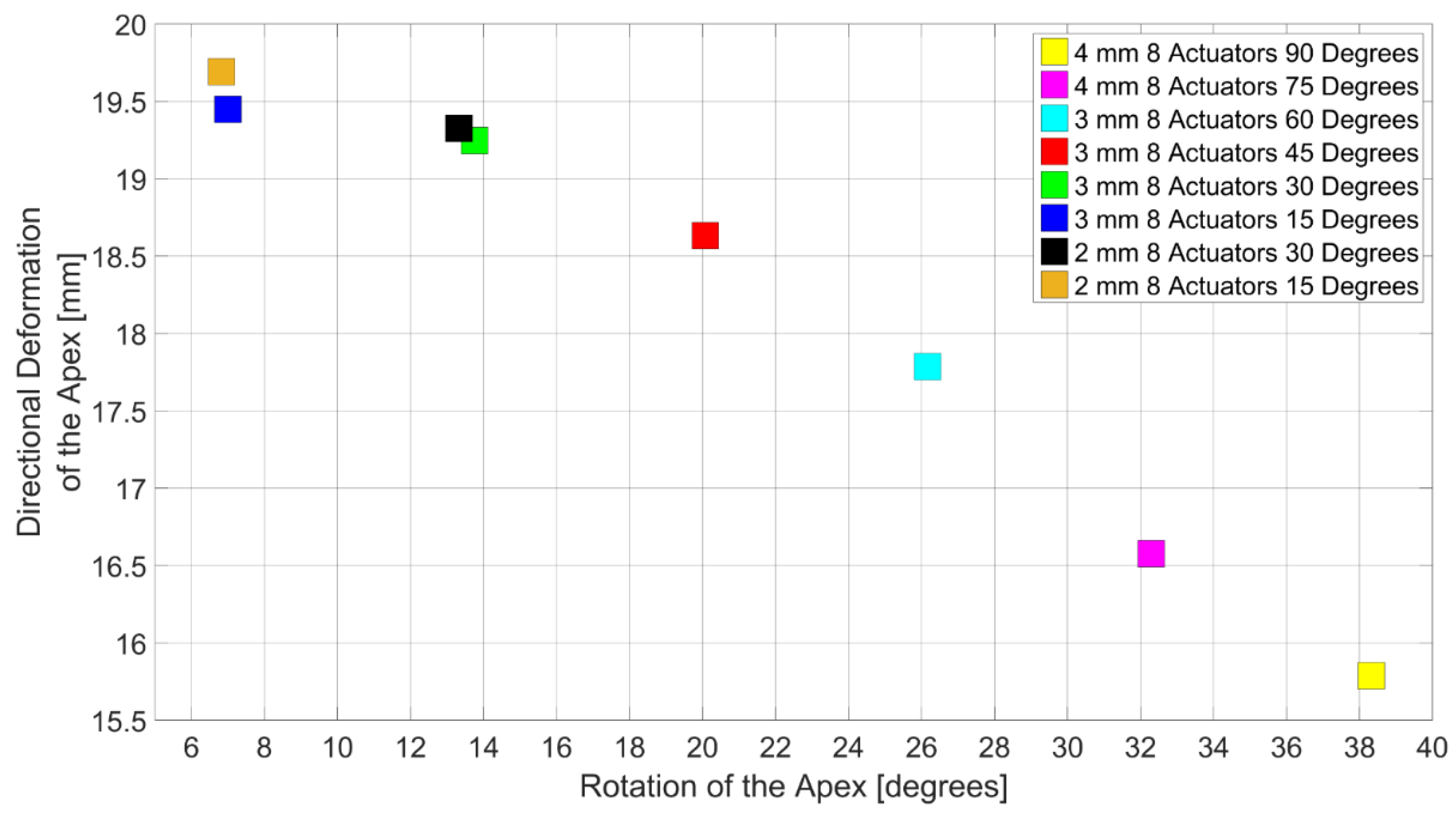
3.3. Design Domain of the LV Simulator
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Bornstein, D.B.; Sui, X.; Hooker, S.P.; Church, T.S.; Lee, C.D.; Lee, D.; Blair, S.N. The impact of combined health factors on cardiovascular disease mortality. Am. Heart J. 2010, 160, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Califano, S.; Pagani, F.D.; Malani, P.N. Left Ventricular Assist Device–Associated Infections. Infect. Dis. Clin. North Am. 2012, 26, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Tonsho, M.; Michel, S.; Ahmed, Z.; Alessandrini, A.; Madsen, J.C. Heart transplantation: challenges facing the field. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Trumble, D.; Han, J.; Trumble, D.R. Cardiac Assist Devices: Early Concepts, Current Technologies, and Future Innovations. Bioengineering 2019, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.; Tsui, S.S.; El-Banayosy, A.; Kormos, R.; Icenogle, T.; Hill, J. The thoratec IVAD: a new implantable ventricular assist device. J. Hear. Lung Transplant. 2004, 23, S149. [Google Scholar] [CrossRef]
- Lad, V.; Elhenawy, A.; Harwood CCP, S.; MacIver, J.R.; Badiwala, M.V.; Vallelonga CCP, M.; Yau MDCM, T.M.; Cusimano, R.J.; Delgado, D.H.; Ross, H.J.; et al. Mechanical circulatory support with the ABIOMED BVS 5000: The Toronto General Hospital experience. Can. J. Cardiol. 2010, 26, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M.; Long, J.W.; et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef]
- HETZER, R.; WENG, Y.; POTAPOV, E.; PASIC, M.; DREWS, T.; JURMANN, M.; HENNIG, E.; MULLER, J. First experiences with a novel magnetically suspended axial flow left ventricular assist device. Eur. J. Cardio-Thoracic Surg. 2004, 25, 964–970. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kihara, S.; Akimoto, T.; Tagusari, O.; Kawai, A.; Umezu, M.; Tomioka, J.; Kormos, R.L.; Griffith, B.P.; Kurosawa, H. EVAHEARTTM: An implantable centrifugal blood pump for long-term circulatory support. Japanese J. Thorac. Cardiovasc. Surg. 2002, 50, 461–465. [Google Scholar] [CrossRef]
- Larose, J.A.; Tamez, D.; Ashenuga, M.; Reyes, C. Design concepts and principle of operation of the HeartWare ventricular assist system. ASAIO J. 2010, 56, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Watterson, P.A.; Woodard, J.C.; Ramsden, V.S.; Reizes, J.A. VentrAssist Hydrodynamically Suspended, Open, Centrifugal Blood Pump. Artif. Organs 2000, 24, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Monreal, G.; Sherwood, L.C.; Sobieski, M.A.; Giridharan, G.A.; Slaughter, M.S.; Koenig, S.C. Large Animal Models for Left Ventricular Assist Device Research and Development. ASAIO J. 2014, 60, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Fan, H.; Liu, Z.; He, J.-Q. Large Mammalian Animal Models of Heart Disease. J. Cardiovasc. Dev. Dis. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Milani-Nejad, N.; Janssen, P.M.L. Small and large animal models in cardiac contraction research: Advantages and disadvantages. Pharmacol. Ther. 2014, 141, 235–249. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, L.; Yang, B.; Li, X.; Ni, K.; Zhou, Q.; Wang, X. In vitro testing of an intra-ventricular assist device. Comput. Assist. Surg. 2019, 1–7. [Google Scholar] [CrossRef]
- Petrou, A.; Granegger, M.; Meboldt, M.; Schmid Daners, M. A Versatile Hybrid Mock Circulation for Hydraulic Investigations of Active and Passive Cardiovascular Implants. ASAIO J. 2019, 65, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Karabegovic, A.; Hinteregger, M.; Janeczek, C.; Reichenfelser, W.; Soragnese, V.; Mohl, W.; Gföhler, M. A Systemic Mock Circulation for In-Vitro Testing of a Pneumatically Operated Left Ventricular Assist Device. IFAC Proc. Vol. 2014, 47, 8409–8414. [Google Scholar] [CrossRef] [Green Version]
- Sénage, T.; Février, D.; Michel, M.; Pichot, E.; Duveau, D.; Tsui, S.; Trochu, J.N.; Roussel, J.C. A mock circulatory system to assess the performance of continuous-flow left ventricular assist devices (LVADs): Does axial flow unload better than centrifugal LVAD? ASAIO J. 2014, 60, 140–147. [Google Scholar] [CrossRef]
- Timms, D.; Hayne, M.; McNeil, K.; Galbraith, A. A complete mock circulation loop for the evaluation of left, right, and biventricular assist devices. Artif. Organs 2005, 29, 564–572. [Google Scholar] [CrossRef]
- Pantalos, G.M.; Koenig, S.C.; Gillars, K.J.; Giridharan, G.A.; Ewert, D.L. Characterization of an Adult Mock Circulation for Testing Cardiac Support Devices. ASAIO J. 2004, 50, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.; Timms, D.; Pearcy, M.J.; Tansley, G. A naturally shaped silicone ventricle evaluated in a mock circulation loop: A preliminary study. J. Med. Eng. Technol. 2009, 33, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Roche, E.T.; Wohlfarth, R.; Overvelde, J.T.B.; Vasilyev, N.V.; Pigula, F.A.; Mooney, D.J.; Bertoldi, K.; Walsh, C.J. A Bioinspired Soft Actuated Material. Adv. Mater. 2014, 26, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Baturalp, T.B. Design and Development of a Systemic Mock Circulation Loop with a Novel Beating Left Ventricular Simulator. Doctoral Dissertation, Texas Tech University, Lubbock, TX, USA, August 2016. [Google Scholar]
- ASTM D412-16 Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers-Tension. Available online: https://www.astm.org/Standards/D412 (accessed on 12 September 2019).
- Xu, B.; He, Q.S.; Yu, S.R. Finite Element Analysis on the Large Deformations of Rubber Structure. Appl. Mech. Mater. 2010, 44, 1487–1491. [Google Scholar] [CrossRef]
- Genovese, K.; Lamberti, L.; Pappalettere, C. Mechanical characterization of hyperelastic materials with fringe projection and optimization techniques. Opt. Lasers Eng. 2006, 44, 423–442. [Google Scholar] [CrossRef]
- Sridar, S.; Majeika, C.J.; Schaffer, P.; Bowers, M.; Ueda, S.; Barth, A.J.; Sorrells, J.L.; Wu, J.T.; Hunt, T.R.; Popovic, M. Hydro Muscle -a novel soft fluidic actuator. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016. [Google Scholar]
- Nakatani, S. Left ventricular rotation and twist: why should we learn? J. Cardiovasc. Ultrasound 2011, 19, 1–6. [Google Scholar] [CrossRef]
- Borazjani, I.; Westerdale, J.; Mcmahon, E.M.; Rajaraman, P.K.; Heys, J.J.; Belohlavek, M. Left Ventricular Flow Analysis: Recent Advances in Numerical Methods and Applications in Cardiac Ultrasound. Comput. Math. Methods Med. 2013, 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Bavo, A.M.; Pouch, A.M.; Degroote, J.; Vierendeels, J.; Gorman, J.H.; Gorman, R.C.; Segers, P. Patient-specific CFD models for intraventricular flow analysis from 3D ultrasound imaging: Comparison of three clinical cases. J. Biomech. 2017, 50, 144–150. [Google Scholar] [CrossRef]
- Lee, C.-H.; Laurence, D.W.; Ross, C.J.; Kramer, K.E.; Babu, A.R.; Johnson, E.L.; Hsu, M.-C.; Aggarwal, A.; Mir, A.; Burkhart, H.M.; et al. Mechanics of the Tricuspid Valve—From Clinical Diagnosis/Treatment, In-Vivo and In-Vitro Investigations, to Patient-Specific Biomechanical Modeling. Bioengineering 2019, 6, 47. [Google Scholar] [CrossRef]
- Khalili, F.; Gamage, P.; Sandler, R.; Mansy, H.; Khalili, F.; Gamage, P.P.T.; Sandler, R.H.; Mansy, H.A. Adverse Hemodynamic Conditions Associated with Mechanical Heart Valve Leaflet Immobility. Bioengineering 2018, 5, 74. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulbulak, U.; Ertas, A. Finite Element Driven Design Domain Identification of a Beating Left Ventricular Simulator. Bioengineering 2019, 6, 83. https://doi.org/10.3390/bioengineering6030083
Gulbulak U, Ertas A. Finite Element Driven Design Domain Identification of a Beating Left Ventricular Simulator. Bioengineering. 2019; 6(3):83. https://doi.org/10.3390/bioengineering6030083
Chicago/Turabian StyleGulbulak, Utku, and Atila Ertas. 2019. "Finite Element Driven Design Domain Identification of a Beating Left Ventricular Simulator" Bioengineering 6, no. 3: 83. https://doi.org/10.3390/bioengineering6030083
APA StyleGulbulak, U., & Ertas, A. (2019). Finite Element Driven Design Domain Identification of a Beating Left Ventricular Simulator. Bioengineering, 6(3), 83. https://doi.org/10.3390/bioengineering6030083