Fibrin Glue Implants Seeded with Dental Pulp and Periodontal Ligament Stem Cells for the Repair of Periodontal Bone Defects: A Preclinical Study
Abstract
:1. Introduction
2. Materials and Methods
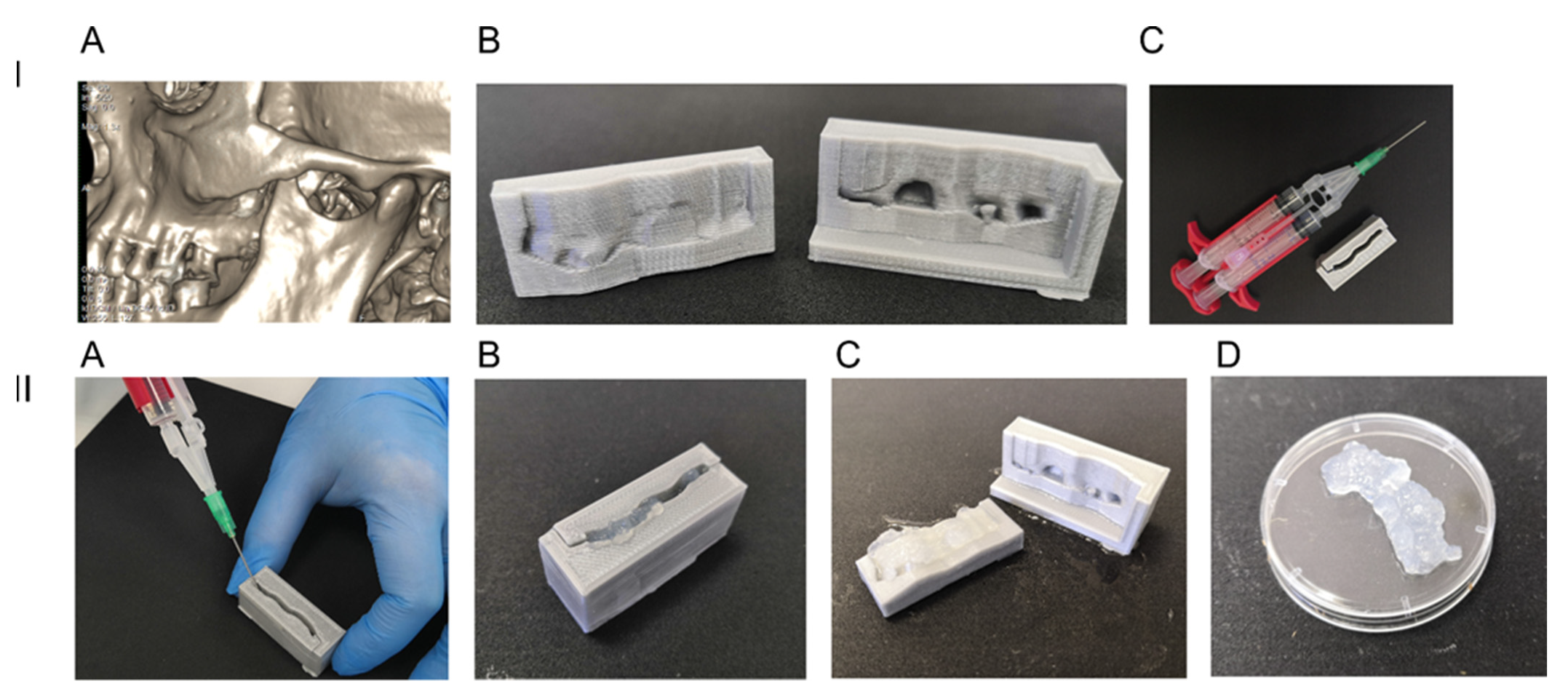
2.1. 3D Printed Anatomical Prototype Molding Form
2.2. Cell Cultures
2.3. Fibrin Glue
2.4. Evaluation of Cells Proliferation, Differentiation and Surface Markers Phenotype
2.5. Bone Defect Animal Model and Cell-Seeded Scaffold Implantation
2.6. Cone Beam Computed Tomography
2.7. Histomorphometric Analysis
2.8. Statistical Analysis
3. Results
3.1. Human DPSC Grown in Fibrin Scaffolds: Proliferation, Immunophenotype and Osteogenic Differentiation
3.2. Alveolar Bone Defect In Vivo. Influence of Cell-Seeded Implant on Bone Remodeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nyberg, E.L.; Farris, A.L.; Hung, B.P.; Dias, M.; Garcia, J.R.; Dorafshar, A.H.; Grayson, W.L. 3D-Printing Technologies for Craniofacial Rehabilitation, Reconstruction, and Regeneration. Ann. Biomed. Eng. 2017, 45, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, A.N.; Norkin, I.A.; Puchin’ian, D.M. The possibilities and perspectives of using scaffold technology for bone regeneration. Tsitologiia 2014, 56, 543–548. [Google Scholar]
- Grimm, W.-D.; Giesenhagen, B.; Hakki, S.; Schau, I.; Sirak, S.; Sletov, A.; Varga, G.; Vukovic, M.A.; Widera, D. Translational Research and Therapeutic Applications of Neural Crest-Derived Stem Cells in Regenerative Periodontology. Curr. Oral Health Rep. 2015, 2, 266–274. [Google Scholar] [CrossRef] [Green Version]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpe, P.T. Dental mesenchymal stem cells. Development 2016, 143, 2273–2280. [Google Scholar] [CrossRef] [Green Version]
- Grimm, W.D.; Dannan, A.; Giesenhagen, B.; Schau, I.; Varga, G.; Vukovic, M.A.; Sirak, S.V. Translational Research: Palatal-derived Ecto-mesenchymal Stem Cells from Human Palate: A New Hope for Alveolar Bone and Cranio-Facial Bone Reconstruction. Int. J. Stem Cells 2014, 7, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Sarrà, E.; Montori, S.; Gil-Recio, C.; Núñez-Toldrà, R.; Costamagna, D.; Rotini, A.; Atari, M.; Luttun, A.; Sampaolesi, M. Human dental pulp pluripotent-like stem cells promote wound healing and muscle regeneration. Stem Cell Res. Ther. 2017, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Atari, M.; Gil-Recio, C.; Fabregat, M.; García-Fernández, D.; Barajas, M.; Carrasco, M.A.; Jung, H.-S.; Alfaro, F.H.; Casals, N.; Prosper, F.; et al. Dental pulp of the third molar: A new source of pluripotent-like stem cells. J. Cell Sci. 2012, 125, 3343–3356. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wei, X.; Ling, J.; Wu, L.; Xiao, Y. Expression pattern of Oct-4, Sox2, and c-Myc in the primary culture of human dental pulp derived cells. J. Endod. 2011, 37, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Zeuner, M.T.; Didenko, N.N.; Humphries, D.; Stergiadis, S.; Morash, T.M.; Patel, K.; Grimm, W.D.; Widera, D. Isolation and characterization of neural crest-derived stem cells from adult ovine palatal tissue. Front. Cell Dev. Biol. 2018, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Sheard, J.J.; Bicer, M.; Meng, Y.; Frigo, A.; Aguilar, R.M.; Vallance, T.M.; Iandolo, D.; Widera, D. Optically Transparent Anionic Nanofibrillar Cellulose Is Cytocompatible with Human Adipose Tissue-Derived Stem Cells and Allows Simple Imaging in 3D. Stem Cells Int. 2019, 2019, 3106929. [Google Scholar] [CrossRef] [PubMed]
- Jockenhoevel, S.; Zund, G.; Hoerstrup, S.P.; Chalabi, K.; Sachweh, J.S.; Demircan, L.; Messmer, B.J.; Turina, M. Fibrin gel—Advantages of a new scaffold in cardiovascular tissue engineering. Eur. J. Cardiothorac. Surg. 2001, 19, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Swartz, D.D.; Russell, J.A.; Andreadis, S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1451–H1460. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- Uehara, K.; Zhao, C.; Gingery, A.; Thoreson, A.R.; An, K.-N.; Amadio, P.C. Effect of Fibrin Formulation on Initial Strength of Tendon Repair and Migration of Bone Marrow Stromal Cells in Vitro. J. Bone Joint Surg. Am. 2015, 97, 1792–1798. [Google Scholar] [CrossRef] [Green Version]
- Enukashvily, N.I.; Aizenshtadt, A.A.; Bagaeva, V.V.; Supilnikova, O.V.; Ivolgin, D.A.; Maslennikova, I.I.; Novikova, S.V.; Adylov, S.F. Assesing the possibility to apply the fibrin glue from cord blood plasma as a scaffold for mesenchymal stem cells transplantation. Her. North West. State Med. Univ. Named I.I. Mechnikov 2017, 9, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Bagaeva, V.V.; Aizenshtadt, A.A.; Savintsev, A.M.; Aleksandrova, L.; Enukashvily, N.I.; Adylov, S.F.; Zolina, T.L.; Urusova, M.E. Method for Obtaining of Two-Component Preparation for Treatment of Joints Damage by Low-Invasive Introduction into Joint Bag and Preparation Obtained by This Method. RU Patent 2 638 796 C1, 8 May 2016. [Google Scholar]
- Dombrovskaya, Y.A.; Enukashvily, N.I.; Kotova, A.V.; Bilyk, S.S.; Kovalenko, A.N.; Silin, A.V. Fibrin scaffolds containing dental pulp stem cells for the repair of periodontal bone defects. Transl. Med. 2020, 7, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Egorikhina, M.N.; Aleynik, D.Y.; Rubtsova, Y.P.; Levin, G.Y.; Charykova, I.N.; Semenycheva, L.L.; Bugrova, M.L.; Zakharychev, E.A. Hydrogel scaffolds based on blood plasma cryoprecipitate and collagen derived from various sources: Structural, mechanical and biological characteristics. Bioact. Mater. 2019, 4, 334–345. [Google Scholar] [CrossRef]
- Tikhomirova, A.V.; Goryachev, D.V.; Merkulov, V.A.; Lysikova, I.V.; Gubenko, A.I.; Zebrev, A.I.; Solovieva, А.P.; Romodanovsky, D.P.; Melnikova, E.V. preclinical and clinical aspects of the development of biomedical cell products. Bull. Sci. Cent. Expert Eval. Med. Prod. 2018, 8, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Brecher, M.E. AABB Technical Manual, 15th ed.; Brecher, M.E., Ed.; AABB: Bethesda, MD, USA, 2005; ISBN 1563951967. [Google Scholar]
- Silver, F.H.; Wang, M.-C.; Pins, G.D. Preparation and use of fibrin glue in surgery. Biomaterials 1995, 16, 891–903. [Google Scholar] [CrossRef]
- Silver, F.H.; Wang, M.C.; Pins, G.D. Preparation of fibrin glue: A study of chemical and physical methods. J. Appl. Biomater. 1995, 6, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Elnager, A.; Abdullah, W.Z.; Hassan, R.; Idris, Z.; Wan Arfah, N.; Sulaiman, S.A.; Mustafa, Z. In Vitro whole blood clot lysis for fibrinolytic activity study using D-dimer and confocal microscopy. Adv. Hematol. 2014, 2014, 814684. [Google Scholar] [CrossRef] [Green Version]
- Bogdanova, M.; Zabirnyk, A.; Malashicheva, A.; Enayati, K.Z.; Karlsen, T.A.; Kaljusto, M.L.; Kvitting, J.P.E.; Dissen, E.; Sullivan, G.J.; Kostareva, A.; et al. Interstitial cells in calcified aortic valves have reduced differentiation potential and stem cell-like properties. Sci. Rep. 2019, 9, 12934. [Google Scholar] [CrossRef]
- Widera, D.; Grimm, W.-D.; Moebius, J.M.; Mikenberg, I.; Piechaczek, C.; Gassmann, G.; Wolff, N.A.; Thévenod, F.; Kaltschmidt, C.; Kaltschmidt, B. Highly Efficient Neural Differentiation of Human Somatic Stem Cells, Isolated by Minimally Invasive Periodontal Surgery. Stem Cells Dev. 2007, 16, 447–460. [Google Scholar] [CrossRef]
- Pauwels, R.; Jacobs, R.; Singer, S.R.; Mupparapu, M. CBCT-based bone quality assessment: Are Hounsfield units applicable? Dentomaxillofacial Radiol. 2015, 44, 20140238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, N.T.; Carlos Munévar, J.; González, J.M.; Infante, C.; Lara, S.J.P. In Vitro response of dental pulp stem cells in 3D scaffolds: A regenerative bone material. Heliyon 2018, 4, e00775. [Google Scholar] [CrossRef]
- Wufsus, A.R.; Rana, K.; Brown, A.; Dorgan, J.R.; Liberatore, M.W.; Neeves, K.B. Elastic behavior and platelet retraction in low- and high-density fibrin gels. Biophys. J. 2015, 108, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Salam, N.; Toumpaniari, S.; Gentile, P.; Ferreira, A.M.; Dalgarno, K.; Partridge, S. Assessment of migration of human mscs through fibrin hydrogels as a tool for formulation optimisation. Materials 2018, 11, 1781. [Google Scholar] [CrossRef] [Green Version]
- Langova, P.; Stembirek, J.; Matalova, E.; Buchtova, M. Tooth autotransplantations-lessons from animal models: A review. Vet. Med. 2015, 60, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Uribe, S.; Rojas, L.; Rosas, C. Accuracy of imaging methods for detection of bone tissue invasion in patients with oral squamous cell carcinoma. Dentomaxillofacial Radiol. 2013, 42, 20120346. [Google Scholar] [CrossRef] [Green Version]
- Porrini, R.; Rocchetti, V.; Vercellino, V.; Cannas, M.; Sabbatini, M. Alveolar bone regeneration in post-extraction socket: A review of materials to postpone dental implant. Biomed. Mater. Eng. 2011, 21, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Khang, G. Handbook of Intelligent Scaffolds for Tissue Engineering and Regenerative Medicine, 2nd ed.; Pan Stanford: Boca Raton, FL, USA, 2017; ISBN 9781315364698. [Google Scholar]
- Park, S.A.; Lee, H.-J.; Kim, K.-S.; Lee, S.J.; Lee, J.-T.; Kim, S.-Y.; Chang, N.-H.; Park, S.-Y. In Vivo Evaluation of 3D-Printed Polycaprolactone Scaffold Implantation Combined with β-TCP Powder for Alveolar Bone Augmentation in a Beagle Defect Model. Materials 2018, 11, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, O.; Kort-Mascort, J.; Lin, Y.; Pham, H.M.; Charbonneau, A.M.; ElKashty, O.A.; Kinsella, J.M.; Tran, S.D. The applications of 3D printing for craniofacial tissue engineering. Micromachines 2019, 10, 480. [Google Scholar] [CrossRef] [Green Version]
- Maroulakos, M.; Kamperos, G.; Tayebi, L.; Halazonetis, D.; Ren, Y. Applications of 3D printing on craniofacial bone repair: A systematic review. J. Dent. 2019, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lee, S.K.; Yoon, J.I.; Kim, D.E.; Kim, M.; Ha, H. Fibrin glue improves the therapeutic effect of MSCs by sustaining survival and paracrine function. Tissue Eng. Part A 2013, 19, 2373–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedrich, H.C.; Simunek, M.; Reisinger, S.; Ferguson, J.; Gulle, H.; Goppelt, A.; Redl, H. Fibrin chain cross-linking, fibrinolysis, and in vivo sealing efficacy of differently structured fibrin sealants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1507–1512. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Gong, L.; Yu, D.; An, C.; Bunpetch, V.; Dai, J.; Huang, H.; Zou, X.; Ouyang, H.; et al. The Plasticity of Mesenchymal Stem Cells in Regulating Surface HLA-I. iScience 2019, 15, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Iohara, K.; Utsunomiya, S.; Kohara, S.; Nakashima, M. Allogeneic transplantation of mobilized dental pulp stem cells with the mismatched dog leukocyte antigen type is safe and efficacious for total pulp regeneration. Stem Cell Res. Ther. 2018, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Amghar-Maach, S.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Regeneration of periodontal bone defects with dental pulp stem cells grafting: Systematic Review. J. Clin. Exp. Dent. 2019, 11, e373–e381. [Google Scholar] [CrossRef] [PubMed]
- Grimm, W.-D.; Dannan, A.; Becher, S.; Gassmann, G.; Arnold, W.; Varga, G.; Dittmar, T. The ability of human periodontium-derived stem cells to regenerate periodontal tissues: A preliminary In Vivo investigation. Int. J. Periodontics Restor. Dent. 2011, 31, e94–e101. [Google Scholar]
- Bujoli, B.; Scimeca, J.C.; Verron, E. Fibrin as a multipurpose physiological platform for bone tissue engineering and targeted delivery of bioactive compounds. Pharmaceutics 2019, 11, 556. [Google Scholar] [CrossRef] [Green Version]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of blood clot on biomaterial implants influences bone healing. Tissue Eng. Part B Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, P.; Mei, S.; Li, C.; Cai, C.; Ding, Y. In Vivo alveolar bone regeneration by bone marrow stem cells/fibrin glue composition. Arch. Oral Biol. 2012, 57, 238–244. [Google Scholar] [CrossRef]
- Van Der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Yu, J.; Rho, J. Bone Loss Triggered by the Cytokine Network in Inflammatory Autoimmune Diseases. J. Immunol. Res. 2015, 2015, 832127. [Google Scholar] [CrossRef]
- Buchta, C.; Hedrich, H.C.; Macher, M.; Höcker, P.; Redl, H. Biochemical characterization of autologous fibrin sealants produced by CryoSeal® and Vivostat® in comparison to the homologous fibrin sealant product Tissucol/Tisseel®. Biomaterials 2005, 26, 6233–6241. [Google Scholar] [CrossRef]
- Harris, D.M.; Siedentop, K.H.; Ham, K.R.; Sanchez, B. Autologous fibrin tissue adhesive biodegration and systemic effects. Laryngoscope 1987, 97, 1141–1144. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Guller, A.E.; Istranov, L.P.; Istranova, E.V.; Butnaru, D.V.; Vinarov, A.Z.; Zakharkina, O.L.; Kurkov, A.V.; Kantimerov, D.F.; Antonov, E.N.; et al. Morphology of collagen matrices for tissue engineering (biocompatibility, biodegradation, tissue response). Arkh. Patol. 2015, 77, 29–38. [Google Scholar] [CrossRef]






| Surface Antigen | DPSC Grown in Fibrin Gel * | DPSC Grown in Standard Conditions * |
|---|---|---|
| CD90 | 99.7 ± 0.52 | 99.3 ± 0.8 |
| CD105 | 99.1 ± 1.1 | 99.8 ± 0.85 |
| CD44 | 99.7 ± 0.82 | 98.9 ± 1.2 |
| CD73 | 98.2 ± 0.84 | 98.8 ± 0.5 |
| CD45 | 0.1 ± 0.07 | 0 |
| CD34 | 0 | 0 |
| CD14 | 1.2 ± 0.0.4 | 1.0 ± 0.2 |
| CD117 | 0.4 ± 0.24 | 0 |
| HLA-DR | 0 | 0 |
| Measured Parameter | Group | p | ||
|---|---|---|---|---|
| No Implants | Cell-Free Fibrin Glue | Cell-Seeded Fibrin Glue | ||
| Inflammation, Me [Q1–Q3] | 0 [0, 2] | 1 [0, 2] | 2 [2, 2] | 0.203 |
| Fibrosis, Me [Q1–Q3] | 2 [2, 3] | 2 [2, 2] | 3 [2, 3] | 0.243 |
| Remodeling of bone, Me [Q1–Q3] | 1 [1, 3] | 1 [1, 2] | 2 [2, 2] | 0.193 |
| Vessels Me [Q1–Q3] | 1 | 3 | 2 | 0.198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enukashvily, N.I.; Dombrovskaya, J.A.; Kotova, A.V.; Semenova, N.; Karabak, I.; Banashkov, R.E.; Baram, D.; Paderina, T.; Bilyk, S.S.; Grimm, W.-D.; et al. Fibrin Glue Implants Seeded with Dental Pulp and Periodontal Ligament Stem Cells for the Repair of Periodontal Bone Defects: A Preclinical Study. Bioengineering 2021, 8, 75. https://doi.org/10.3390/bioengineering8060075
Enukashvily NI, Dombrovskaya JA, Kotova AV, Semenova N, Karabak I, Banashkov RE, Baram D, Paderina T, Bilyk SS, Grimm W-D, et al. Fibrin Glue Implants Seeded with Dental Pulp and Periodontal Ligament Stem Cells for the Repair of Periodontal Bone Defects: A Preclinical Study. Bioengineering. 2021; 8(6):75. https://doi.org/10.3390/bioengineering8060075
Chicago/Turabian StyleEnukashvily, Natella I., Julia A. Dombrovskaya, Anastasia V. Kotova, Natalia Semenova, Irina Karabak, Roman E. Banashkov, Dmitry Baram, Tatiana Paderina, Stanislav S. Bilyk, Wolf-Dieter Grimm, and et al. 2021. "Fibrin Glue Implants Seeded with Dental Pulp and Periodontal Ligament Stem Cells for the Repair of Periodontal Bone Defects: A Preclinical Study" Bioengineering 8, no. 6: 75. https://doi.org/10.3390/bioengineering8060075








