Different Microfluidic Environments for In Vitro Testing of Lipid Nanoparticles against Osteosarcoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Fabrication of the Microfluidic Platform
2.2. Reagents for Cell Culture and MTX-Lipid Nanoparticles
2.3. Experimental Set-Ups and Procedures
- No treatment (1), keeping cells under recirculating media in order to determine their normal proliferation.
- Regular treatment (2), where cells were subjected to recirculating free MTX to determine the cytotoxic effect of the circulation of different drug concentrations.
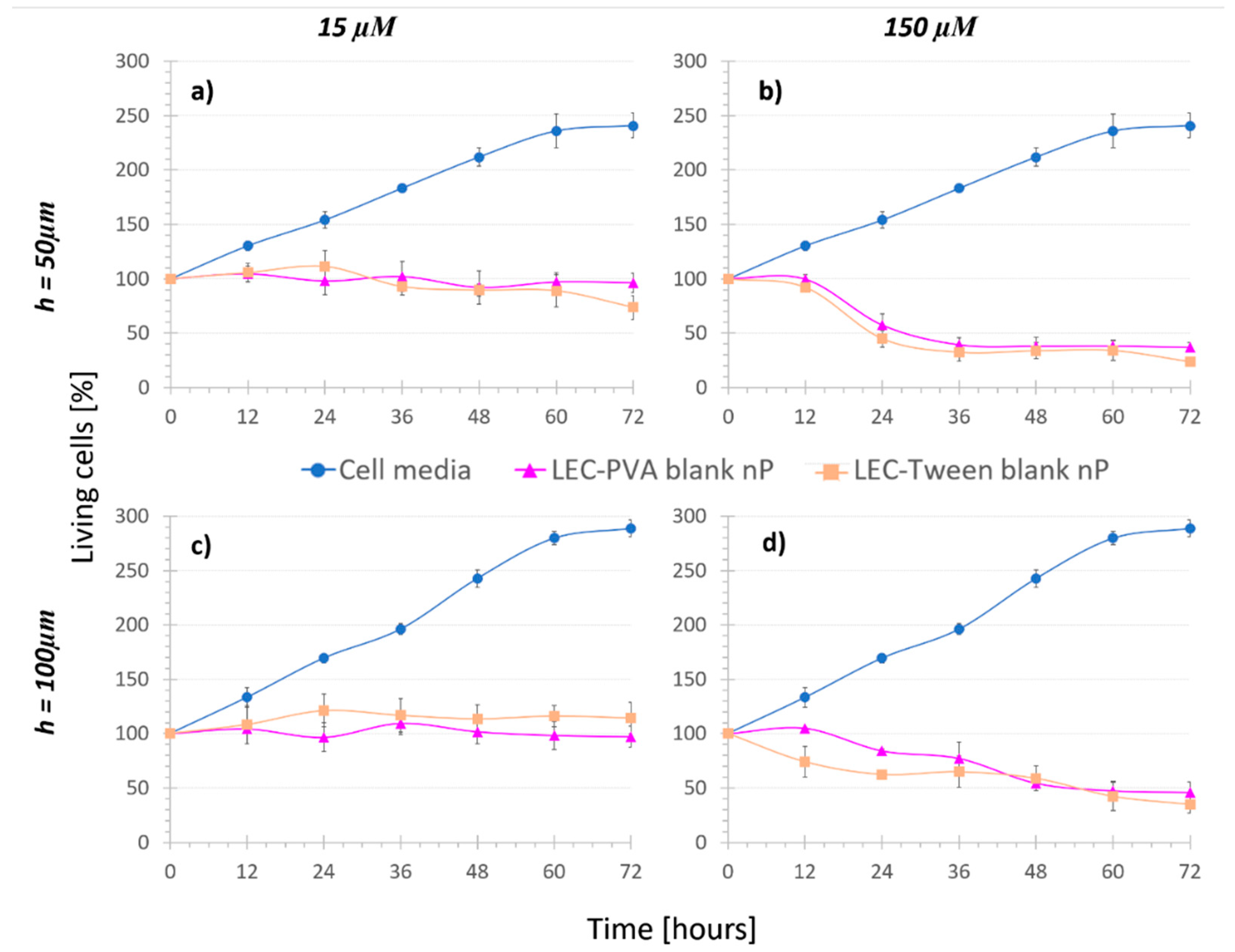
- Nanoparticle controls (3), namely cells were kept under the recirculation of nanoparticles with no MTX inside (blank nanoparticles). In this case, both LEC-PVA and LEC-Tween type blank nanoparticles were used.
- Nanoparticle treatments (4), where cells were subjected to the recirculation of either LEC-PVA or LEC-Tween encapsulated MTX in different concentrations.
2.4. Statistical Analysis
3. Results and Discussion
3.1. Cell Velocity during Insertion
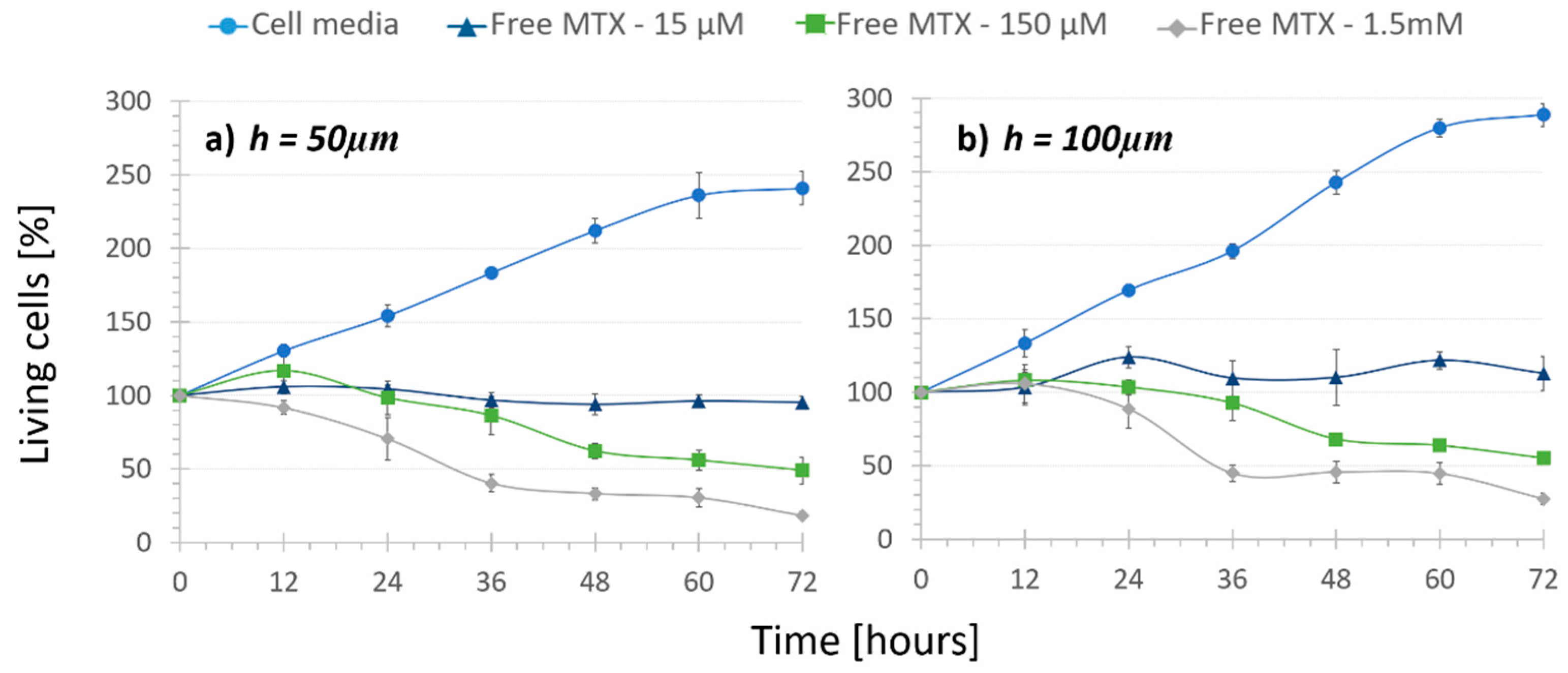
3.2. Treatment Comparison: In Vitro Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peris-Bonet, R.; Salmeron, D.; Martinez-Beneito, M.A.; Galceran, J.; Marcos-Gragera, R.; Felipe, S.; Gonzalez, V.; Sanchez de Toledo Codina, J. Childhood cancer incidence and survival in Spain. Ann. Oncol. 2010, 21 (Suppl. S3), iii103–iii110. [Google Scholar] [CrossRef]
- Jaffe, N. Historical perspective on the introduction and use of chemotherapy for the treatment of osteosarcoma. Adv. Exp. Med. Biol. 2014, 804, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Sanna, V.; Pala, N.; Sechi, M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Aryal, S.; Zhang, L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010, 1, 323–334. [Google Scholar] [CrossRef]
- Livney, Y.D.; Assaraf, Y.G. Rationally designed nanovehicles to overcome cancer chemoresistance. Adv. Drug Deliv. Rev. 2013, 65, 1716–1730. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Raghuvanshi, S. Oral bioavailability: Issues and solutions via nanoformulations. Clin. Pharmacokinet. 2015, 54, 325–357. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef] [Green Version]
- De Mendoza, A.E.-H.; Campanero, M.A.; Mollinedo, F.; Blanco-Prieto, M.J. Lipid Nanomedicines for Anticancer Drug Therapy. J. Biomed. Nanotechnol. 2009, 5, 323–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasa-Saracibar, B.; de Mendoza, A.E.-H.; Guada, M.; Dios-Vieitez, C.; Blanco-Prieto, M.J. Lipid nanoparticles for cancer therapy: State of the art and future prospects. Expert Opin. Drug Deliv. 2012, 9, 1245–1261. [Google Scholar] [CrossRef] [Green Version]
- De Mendoza, A.E.-H.; Preat, V.; Mollinedo, F.; Blanco-Prieto, M.J. In vitro and in vivo efficacy of edelfosine-loaded lipid nanoparticles against glioma. J. Control. Release 2011, 156, 421–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- De Mendoza, A.E.-H.; Campanero, M.A.; Lana, H.; Villa-Pulgarin, J.A.; de la Iglesia-Vicente, J.; Mollinedo, F.; Blanco-Prieto, M.J. Complete inhibition of extranodal dissemination of lymphoma by edelfosine-loaded lipid nanoparticles. Nanomedicine 2012, 7, 679–690. [Google Scholar] [CrossRef]
- González-Fernández, Y.; Zalacain, M.; Imbuluzqueta, E.; Sierrasesumaga, L.; Patiño-García, A.; Blanco-Prieto, M.J. Lipid nanoparticles enhance the efficacy of chemotherapy in primary and metastatic human osteosarcoma cells. J. Drug Deliv. Sci. Technol. 2015, 30, 435–442. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, Y.; Imbuluzqueta, E.; Zalacain, M.; Mollinedo, F.; Patino-Garcia, A.; Blanco-Prieto, M.J. Doxorubicin and edelfosine lipid nanoparticles are effective acting synergistically against drug-resistant osteosarcoma cancer cells. Cancer Lett. 2017, 388, 262–268. [Google Scholar] [CrossRef]
- Kang, K.W.; Chun, M.-K.; Kim, O.; Subedi, R.K.; Ahn, S.-G.; Yoon, J.-H.; Choi, H.-K. Doxorubicin-loaded solid lipid nanoparticles to overcome multidrug resistance in cancer therapy. Nanomedicine 2010, 6, 210–213. [Google Scholar] [CrossRef]
- Wong, H.L.; Rauth, A.M.; Bendayan, R.; Manias, J.L.; Ramaswamy, M.; Liu, Z.; Erhan, S.Z.; Wu, X.Y. A new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm. Res. 2006, 23, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Vandita, K.; Shashi, B.; Santosh, K.G.; Pal, K.I. Enhanced apoptotic effect of curcumin loaded solid lipid nanoparticles. Mol. Pharm. 2012, 9, 3411–3421. [Google Scholar] [CrossRef]
- Puglia, C.; Frasca, G.; Musumeci, T.; Rizza, L.; Puglisi, G.; Bonina, F.; Chiechio, S. Curcumin loaded NLC induces histone hypoacetylation in the CNS after intraperitoneal administration in mice. Eur. J. Pharm. Biopharm. 2012, 81, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Cai, S.; Yang, Q.; Bagby, T.R.; Forrest, M.L. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Wissing, S.A.; Muller, R.H. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity—In vivo study. Eur. J. Pharm. Biopharm. 2003, 56, 67–72. [Google Scholar] [CrossRef]
- Kumar, S.; Dilbaghi, N.; Saharan, R.; Bhanjana, G. Nanotechnology as Emerging Tool for Enhancing Solubility of Poorly Water-Soluble Drugs. Bionanoscience 2012, 2, 227–250. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C 2016, 68, 982–994. [Google Scholar] [CrossRef]
- Hu, F.Q.; Hong, Y.; Yuan, H. Preparation and characterization of solid lipid nanoparticles containing peptide. Int. J. Pharm. 2004, 273, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Han, L.; Deng, L.; Zhang, Y.; Shen, H.; Gong, T.; Zhang, Z.; Sun, X. Dual drugs (microRNA-34a and paclitaxel)-loaded functional solid lipid nanoparticles for synergistic cancer cell suppression. J. Control. Release 2014, 194, 228–237. [Google Scholar] [CrossRef]
- Fadda, P.; Monduzzi, M.; Caboi, F.; Piras, S.; Lazzari, P. Solid lipid nanoparticle preparation by a warm microemulsion based process: Influence of microemulsion microstructure. Int. J. Pharm. 2013, 446, 166–175. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, F.; Bu, H.; Xiao, J.; Li, Y. Solid lipid nanoparticles loading candesartan cilexetil enhance oral bioavailability: In vitro characteristics and absorption mechanism in rats. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 740–747. [Google Scholar] [CrossRef]
- Dwivedi, P.; Khatik, R.; Khandelwal, K.; Taneja, I.; Raju, K.S.R.; Wahajuddin; Paliwal, S.K.; Dwivedi, A.K.; Mishra, P.R. Pharmacokinetics study of arteether loaded solid lipid nanoparticles: An improved oral bioavailability in rats. Int. J. Pharm. 2014, 466, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Teskac, K.; Kristl, J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010, 390, 61–69. [Google Scholar] [CrossRef]
- Jain, A.K.; Jain, A.; Garg, N.K.; Agarwal, A.; Jain, A.; Jain, S.A.; Tyagi, R.K.; Jain, R.K.; Agrawal, H.; Agrawal, G.P. Adapalene loaded solid lipid nanoparticles gel: An effective approach for acne treatment. Colloids Surf. B Biointerfaces 2014, 121, 222–229. [Google Scholar] [CrossRef]
- Severino, P.; Andreani, T.; Jäger, A.; Chaud, M.V.; Santana, M.H.A.; Silva, A.M.; Souto, E.B. Solid lipid nanoparticles for hydrophilic biotech drugs: Optimization and cell viability studies (Caco-2 & HEPG-2 cell lines). Eur. J. Med. Chem. 2014, 81, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Singh, K.K.; Müller, R.H. Production & stability of stavudine solid lipid nanoparticles—From lab to industrial scale. Int. J. Pharm. 2011, 416, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.P.; Manjunath, K.; Bhagawati, S.T.; Thippeswamy, B.S. Bixin loaded solid lipid nanoparticles for enhanced hepatoprotection--preparation, characterisation and in vivo evaluation. Int. J. Pharm. 2014, 473, 485–492. [Google Scholar] [CrossRef]
- Liu, D.; Liu, C.; Zou, W.; Zhang, N. Enhanced gastrointestinal absorption of N3-O-toluyl-fluorouracil by cationic solid lipid nanoparticles. J. Nanoparticle Res. 2010, 12, 975–984. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.; Wang, L.; Zhang, C.; Zhang, N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf. B Biointerfaces 2011, 85, 262–269. [Google Scholar] [CrossRef]
- Liu, C.; Liu, D.; Bai, F.; Zhang, J.; Zhang, N. In vitro and in vivo studies of lipid-based nanocarriers for oral N3-o-toluyl-fluorouracil delivery. Drug Deliv. 2010, 17, 352–363. [Google Scholar] [CrossRef]
- Varshosaz, J.; Minayian, M.; Moazen, E. Enhancement of oral bioavailability of pentoxifylline by solid lipid nanoparticles. J. Liposome Res. 2010, 20, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.F.; Chen, D.W.; Ren, L.X.; Zhao, X.L.; Qin, J. Solid lipid nanoparticles for enhancing vinpocetine’s oral bioavailability. J. Control. Release 2006, 114, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.-F.; Yuan, M.; Chen, M.-S.; Liu, S.-M.; Zhu, L.; Huang, B.-Y.; Liu, X.-W.; Xiong, W. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. Int. J. Pharm. 2011, 410, 138–144. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef]
- Kheradmandnia, S.; Vasheghani-Farahani, E.; Nosrati, M.; Atyabi, F. Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 753–759. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.-J.; Peng, D.-Y.; Li, Q.-L.; Wang, X.-S.; Wang, D.-L.; Chen, W.-D. Solid lipid nanoparticles as delivery systems for Gambogenic acid. Colloids Surf. B Biointerfaces 2013, 102, 391–397. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Wang, C.-C. Cationic solid lipid nanoparticles with primary and quaternary amines for release of saquinavir and biocompatibility with endothelia. Colloids Surf. B Biointerfaces 2013, 101, 101–105. [Google Scholar] [CrossRef]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H. Preparation of solid lipid nanoparticles as drug carriers for levothyroxine sodium with in vitro drug delivery kinetic characterization. Mol. Biol. Rep. 2014, 41, 3521–3527. [Google Scholar] [CrossRef] [PubMed]
- Venishetty, V.K.; Komuravelli, R.; Kuncha, M.; Sistla, R.; Diwan, P.V. Increased brain uptake of docetaxel and ketoconazole loaded folate-grafted solid lipid nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 111–121. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the Antitumor Activity of Berberine Hydrochloride by Solid Lipid Nanoparticle Encapsulation. AAPS PharmSciTech 2014, 15, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zeng, S.; Jia, H.; He, X.; Fang, Y.; Jing, Z.; Cai, X. Adjuvant effect enhancement of porcine interleukin-2 packaged into solid lipid nanoparticles. Res. Vet. Sci. 2014, 96, 62–68. [Google Scholar] [CrossRef]
- Nafee, N.; Husari, A.; Maurer, C.K.; Lu, C.; de Rossi, C.; Steinbach, A.; Hartmann, R.W.; Lehr, C.-M.; Schneider, M. Antibiotic-free nanotherapeutics: Ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J. Control. Release 2014, 192, 131–140. [Google Scholar] [CrossRef]
- Ravi, P.R.; Vats, R.; Dalal, V.; Murthy, A.N. A hybrid design to optimize preparation of lopinavir loaded solid lipid nanoparticles and comparative pharmacokinetic evaluation with marketed lopinavir/ritonavir coformulation. J. Pharm. Pharmacol. 2014, 66, 912–926. [Google Scholar] [CrossRef]
- Soares, S.; Fonte, P.; Costa, A.; Andrade, J.; Seabra, V.; Ferreira, D.; Reis, S.; Sarmento, B. Effect of freeze-drying, cryoprotectants and storage conditions on the stability of secondary structure of insulin-loaded solid lipid nanoparticles. Int. J. Pharm. 2013, 456, 370–381. [Google Scholar] [CrossRef]
- Xie, S.; Zhu, L.; Dong, Z.; Wang, X.; Wang, Y.; Li, X.; Zhou, W. Preparation, characterization and pharmacokinetics of enrofloxacin-loaded solid lipid nanoparticles: Influences of fatty acids. Colloids Surf. B Biointerfaces 2011, 83, 382–387. [Google Scholar] [CrossRef]
- Zariwala, M.G.; Elsaid, N.; Jackson, T.L.; López, F.C.; Farnaud, S.; Somavarapu, S.; Renshaw, D. A novel approach to oral iron delivery using ferrous sulphate loaded solid lipid nanoparticles. Int. J. Pharm. 2013, 456, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Bhise, N.S.; Ribas, J.; Manoharan, V.; Zhang, Y.S.; Polini, A.; Massa, S.; Dokmeci, M.R.; Khademhosseini, A. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release 2014, 190, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.U.; Serra, C.A.; Anton, N.; Vandamme, T. Microfluidics: A focus on improved cancer targeted drug delivery systems. J. Control. Release 2013, 172, 1065–1074. [Google Scholar] [CrossRef]
- Riahi, R.; Tamayol, A.; Shaegh, S.A.M.; Ghaemmaghami, A.M.; Dokmeci, M.R.; Khademhosseini, A. Microfluidics for advanced drug delivery systems. Curr. Opin. Chem. Eng. 2015, 7, 101–112. [Google Scholar] [CrossRef]
- Rivet, C.; Lee, H.; Hirsch, A.; Hamilton, S.; Lu, H. Microfluidics for medical diagnostics and biosensors. Chem. Eng. Sci. 2011, 66, 1490–1507. [Google Scholar] [CrossRef]
- Streets, A.M.; Huang, Y. Microfluidics for biological measurements with single-molecule resolution. Curr. Opin. Biotechnol. 2014, 25, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Tarn, M.D.; Pamme, N. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780124095472. [Google Scholar]
- Mitxelena-Iribarren, O.; Hisey, C.L.; Errazquin-Irigoyen, M.; Gonzalez-Fernandez, Y.; Imbuluzqueta, E.; Mujika, M.; Blanco-Prieto, M.J.; Arana, S. Effectiveness of nanoencapsulated methotrexate against osteosarcoma cells: In vitro cytotoxicity under dynamic conditions. Biomed. Microdevices 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Current developments and applications of microfluidic technology toward clinical translation of nanomedicines. Adv. Drug Deliv. Rev. 2017. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Huang, T.J. Microfluidic diagnostics for the developing world. Lab Chip 2012, 12, 1412. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Khademhosseini, A.; Jon, S.; Hermmann, A.; Cheng, J.; Chin, C.; Kiselyuk, A.; Teply, B.; Eng, G.; Langer, R. Microfluidic system for studying the interaction of nanoparticles and microparticles with cells. Anal. Chem. 2005, 77, 5453–5459. [Google Scholar] [CrossRef]
- Gomez-Aranzadi, M.; Arana, S.; Mujika, M.; Hansford, D. Integrated Microstructures to Improve Surface-Sample Interaction in Planar Biosensors. IEEE Sens. J. 2015, 15, 1216–1223. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, Y.; Hao, Y.; Li, E.; Wang, Y.; Zhang, J.; Wang, W.; Gao, Z.; Wang, Q. Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials 2013, 34, 4109–4117. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.H.; Kim, Y.-C.; Choi, I.; Sung, J.H. Fabrication and characterization of microfluidic liver-on-a-chip using microsomal enzymes. Enzyme Microb. Technol. 2013, 53, 159–164. [Google Scholar] [CrossRef]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef]
- Mujika Garmendia, M.; Benavente-Babace, A.; González-Fernández, Y.; Imbuluzqueta Iturbura, E.; Pérez-Lorenzo, E.; Arana Alonso, S.; Blanco-Prieto, M.J. Plataforma microfluídica para estudios celulares dinámicos in vitro. In Proceedings of the Congreso Anual de la Sociedad Española de Ingeniería Biomédica CASEIB 2014, Barcelona, Spain, 26–28 November 2014. [Google Scholar]
- National Center for Biotechnology Information PubChem Compound Database, CID=443315. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/443315#section=Top (accessed on 29 November 2017).
- National Center for Biotechnology Information PubChem Compound Database, CID=11199. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11199 (accessed on 29 November 2017).
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of Nanoparticles Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Wang, J.; Feng, Q.; Liu, D.; Yin, Q.; Xu, D.; Wei, Y.; Ding, B.; Shi, X.; et al. Tunable Rigidity of (Polymeric Core)–(Lipid Shell) Nanoparticles for Regulated Cellular Uptake. Adv. Mater. 2015, 27, 1402–1407. [Google Scholar] [CrossRef]




| MTX Concentration | Free MTX | MTX-LEC-PVA Nanoparticles | MTX-LEC-Tween Nanoparticles |
|---|---|---|---|
| 15 µM | 6.82 µg/mL | 262.6 µg/mL | 343.5 µg/mL |
| 150 µM | 68.2 µg/mL | 2.626 mg/mL | 3.435 mg/mL |
| 1.5 mM | 682 µg/mL | 26.26 mg/mL | 34.35 mg/mL |
| E | Nanoparticle Type | 15 µM | 150 µM | ||
|---|---|---|---|---|---|
| 50 µm | 100 µm | 50 µm | 100 µm | ||
| e1 | LEC-PVA | 72.4% | 78.8% | 84.7% | 84.2% |
| LEC-Tween | 70.2% | 61.3% | 90.2% | 88.1% | |
| e2 | LEC-PVA | 27.6% | 21.2% | 15.3% | 15.8% |
| LEC-Tween | 29.8% | 38.7% | 9.8% | 11.9% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitxelena-Iribarren, O.; Lizarbe-Sancha, S.; Campisi, J.; Arana, S.; Mujika, M. Different Microfluidic Environments for In Vitro Testing of Lipid Nanoparticles against Osteosarcoma. Bioengineering 2021, 8, 77. https://doi.org/10.3390/bioengineering8060077
Mitxelena-Iribarren O, Lizarbe-Sancha S, Campisi J, Arana S, Mujika M. Different Microfluidic Environments for In Vitro Testing of Lipid Nanoparticles against Osteosarcoma. Bioengineering. 2021; 8(6):77. https://doi.org/10.3390/bioengineering8060077
Chicago/Turabian StyleMitxelena-Iribarren, Oihane, Sara Lizarbe-Sancha, Jay Campisi, Sergio Arana, and Maite Mujika. 2021. "Different Microfluidic Environments for In Vitro Testing of Lipid Nanoparticles against Osteosarcoma" Bioengineering 8, no. 6: 77. https://doi.org/10.3390/bioengineering8060077
APA StyleMitxelena-Iribarren, O., Lizarbe-Sancha, S., Campisi, J., Arana, S., & Mujika, M. (2021). Different Microfluidic Environments for In Vitro Testing of Lipid Nanoparticles against Osteosarcoma. Bioengineering, 8(6), 77. https://doi.org/10.3390/bioengineering8060077






