Effects of Different Titanium Surfaces Created by 3D Printing Methods, Particle Sizes, and Acid Etching on Protein Adsorption and Cell Adhesion, Proliferation, and Differentiation
Abstract
:1. Introduction
2. Materials and Methods
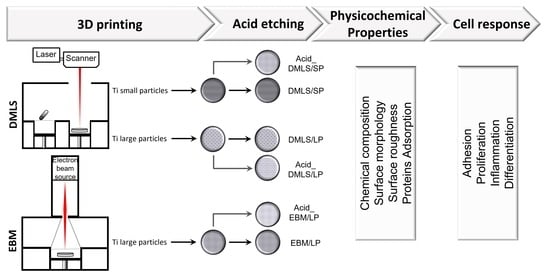
2.1. Fabrication of 3D Printed Ti Discs
- DMLS/SP: the disc printed by DMLS with small particle powders Ti6Al4V (average size of 22.5 µm);
- DMLS/LP: the disc printed by DMLS with large particle powders Ti6Al4V (average size of 100 µm);
- EBM/LP: the disc printed by EBM with large particle powders Ti6Al4V (average size of 100 µm).
2.2. Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Spectroscopy (EDX), and Surface Roughness
2.2.1. SEM and EDX Analysis
2.2.2. Surface Roughness
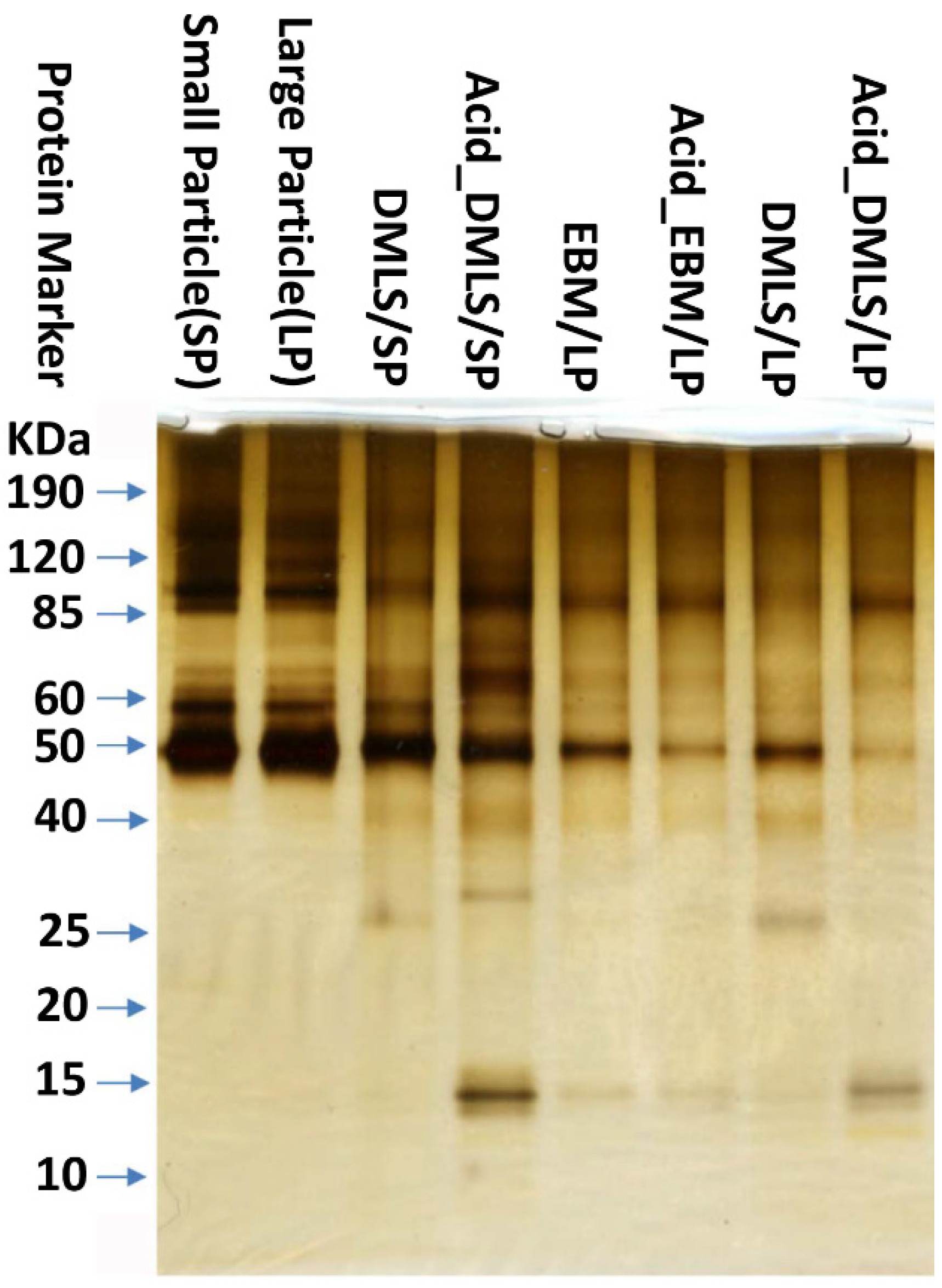
2.3. Protein Adsorption
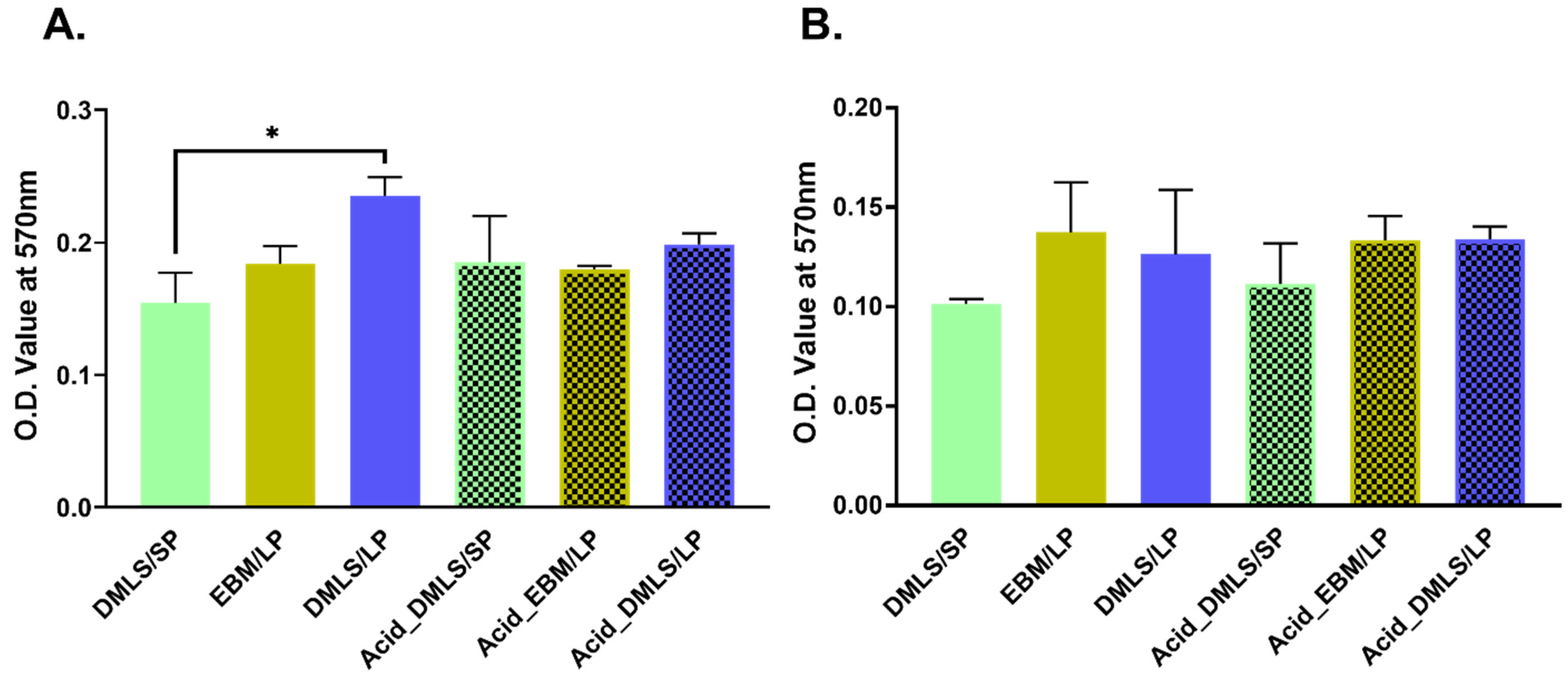
2.4. Cell Adhesion
2.5. Cell Proliferation
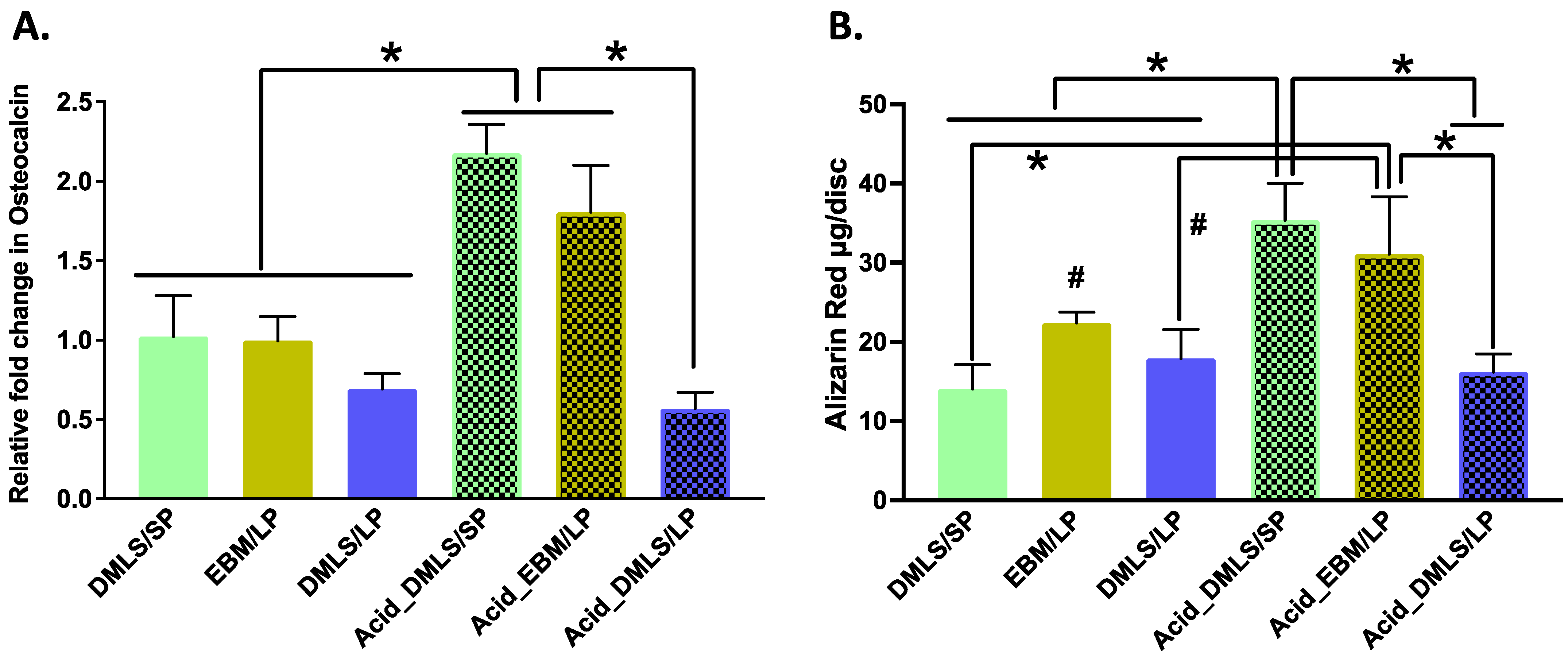
2.6. Osteocalcin Gene Expression and Alizarin Red Measurement
2.6.1. Osteocalcin Gene Expression
2.6.2. Alizarin Red Staining
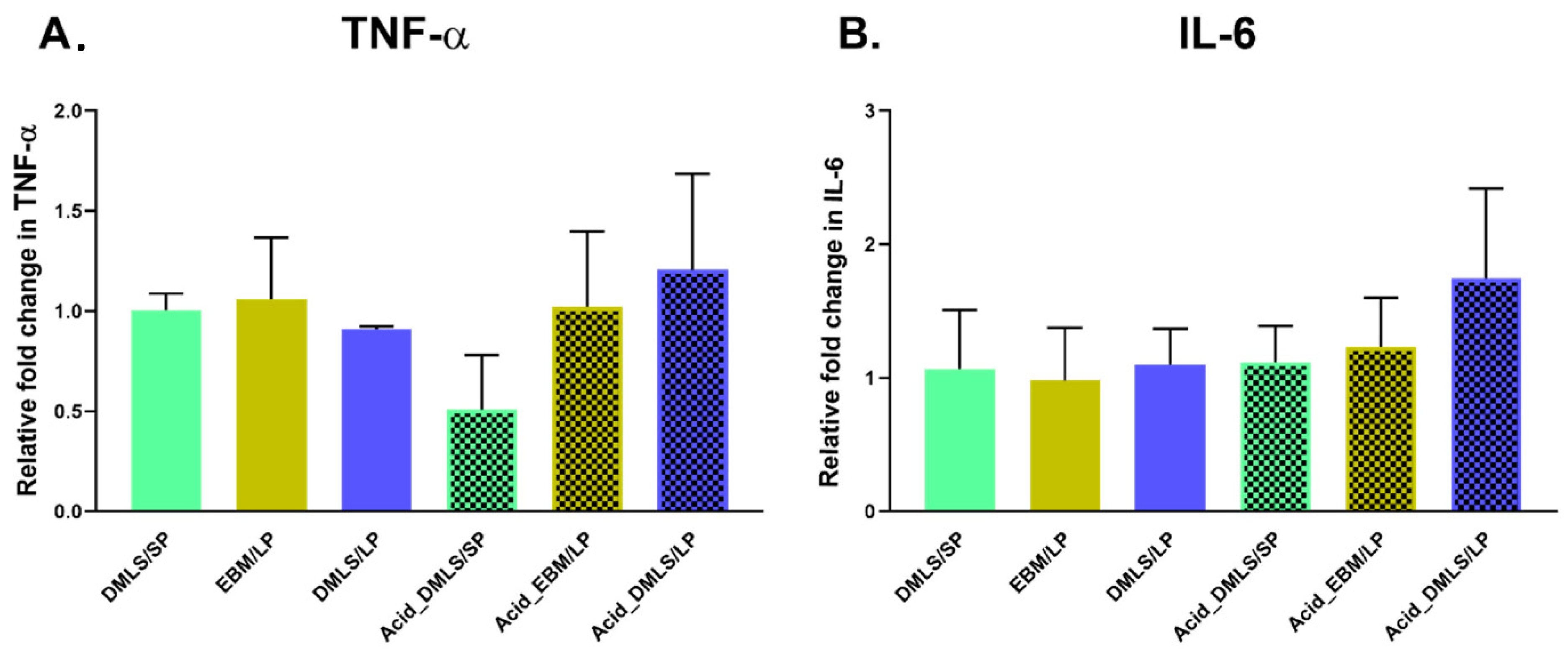
2.7. TNF-α and IL-6 Genes Expression
2.8. Statistics Analysis
3. Results
3.1. Ultramorphology, Chemical Composition, and Surface Roughness
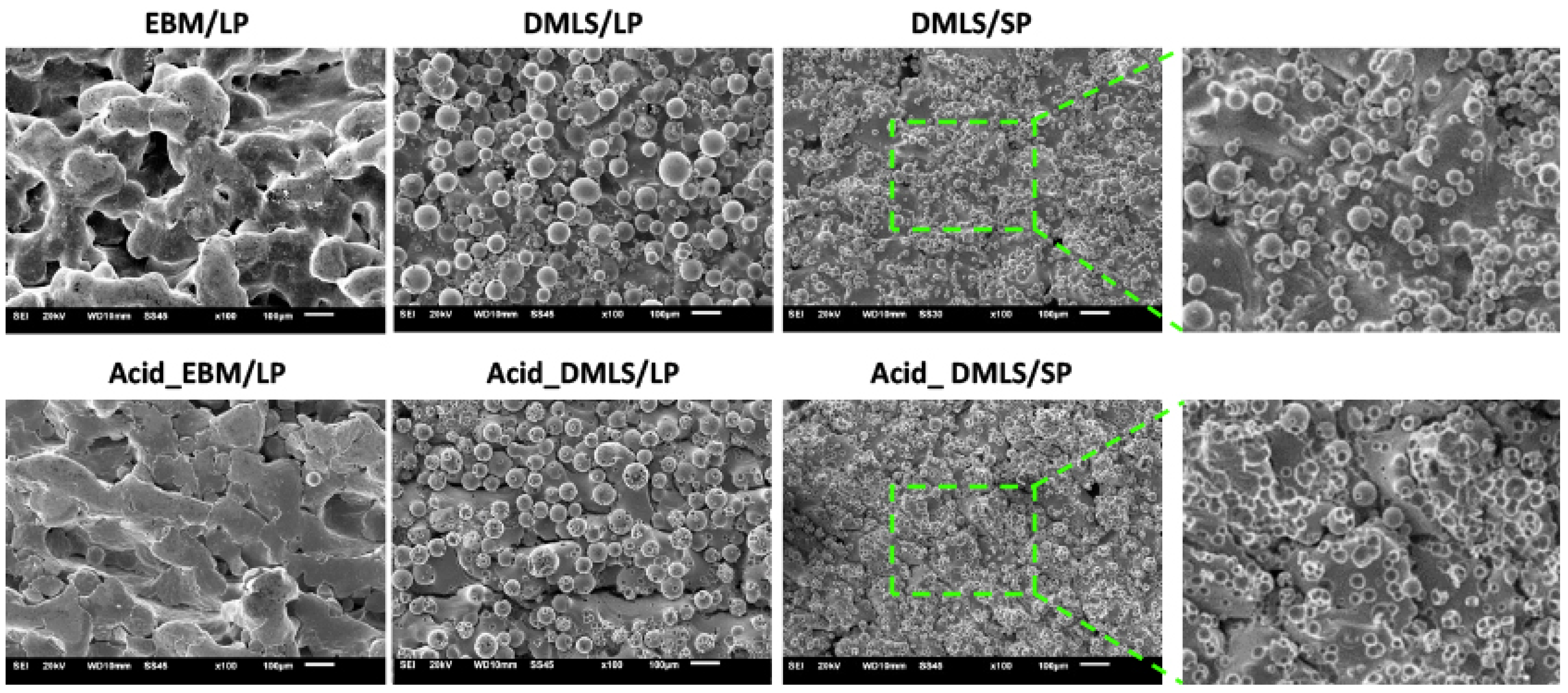
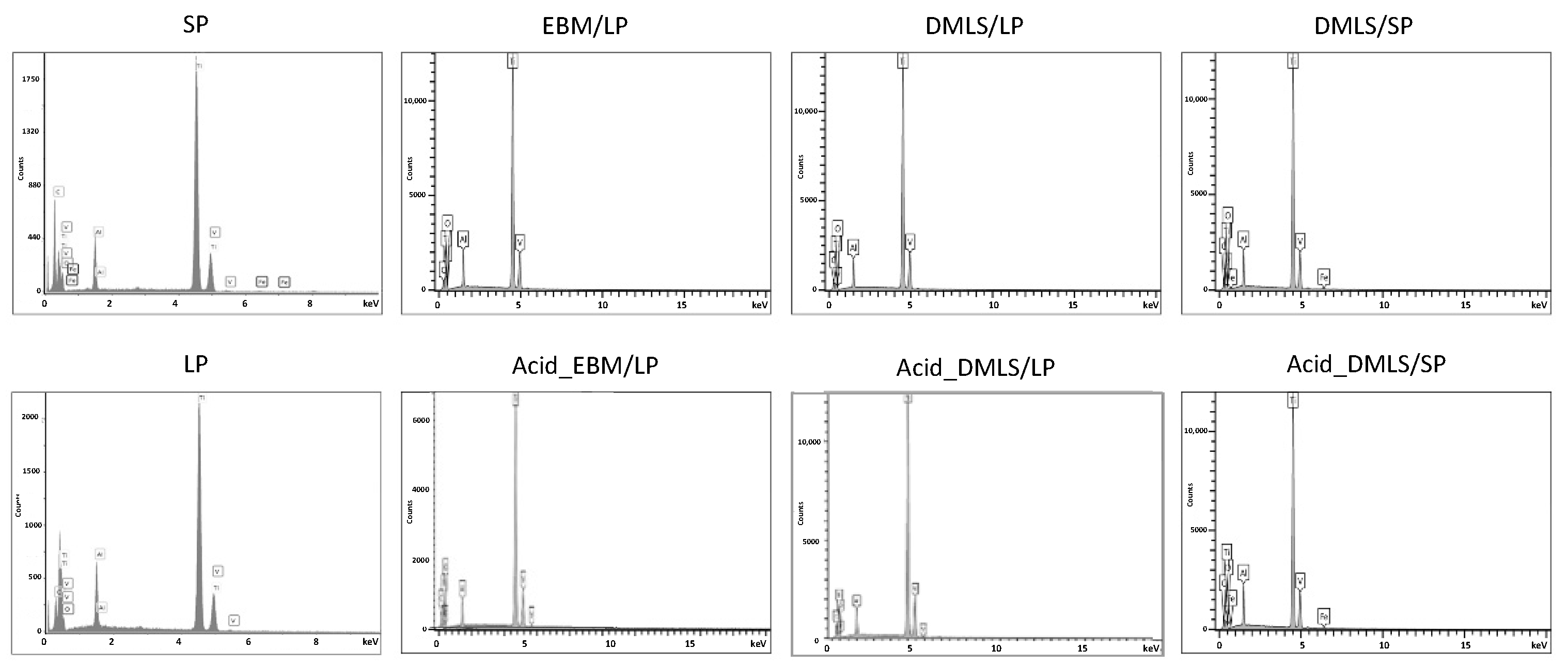
3.1.1. SEM and EDX Analysis
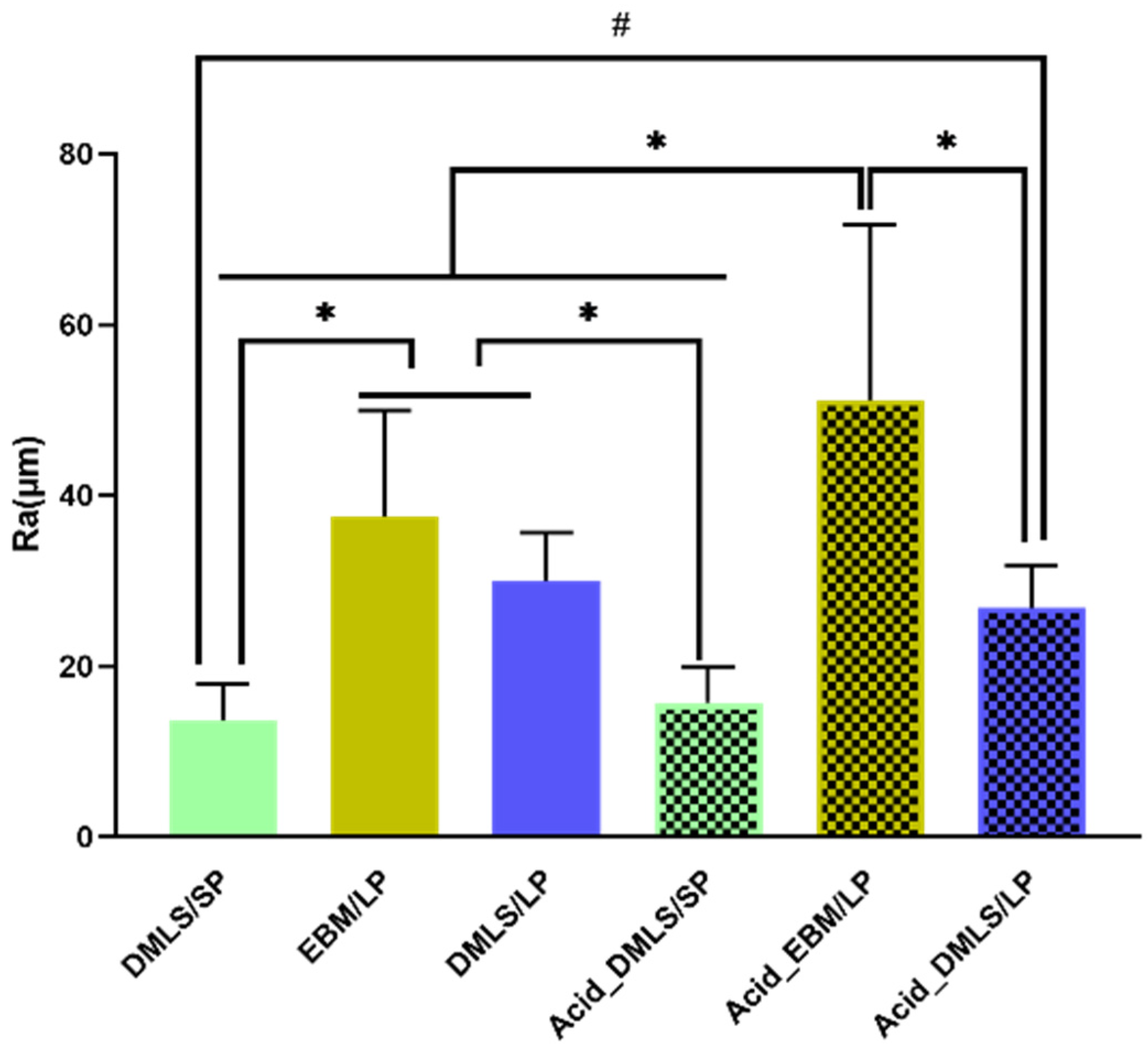
3.1.2. Surface Roughness
3.2. Protein Adsorption
3.3. Cell Adhesion and Proliferation
3.4. TNF-α and IL-6 Gene Expressions
3.5. Mineralization and Nodule Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Ong, K.L.; Schmier, J.; Mowat, F.; Saleh, K.; Dybvik, E.; Karrholm, J.; Garellick, G.; Havelin, L.I.; Furnes, O.; et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J. Bone Jt. Surg. Am. 2007, 89 (Suppl. 3), 144–151. [Google Scholar] [CrossRef]
- Sadoghi, P.; Liebensteiner, M.; Agreiter, M.; Leithner, A.; Bohler, N.; Labek, G. Revision surgery after total joint arthroplasty: A complication-based analysis using worldwide arthroplasty registers. J. Arthroplast. 2013, 28, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.; Roebuck, K.A.; Archibeck, M.; Hallab, N.J.; Glant, T.T. Osteolysis: Basic science. Clin. Orthop. Relat. Res. 2001, 71–77. [Google Scholar] [CrossRef]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodapour, J.; Montazerian, H.; Darabi, A.; Anaraki, A.P.; Ahmadi, S.M.; Zadpoor, A.A.; Schmauder, S. Failure mechanisms of additively manufactured porous biomaterials: Effects of porosity and type of unit cell. J. Mech. Behav. Biomed. Mater. 2015, 50, 180–191. [Google Scholar] [CrossRef]
- Bari, K.; Arjunan, A. Extra low interstitial titanium based fully porous morphological bone scaffolds manufactured using selective laser melting. J. Mech. Behav. Biomed. Mater. 2019, 95, 1–12. [Google Scholar] [CrossRef]
- Horn, T.J.; Harrysson, O.L. Overview of current additive manufacturing technologies and selected applications. Sci. Prog. 2012, 95, 255–282. [Google Scholar] [CrossRef]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Dourado, N.; Pereira, F.; Alves, N.; Miranda, G.; Silva, F.S. Additive manufactured porous biomaterials targeting orthopedic implants: A suitable combination of mechanical, physical and topological properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110342. [Google Scholar] [CrossRef]
- Elias, C.; Lima, J.; Valiev, R.; Meyers, M. Biomedical applications of titanium and its alloys. Jom 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Schiff, N.; Grosgogeat, B.; Lissac, M.; Dalard, F. Influence of fluoride content and pH on the corrosion resistance of titanium and its alloys. Biomaterials 2002, 23, 1995–2002. [Google Scholar] [CrossRef]
- Sommer, U.; Laurich, S.; de Azevedo, L.; Viehoff, K.; Wenisch, S.; Thormann, U.; Alt, V.; Heiss, C.; Schnettler, R. In Vitro and In Vivo Biocompatibility Studies of a Cast and Coated Titanium Alloy. Molecules 2020, 25, 3399. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.K.; Armstrong, N.R. Electrochemical and Surface Analytical Characterization of Titanium and Titanium Hydride Thin Film Electrode Oxidation. J. Electrochem. Soc. 1978, 125, 1790. [Google Scholar] [CrossRef]
- Tuomi, J.T.; Bjorkstrand, R.V.; Pernu, M.L.; Salmi, M.V.; Huotilainen, E.I.; Wolff, J.E.; Vallittu, P.K.; Makitie, A.A. In vitro cytotoxicity and surface topography evaluation of additive manufacturing titanium implant materials. J. Mater. Sci. Mater. Med. 2017, 28, 53. [Google Scholar] [CrossRef]
- Haslauer, C.M.; Springer, J.C.; Harrysson, O.L.; Loboa, E.G.; Monteiro-Riviere, N.A.; Marcellin-Little, D.J. In vitro biocompatibility of titanium alloy discs made using direct metal fabrication. Med. Eng. Phys. 2010, 32, 645–652. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants-A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, B.; Liu, C.; Wang, C.; Tan, X.; Hu, M. A Comparison of Biocompatibility of a Titanium Alloy Fabricated by Electron Beam Melting and Selective Laser Melting. PLoS ONE 2016, 11, e0158513. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.; Qiao, N.; Wang, C.; Hu, M. Corrosion resistance characteristics of a Ti-6Al-4V alloy scaffold that is fabricated by electron beam melting and selective laser melting for implantation in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 832–841. [Google Scholar] [CrossRef]
- Tunchel, S.; Blay, A.; Kolerman, R.; Mijiritsky, E.; Shibli, J.A. 3D Printing/Additive Manufacturing Single Titanium Dental Implants: A Prospective Multicenter Study with 3 Years of Follow-Up. Int. J. Dent. 2016, 2016, 8590971. [Google Scholar] [CrossRef] [Green Version]
- Mangano, F.; Mangano, C.; Piattelli, A.; Iezzi, G. Histological Evidence of the Osseointegration of Fractured Direct Metal Laser Sintering Implants Retrieved after 5 Years of Function. Biomed. Res. Int. 2017, 2017, 9732136. [Google Scholar] [CrossRef] [PubMed]
- So, E.; Mandas, V.H.; Hlad, L. Large Osseous Defect Reconstruction Using a Custom Three-Dimensional Printed Titanium Truss Implant. J. Foot Ankle Surg. 2018, 57, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Sun, R.; Jia, Y.; Chen, X.; Liu, Y.; Oyang, H.; Feng, L. A new 3D printed titanium metal trabecular bone reconstruction system for early osteonecrosis of the femoral head. Medicine 2018, 97, e11088. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontol. 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Adell, R.; Lekholm, U.; Rockler, B.; Branemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral. Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Grossi-Oliveira, G.A.; Antunes, A.A.; Elias, C.N.; Wennerberg, A.; Sennerby, L.; Salata, L.A. Early Osseointegration Events on Neoss(R) ProActive and Bimodal Implants: A Comparison of Different Surfaces in an Animal Model. Clin. Implant. Dent. Relat. Res. 2015, 17, 1060–1072. [Google Scholar] [CrossRef]
- Rosa, M.B.; Albrektsson, T.; Francischone, C.E.; Schwartz Filho, H.O.; Wennerberg, A. The influence of surface treatment on the implant roughness pattern. J. Appl. Oral Sci. Rev. FOB 2012, 20, 550–555. [Google Scholar] [CrossRef]
- Townsend, A.; Senin, N.; Blunt, L.; Leach, R.K.; Taylor, J.S. Surface texture metrology for metal additive manufacturing: A review. Precis. Eng. 2016, 46, 34–47. [Google Scholar] [CrossRef]
- Bitar, D.; Parvizi, J. Biological response to prosthetic debris. World J. Orthop. 2015, 6, 172–189. [Google Scholar] [CrossRef]
- Buly, R.L.; Huo, M.H.; Salvati, E.; Brien, W.; Bansal, M. Titanium wear debris in failed cemented total hip arthroplasty. An analysis of 71 cases. J. Arthroplast. 1992, 7, 315–323. [Google Scholar] [CrossRef]
- Zhang, L.; Haddouti, E.M.; Welle, K.; Burger, C.; Wirtz, D.C.; Schildberg, F.A.; Kabir, K. The Effects of Biomaterial Implant Wear Debris on Osteoblasts. Front. Cell Dev. Biol. 2020, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, D.S.; Harrysson, O.L.A.; Marcellin-Little, D.J.; Abumoussa, S.; Dahners, L.E.; Weinhold, P.S. Osseointegration of Coarse and Fine Textured Implants Manufactured by Electron Beam Melting and Direct Metal Laser Sintering. 3D Print Addit. Manuf. 2017, 4, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Das, S. Numerical modeling of scanning laser-induced melting, vaporization and resolidification in metals subjected to step heat flux input. Int. J. Heat Mass Transf. 2004, 47, 4153–4164. [Google Scholar] [CrossRef]
- ResendeI, C.; LimaII, I.; GemelliIII, E.; GranjeiroIII, J.; SoaresI, G. Cell adhesion on different titanium-coated surfaces. Matéria 2010, 5, 368–391. [Google Scholar] [CrossRef]
- Miki, I.; Ishihara, N.; Otoshi, M.; Kase, H. Simple colorimetric cell-cell adhesion assay using MTT-stained leukemia cells. J. Immunol. Methods 1993, 164, 255–261. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Yang, C.; Li, Y.; Dai, Z. An overview of osteocalcin progress. J. Bone Miner. Metab. 2016, 34, 367–379. [Google Scholar] [CrossRef]
- Ginestra, P.; Ferraro, R.M.; Zohar-Hauber, K.; Abeni, A.; Giliani, S.; Ceretti, E. Selective Laser Melting and Electron Beam Melting of Ti6Al4V for Orthopedic Applications: A Comparative Study on the Applied Building Direction. Materials 2020, 13, 5584. [Google Scholar] [CrossRef]
- Trevisan, F.; Calignano, F.; Aversa, A.; Marchese, G.; Lombardi, M.; Biamino, S.; Ugues, D.; Manfredi, D. Additive manufacturing of titanium alloys in the biomedical field: Processes, properties and applications. J. Appl. Biomater. Funct. Mater. 2018, 16, 57–67. [Google Scholar] [CrossRef]
- Shan, L.; Kadhum, A.A.H.; Al-Furjan, M.S.H.; Weng, W.; Gong, Y.; Cheng, K.; Zhou, M.; Dong, L.; Chen, G.; Takriff, M.S.; et al. In Situ Controlled Surface Microstructure of 3D Printed Ti Alloy to Promote Its Osteointegration. Materials 2019, 12, 815. [Google Scholar] [CrossRef] [Green Version]
- Weissmann, V.; Drescher, P.; Seitz, H.; Hansmann, H.; Bader, R.; Seyfarth, A.; Klinder, A.; Jonitz-Heincke, A. Effects of Build Orientation on Surface Morphology and Bone Cell Activity of Additively Manufactured Ti6Al4V Specimens. Materials 2018, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, B.; Idaszek, J.; Szlazak, K.; Strzelczyk, K.; Brynk, T.; Kurzydlowski, K.J.; Swieszkowski, W. Post Processing and Biological Evaluation of the Titanium Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Yang, D.; Maher, S.; Lima-Marques, L.; Zhou, Y.; Chen, Y.; Atkins, G.J.; Losic, D. Micro- and nano-structured 3D printed titanium implants with a hydroxyapatite coating for improved osseointegration. J. Mater. Chem. B 2018, 6, 3136–3144. [Google Scholar] [CrossRef] [PubMed]
- Skoog, S.A.; Kumar, G.; Narayan, R.J.; Goering, P.L. Biological responses to immobilized microscale and nanoscale surface topographies. Pharmacol. Ther. 2018, 182, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhou, C.; Hong, Y.; Zhang, X. A review of protein adsorption on bioceramics. Interface Focus 2012, 2, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.M.; Alabre, C.I.; Rubash, H.E.; Shanbhag, A.S. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: Analysis of multiple cytokines using protein arrays. J. Biomed. Mater. Res. A 2008, 84, 464–474. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Lee, E.M.; Smith, K.; Hyzy, S.L.; Doroudi, M.; Williams, J.K.; Gall, K.; Boyan, B.D.; Schwartz, Z. Substrate Stiffness Controls Osteoblastic and Chondrocytic Differentiation of Mesenchymal Stem Cells without Exogenous Stimuli. PLoS ONE 2017, 12, e0170312. [Google Scholar] [CrossRef]
- Shanbhag, A.S.; Kaufman, A.M.; Hayata, K.; Rubash, H.E. Assessing osteolysis with use of high-throughput protein chips. J. Bone Joint Surg. Am. 2007, 89, 1081–1089. [Google Scholar] [CrossRef]
- Ghassib, I.; Chen, Z.; Zhu, J.; Wang, H.L. Use of IL-1 beta, IL-6, TNF-alpha, and MMP-8 biomarkers to distinguish peri-implant diseases: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2019, 21, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Ingham, E.; Fisher, J. Biological reactions to wear debris in total joint replacement. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2000, 214, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Kaluderovic, M.R.; Mojic, M.; Schreckenbach, J.P.; Maksimovic-Ivanic, D.; Graf, H.L.; Mijatovic, S. A Key Role of Autophagy in Osteoblast Differentiation on Titanium-Based Dental Implants. Cells Tissues Organs. 2014, 200, 265–277. [Google Scholar] [CrossRef] [PubMed]

| Gene | Assay ID |
|---|---|
| GAPDH | Mm99999915_g1 |
| TNF-α | Mm00443258_m1 |
| IL-6 | Mm00446190_m1 |
| OCN | Mm00649782_gH |
| Elements | Atomic Percentage (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| SP | LP | EBM/LP | Acid_ EBM/LP | DMLS/LP | Acid_ DMLS/LP | DMLS/SP | Acid_ DMLS/SP | |
| Ti | 74.64 | 78.35 | 78.8 | 80.5 | 81.4 | 80.4 | 80.2 | 78.8 |
| Al | 4.12 | 6.12 | 5.9 | 4.2 | 4.7 | 4.7 | 6 | 6.5 |
| V | 3.98 | 3.91 | 3.4 | 3.7 | 3.6 | 4 | 4 | 3.8 |
| O | 6.24 | 4.18 | 5.5 | 4.8 | 4.1 | 5.2 | 4.7 | 5.3 |
| C | 10.53 | 7.45 | 6.5 | 6.8 | 6.1 | 5.7 | 4.3 | 5.1 |
| Fe | 0.5 | 0.8 | 0.5 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.; Chung, H.; Kwon, P.; Akkouch, A. Effects of Different Titanium Surfaces Created by 3D Printing Methods, Particle Sizes, and Acid Etching on Protein Adsorption and Cell Adhesion, Proliferation, and Differentiation. Bioengineering 2022, 9, 514. https://doi.org/10.3390/bioengineering9100514
Jin M, Chung H, Kwon P, Akkouch A. Effects of Different Titanium Surfaces Created by 3D Printing Methods, Particle Sizes, and Acid Etching on Protein Adsorption and Cell Adhesion, Proliferation, and Differentiation. Bioengineering. 2022; 9(10):514. https://doi.org/10.3390/bioengineering9100514
Chicago/Turabian StyleJin, Max, Haseung Chung, Patrick Kwon, and Adil Akkouch. 2022. "Effects of Different Titanium Surfaces Created by 3D Printing Methods, Particle Sizes, and Acid Etching on Protein Adsorption and Cell Adhesion, Proliferation, and Differentiation" Bioengineering 9, no. 10: 514. https://doi.org/10.3390/bioengineering9100514
APA StyleJin, M., Chung, H., Kwon, P., & Akkouch, A. (2022). Effects of Different Titanium Surfaces Created by 3D Printing Methods, Particle Sizes, and Acid Etching on Protein Adsorption and Cell Adhesion, Proliferation, and Differentiation. Bioengineering, 9(10), 514. https://doi.org/10.3390/bioengineering9100514