Recent Trends in Injury Models to Study Skeletal Muscle Regeneration and Repair
Abstract
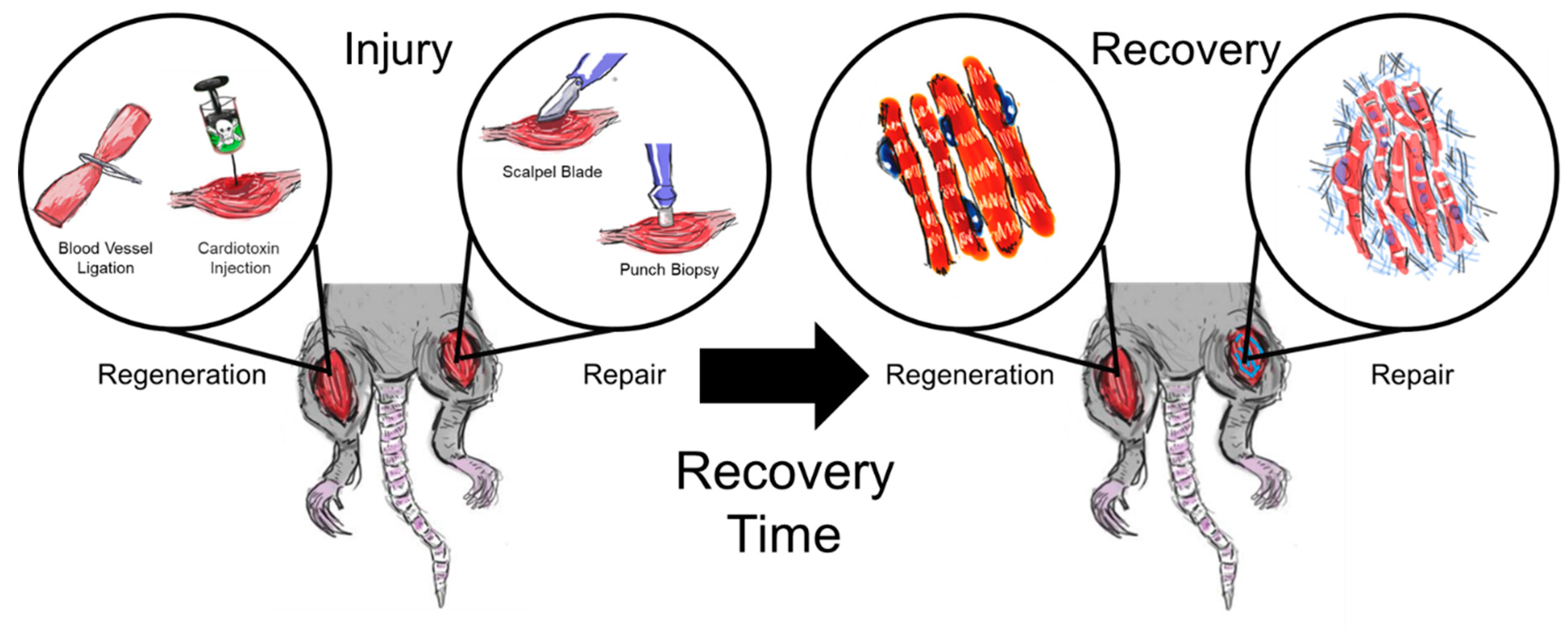
:1. Introduction
2. Myotoxins
3. Ischemia
4. VML Injury Models
4.1. Animal Species Used in VML Studies
4.2. Variations in Muscle Group
4.3. Size and Induction of Injury
5. Summary and Future Considerations
Author Contributions
Funding
Conflicts of Interest
References
- Grogan, B.F.; Hsu, J.R.; Skeletal, C. Trauma Research, Volumetric muscle loss. J. Am. Acad. Orthop. Surg. 2011, 19 (Suppl. 1), S35–S37. [Google Scholar] [CrossRef] [PubMed]
- Grasman, J.M.; Zayas, M.J.; Page, R.L.; Pins, G.D. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015, 25, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Pantelic, M.N.; Larkin, L.M. Stem Cells for Skeletal Muscle Tissue Engineering. Tissue Eng. Part B Rev. 2018, 24, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Honick, J.; Grayson, W. Vascularized and Innervated Skeletal Muscle Tissue Engineering. Adv. Healthc. Mater. 2020, 9, e1900626. [Google Scholar] [CrossRef]
- Gholobova, D.; Terrie, L.; Gerard, M.; Declercq, H.; Thorrez, L. Vascularization of tissue-engineered skeletal muscle constructs. Biomaterials 2020, 235, 119708. [Google Scholar] [CrossRef]
- Dunn, A.; Talovic, M.; Patel, K.; Patel, A.; Marcinczyk, M.; Garg, K. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J. Orthop. Res. 2019, 37, 1246–1262. [Google Scholar] [CrossRef]
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: Results of a 13-patient cohort study. NPJ Regen. Med. 2016, 1, 16008. [Google Scholar] [CrossRef]
- Lomonte, B.; Rangel, J. Snake venom Lys49 myotoxins: From phospholipases A(2) to non-enzymatic membrane disruptors. Toxicon 2012, 60, 520–530. [Google Scholar] [CrossRef]
- Vignaud, A.; Cebrian, J.; Martelly, I.; Caruelle, J.P.; Ferry, A. Effect of anti-inflammatory and antioxidant drugs on the long-term repair of severely injured mouse skeletal muscle. Exp. Physiol. 2005, 90, 487–495. [Google Scholar] [CrossRef]
- Buono, R.; Vantaggiato, C.; Pisa, V.; Azzoni, E.; Bassi, M.T.; Brunelli, S.; Sciorati, C.; Clementi, E. Nitric oxide sustains long-term skeletal muscle regeneration by regulating fate of satellite cells via signaling pathways requiring Vangl2 and cyclic GMP. Stem Cells 2012, 30, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Juhas, M.; Abutaleb, N.; Wang, J.T.; Ye, J.; Shaikh, Z.; Sriworarat, C.; Qian, Y.; Bursac, N. Incorporation of macrophages into engineered skeletal muscle enables enhanced muscle regeneration. Nat. Biomed. Eng. 2018, 2, 942–954. [Google Scholar] [CrossRef]
- Garry, G.A.; Antony, M.L.; Garry, D.J. Cardiotoxin Induced Injury and Skeletal Muscle Regeneration. Methods Mol. Biol. 2016, 1460, 61–71. [Google Scholar] [PubMed]
- Foltz, S.J.; Modi, J.N.; Melick, G.A.; Abousaud, M.I.; Luan, J.; Fortunato, M.J.; Beedle, A.M. Abnormal Skeletal Muscle Regeneration plus Mild Alterations in Mature Fiber Type Specification in Fktn-Deficient Dystroglycanopathy Muscular Dystrophy Mice. PLoS ONE 2016, 11, e0147049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, N.; Martin, P.T. A role for Galgt1 in skeletal muscle regeneration. Skelet. Muscle 2015, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Mofarrahi, M.; McClung, J.M.; Kontos, C.D.; Davis, E.C.; Tappuni, B.; Moroz, N.; Pickett, A.E.; Huck, L.; Harel, S.; Danialou, G.; et al. Angiopoietin-1 enhances skeletal muscle regeneration in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R576–R589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, E.S.; Santana, T.A.; Diniz, P.B.; Montalvao, M.M.; Bani, C.C.; Thomazzi, S.M. Thymol accelerates the recovery of the skeletal muscle of mice injured with cardiotoxin. J. Pharm. Pharmacol. 2016, 68, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Bouviere, J.; Trignol, A.; Hoang, D.H.; del Carmine, P.; Goriot, M.E.; Larbi, S.B.; Barritault, D.; Banzet, S.; Chazaud, B. Heparan sulfate mimetic accelerate post-injury skeletal muscle regeneration. Tissue Eng. Part A 2019, 25, 1667–1676. [Google Scholar] [CrossRef]
- Borselli, C.; Storrie, H.; Benesch-Lee, F.; Shvartsman, D.; Cezar, C.; Lichtman, J.W.; Vandenburgh, H.H.; Mooney, D.J. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. USA 2010, 107, 3287–3292. [Google Scholar] [CrossRef] [Green Version]
- Shvartsman, D.; Storrie-White, H.; Lee, K.; Kearney, C.; Brudno, Y.; Ho, N.; Cezar, C.; McCann, C.; Anderson, E.; Koullias, J.; et al. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol. Ther. 2014, 22, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Corona, B.T.; Rathbone, C.R. Accelerated functional recovery after skeletal muscle ischemia-reperfusion injury using freshly isolated bone marrow cells. J. Surg. Res. 2014, 188, 100–109. [Google Scholar] [CrossRef]
- Blaisdell, F. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc. Surg. 2002, 10, 620–630. [Google Scholar] [CrossRef]
- Zeng, Q.; Fu, Q.; Wang, X.; Zhao, Y.; Liu, H.; Li, Z.; Li, F. Protective Effects of Sonic Hedgehog Against Ischemia/Reperfusion Injury in Mouse Skeletal Muscle via AKT/mTOR/p70S6K Signaling. Cell. Physiol. Biochem. 2017, 43, 1813–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignaud, A.; Hourde, C.; Medja, F.; Agbulut, O.; Butler-Browne, G.; Ferry, A. Impaired skeletal muscle repair after ischemia-reperfusion injury in mice. J. Biomed. Biotechnol. 2010, 2010, 724914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, H.; Wang, M.; Meng, G.; Wang, Z.; Deng, J.; Wang, M.; Zhang, Q.; Yang, S.; Jiang, H. Vagus Nerve Stimulation Attenuates Acute Skeletal Muscle Injury Induced by Ischemia-Reperfusion in Rats. Oxid. Med. Cell. Longev. 2019, 2019, 9208949. [Google Scholar] [CrossRef] [PubMed]
- Greising, S.M.; Corona, B.T.; McGann, C.; Frankum, J.K.; Warren, G.L. Therapeutic Approaches for Volumetric Muscle Loss Injury: A Systematic Review and Meta-Analysis. Tissue Eng. Part B Rev. 2019, 25, 510–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corona, B.T.; Henderson, B.E.; Ward, C.L.; Greising, S.M. Contribution of minced muscle graft progenitor cells to muscle fiber formation after volumetric muscle loss injury in wild-type and immune deficient mice. Physiol. Rep. 2017, 5, e13249. [Google Scholar] [CrossRef]
- Grasman, J.M.; Do, D.M.; Page, R.L.; Pins, G.D. Rapid release of growth factors regenerates force output in volumetric muscle loss injuries. Biomaterials 2015, 72, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Quarta, M.; Cromie, M.; Chacon, R.; Blonigan, J.; Garcia, V.; Akimenko, I.; Hamer, M.; Paine, P.; Stok, M.; Shrager, J.B.; et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat. Commun. 2017, 8, 15613. [Google Scholar] [CrossRef]
- Mintz, E.L.; Passipieri, J.A.; Franklin, I.R.; Toscano, V.M.; Afferton, E.C.; Sharma, P.R.; Christ, G.J. Long-Term Evaluation of Functional Outcomes Following Rat Volumetric Muscle Loss Injury and Repair. Tissue Eng. Part A 2020, 26, 140–156. [Google Scholar] [CrossRef]
- VanDusen, K.W.; Syverud, B.C.; Williams, M.L.; Lee, J.D.; Larkin, L.M. Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng. Part A 2014, 20, 2920–2930. [Google Scholar] [CrossRef] [Green Version]
- Kasukonis, B.; Kim, J.; Brown, L.; Jones, J.; Ahmadi, S.; Washington, T.; Wolchok, J. Codelivery of Infusion Decellularized Skeletal Muscle with Minced Muscle Autografts Improved Recovery from Volumetric Muscle Loss Injury in a Rat Model. Tissue Eng. Part A 2016, 22, 1151–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passipieri, J.A.; Hu, X.; Mintz, E.; Dienes, J.; Baker, H.B.; Wallace, C.H.; Blemker, S.S.; Christ, G.J. In Silico and In Vivo Studies Detect Functional Repair Mechanisms in a Volumetric Muscle Loss Injury. Tissue Eng. Part A 2019, 25, 1272–1288. [Google Scholar] [CrossRef] [PubMed]
- Li, M.T.; Willett, N.J.; Uhrig, B.A.; Guldberg, R.E.; Warren, G.L. Functional analysis of limb recovery following autograft treatment of volumetric muscle loss in the quadriceps femoris. J. Biomech. 2014, 47, 2013–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, S.E.; Han, W.M.; Srinivasa, V.; Mohiuddin, M.; Ruehle, M.A.; Moon, J.Y.; Shin, E.; San, C.L.; Emeterio, M.E.; Ogle, E.A.; et al. Determination of a Critical Size Threshold for Volumetric Muscle Loss in the Mouse Quadriceps. Tissue Eng. Part C Methods 2019, 25, 59–70. [Google Scholar] [CrossRef]
- Ward, C.L.; Pollot, B.E.; Goldman, S.M.; Greising, S.M.; Wenke, J.C.; Corona, B.T. Autologous Minced Muscle Grafts Improve Muscle Strength in a Porcine Model of Volumetric Muscle Loss Injury. J. Orthop. Trauma 2016, 30, e396–e403. [Google Scholar] [CrossRef]
- Novakova, S.S.; Rodriguez, B.L.; Vega-Soto, E.E.; Nutter, G.P.; Armstrong, R.E.; Macpherson, P.C.D.; Larkin, L.M. Repairing Volumetric Muscle Loss in the Ovine Peroneus Tertius Following a 3-Month Recovery. Tissue Eng. Part A 2020. [Google Scholar] [CrossRef]
- Sarrafian, T.L.; Bodine, S.C.; Murphy, B.; Grayson, J.K.; Stover, S.M. Extracellular matrix scaffolds for treatment of large volume muscle injuries: A review. Vet. Surg. 2018, 47, 524–535. [Google Scholar] [CrossRef]
- Matthias, N.; Hunt, S.D.; Wu, J.; Lo, J.; Callahan, L.A.S.; Li, Y.; Huard, J.; Darabi, R. Volumetric muscle loss injury repair using in situ fibrin gel cast seeded with muscle-derived stem cells (MDSCs). Stem Cell Res. 2018, 27, 65–73. [Google Scholar] [CrossRef]
- Page, R.L.; Malcuit, C.; Vilner, L.; Vojtic, I.; Shaw, S.; Hedblom, E.; Hu, J.; Pins, G.D.; Rolle, M.W.; Dominko, T. Restoration of skeletal muscle defects with adult human cells delivered on fibrin microthreads. Tissue Eng. Part A 2011, 17, 2629–2640. [Google Scholar] [CrossRef]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef]
- Goldman, S.M.; Corona, B.T. Co-delivery of micronized urinary bladder matrix damps regenerative capacity of minced muscle grafts in the treatment of volumetric muscle loss injuries. PLoS ONE 2017, 12, e0186593. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.K.; Walters, T.J. Muscle-derived decellularised extracellular matrix improves functional recovery in a rat latissimus dorsi muscle defect model. J. Plast. Reconstr. Aesthet. Surg. JPRAS 2013, 66, 1750–1758. [Google Scholar] [CrossRef]
- Corona, B.T.; Machingal, M.A.; Criswell, T.; Vadhavkar, M.; Dannahower, A.C.; Bergman, C.; Zhao, W.; Christ, G.J. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng. Part A 2012, 18, 1213–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, P.L.; Roy, R.R.; Kanim, P.; Bello, M.A.; Edgerton, V.R. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, M.A.; Chapman, M.A.; Hentzen, E.R.; Friden, J.; Lieber, R.L. Anatomical, architectural, and biochemical diversity of the murine forelimb muscles. J. Anat. 2012, 221, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Honick, J.; Iyer, S.R.; Somers, S.M.; Lovering, R.M.; Wagner, K.; Mao, H.Q.; Grayson, W.L. Engineering functional and histological regeneration of vascularized skeletal muscle. Biomaterials 2018, 164, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.H.; Alcazar, C.; Yang, G.; Quarta, M.; Paine, P.; Doan, L.; Davies, A.; Rando, T.A.; Huang, N.F. Rehabilitative exercise and spatially patterned nanofibrillar scaffolds enhance vascularization and innervation following volumetric muscle loss. NPJ Regen. Med. 2018, 3, 16. [Google Scholar] [CrossRef]
- Sicari, B.M.; Rubin, J.P.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; Wyse, A.; et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 2014, 6, 234ra58. [Google Scholar] [CrossRef] [Green Version]
- Corona, B.T.; Ward, C.L.; Baker, H.B.; Walters, T.J.; Christ, G.J. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng. Part A 2014, 20, 705–715. [Google Scholar]
| Injury Model | Treatment | Results | Reference |
|---|---|---|---|
| Mice CTX gastrocnemius muscle | Thymol | Significant decrease in the number of mast cells and the final percent of collagen after 10 days. | [16] |
| Mice CTX TA muscle | Ang-1 | Ang-1 increases muscle contractility, fiber regeneration, and capillary density after 14 days. | [15] |
| Mice CTX TA muscle | Galgt1 | Galgt1 expression resulted in larger myofiber diameters and more markers of muscle regeneration after 2–4 weeks. | [14] |
| Mice CTX TA muscle | Glycosaminoglycan mimetics | Injection of mimetics increased nuclei per myofiber and enhanced capillary formation within muscles. | [17] |
| Injury Model | Treatment | Results | Reference |
|---|---|---|---|
| Mice Ischemia ligation Hindlimb | VEGF and IGF1 | Improvement in vasculature as well as muscle contractility and myofiber diameter after 7 weeks. | [18] |
| Mice Ischemia ligation TA muscle | VEGF | Enhanced vascularization of muscle, leading to expression of neurotrophic factors to maintain innervation. | [19] |
| Mice IR Upper hindlimb | Bone marrow cells | After 4 weeks, there was an increase in maximal tetanic torque and force production and a decrease in centrally located nuclei. | [20] |
| Rats IR Upper hindlimb | Vagus nerve stimulation | Stimulation led to reduction in apoptosis and inflammation, as well as protection of vascular endothelial function. | [24] |
| Mice IR Hindlimb | Shh | Improved myofiber recovery, inhibited apoptosis, and reduced fibrosis. | [22] |
| Animal Model | Location | Injury Size | Treatment | Results | Reference |
|---|---|---|---|---|---|
| IC and ID mice | TA | 2 mm biopsy punch (20% by mass) | Minced muscle from GFP+ mice | Muscle repair response was similar in both strains despite no improvement in TA muscle strength. | [26] |
| ID mice | TA | 4 × 2 × 2 mm3 (50% reduction in force) | Crosslinked fibrin microthreads loaded with HGF | Recovery of 200% of force production and sustained angiogenesis after 2 months. | [27] |
| ID mice | TA | 2 × 7 × 2 mm3 (40% by mass) | Human MuSC+/MRC+ constructs | Increase in functional recovery with exercise and improved vascularization and innervation after 1 month. | [28] |
| IC rats | TA | 10 × 5 × 7 mm3 (20% by mass) | BAM or TEMR | Significant functional recovery in TEMR responders, with mature muscle found in injury site after 6 months. | [29] |
| IC rats | TA | 30% by volume (longitudinal cut) | SMUs | Significant functional recovery with evidence of nerve and blood vessel infiltration within SMU after 1 month. | [30] |
| IC rats | TA | 8 × 3 mm2 deep biopsy punch (20% by mass) | Muscle-derived ECM with minced muscle | Significant functional recovery and reduced fibrotic response. | [31] |
| IC rats | LD | 1.5 × 1.1 cm2 (13% by mass) | BAM or TEMR | Recovery of 71% of force production; enhanced angiogenesis after 2 months. | [32] |
| IC rats | Q | 8 mm biopsy punch | Muscle autograft | No significant recovery of muscle function. | [33] |
| IC mice | Q | 2, 3, or 4 mm biopsy punch, full thickness (5%, 15%, or 30% by mass) | No treatment | Threshold for VML defect was 3 mm biopsy punch. | [34] |
| Yorkshire-cross pigs | PT | 3 × 3 × 1.5 cm3 (20% by mass) | Autologous minced muscle | 32% strength increase after 4 months; extensive fibrotic tissue deposition. | [35] |
| Polypay sheep | PT | 30% by volume (longitudinal cut) | SMUs + ENCs | Implants significantly increased force production after 3 months. | [36] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sicherer, S.T.; Venkatarama, R.S.; Grasman, J.M. Recent Trends in Injury Models to Study Skeletal Muscle Regeneration and Repair. Bioengineering 2020, 7, 76. https://doi.org/10.3390/bioengineering7030076
Sicherer ST, Venkatarama RS, Grasman JM. Recent Trends in Injury Models to Study Skeletal Muscle Regeneration and Repair. Bioengineering. 2020; 7(3):76. https://doi.org/10.3390/bioengineering7030076
Chicago/Turabian StyleSicherer, Sydnee T., Rashmi S. Venkatarama, and Jonathan M. Grasman. 2020. "Recent Trends in Injury Models to Study Skeletal Muscle Regeneration and Repair" Bioengineering 7, no. 3: 76. https://doi.org/10.3390/bioengineering7030076
APA StyleSicherer, S. T., Venkatarama, R. S., & Grasman, J. M. (2020). Recent Trends in Injury Models to Study Skeletal Muscle Regeneration and Repair. Bioengineering, 7(3), 76. https://doi.org/10.3390/bioengineering7030076






