Glucose-Dependent Insulin Secretion from β Cell Spheroids Is Enhanced by Embedding into Softer Alginate Hydrogels Functionalised with RGD Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Husbandry
2.2. Islet Preparation
2.3. Formation of β Cell Spheroids
2.4. Preparation of Sodium Alginate Hydrogels
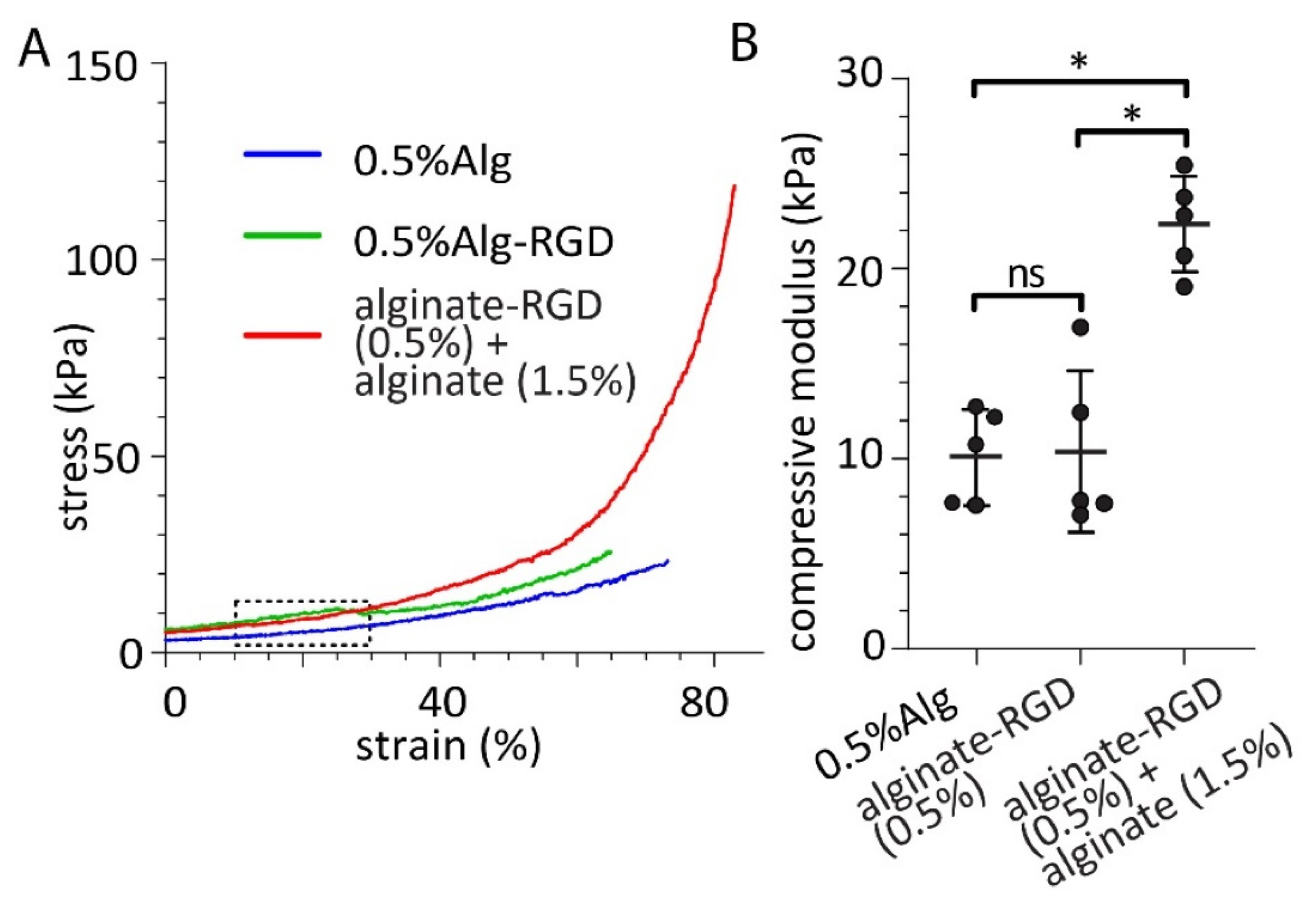
2.5. Mechanical Testing of Alginate Hydrogels
2.6. Glucose-Stimulated Insulin Secretion (GSIS) and HTRF Insulin Assay
2.7. Spheroid Fixation and Immunofluorescent Staining
2.8. Statistical Analyses
3. Results
3.1. Production of β Cell Spheroids of Consistent Diameter
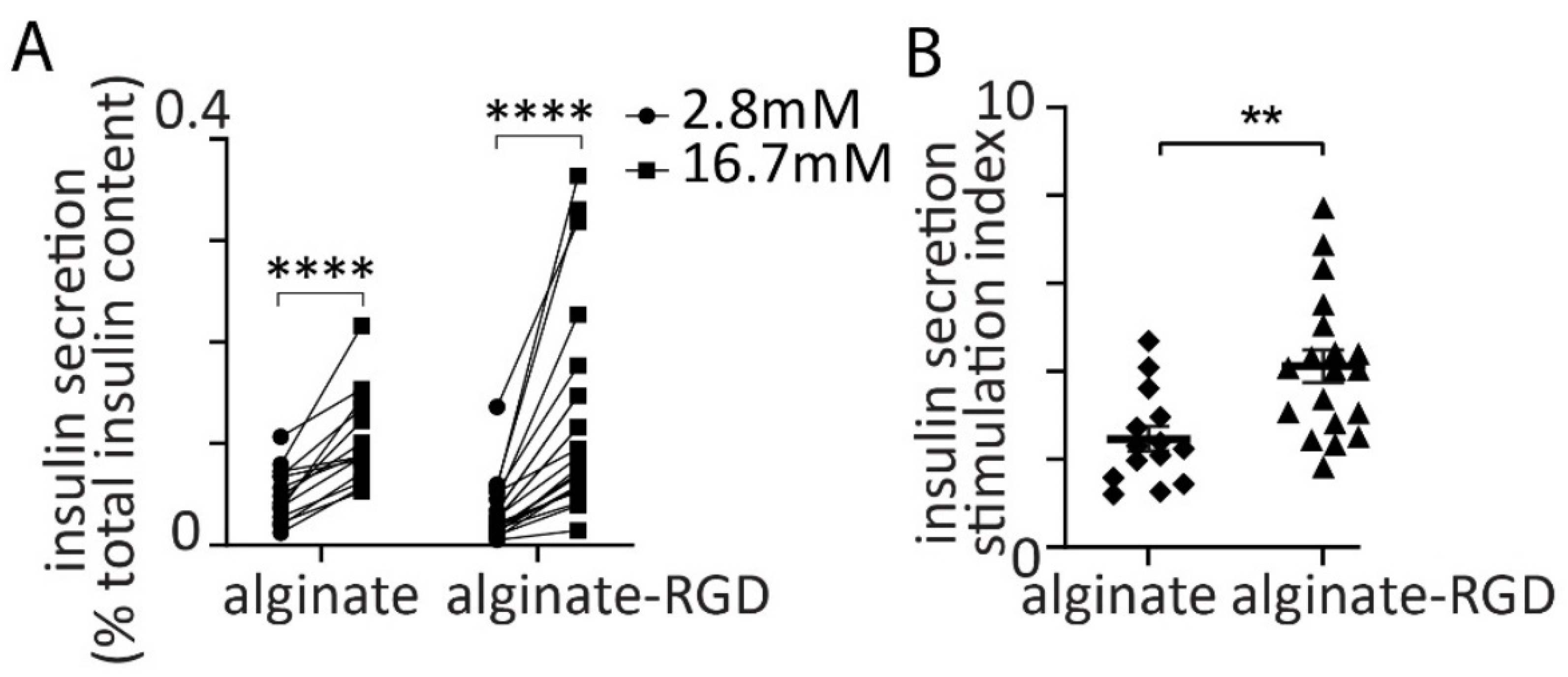
3.2. Glucose-Dependent Insulin Secretion from Spheroids
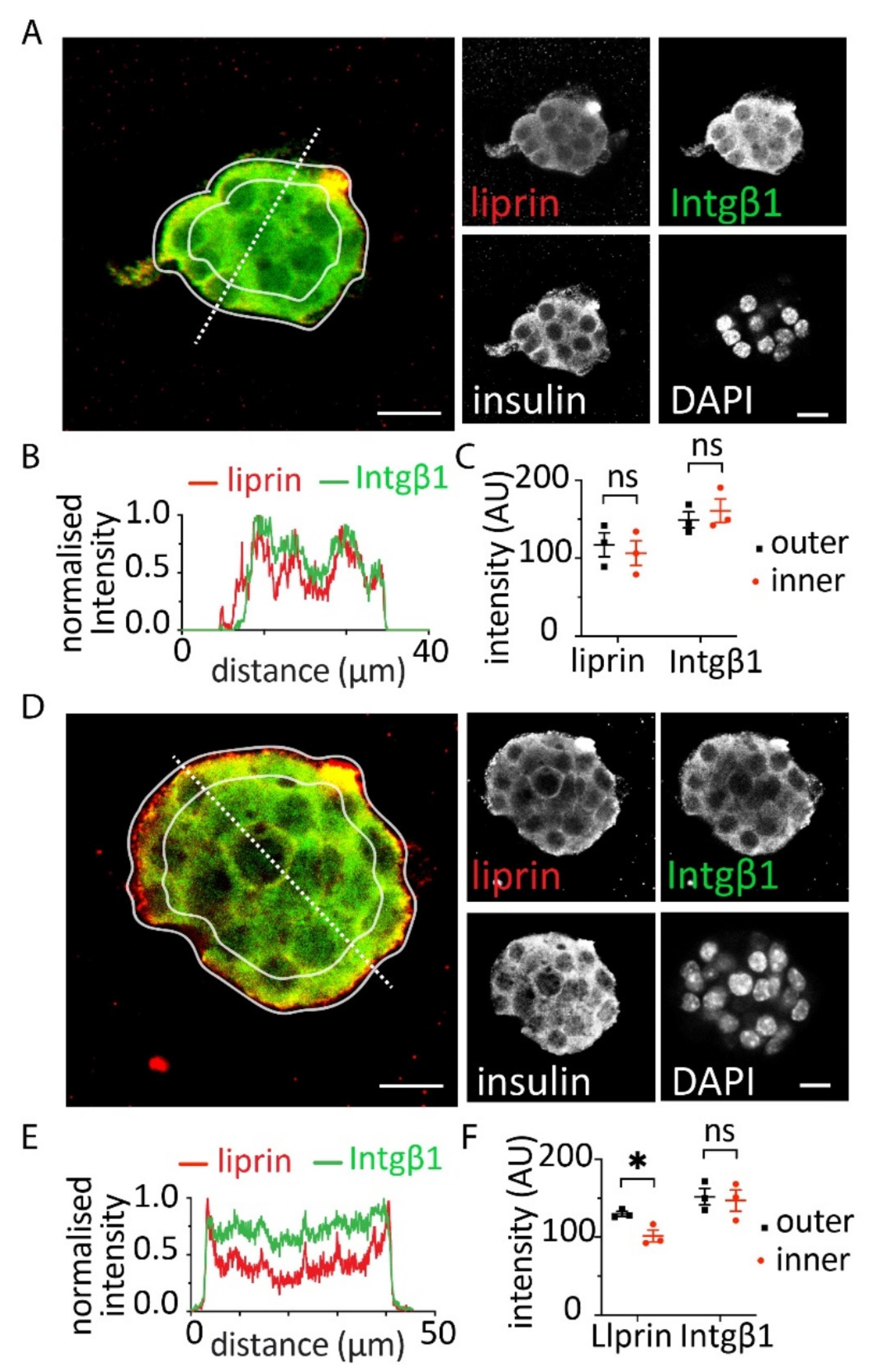
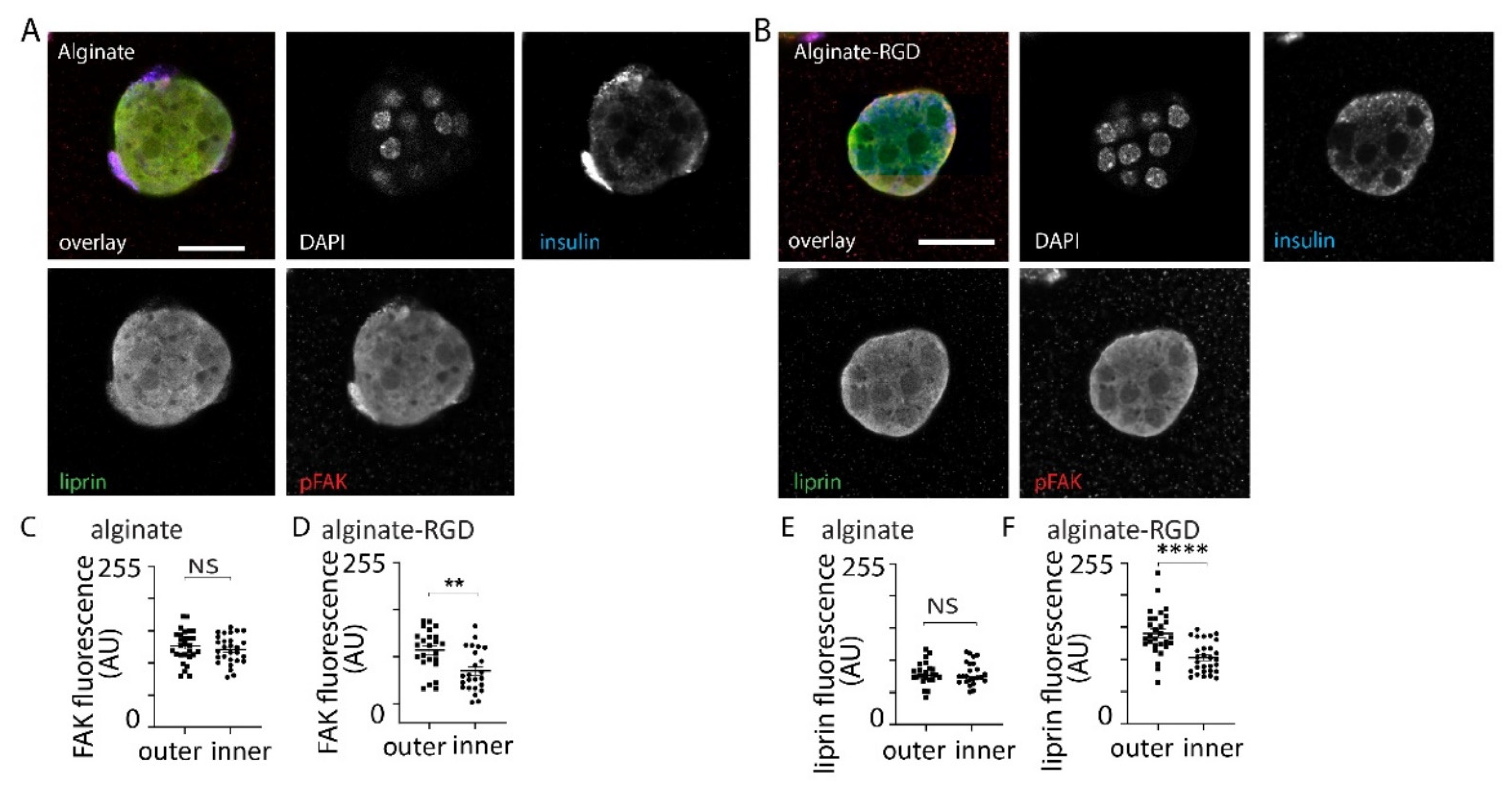
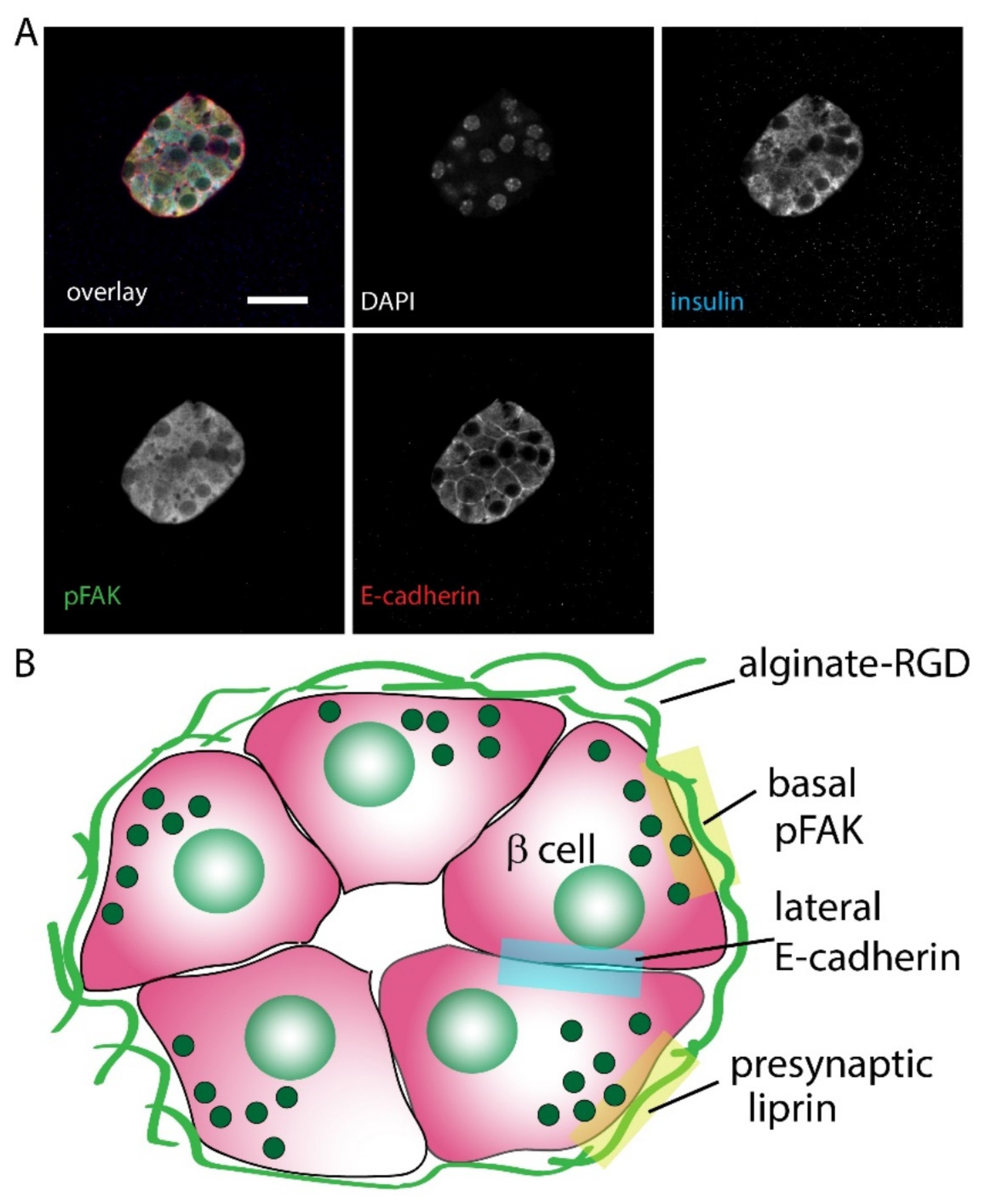
3.3. Effect of Alginates on β Cell Structure within Spheroids
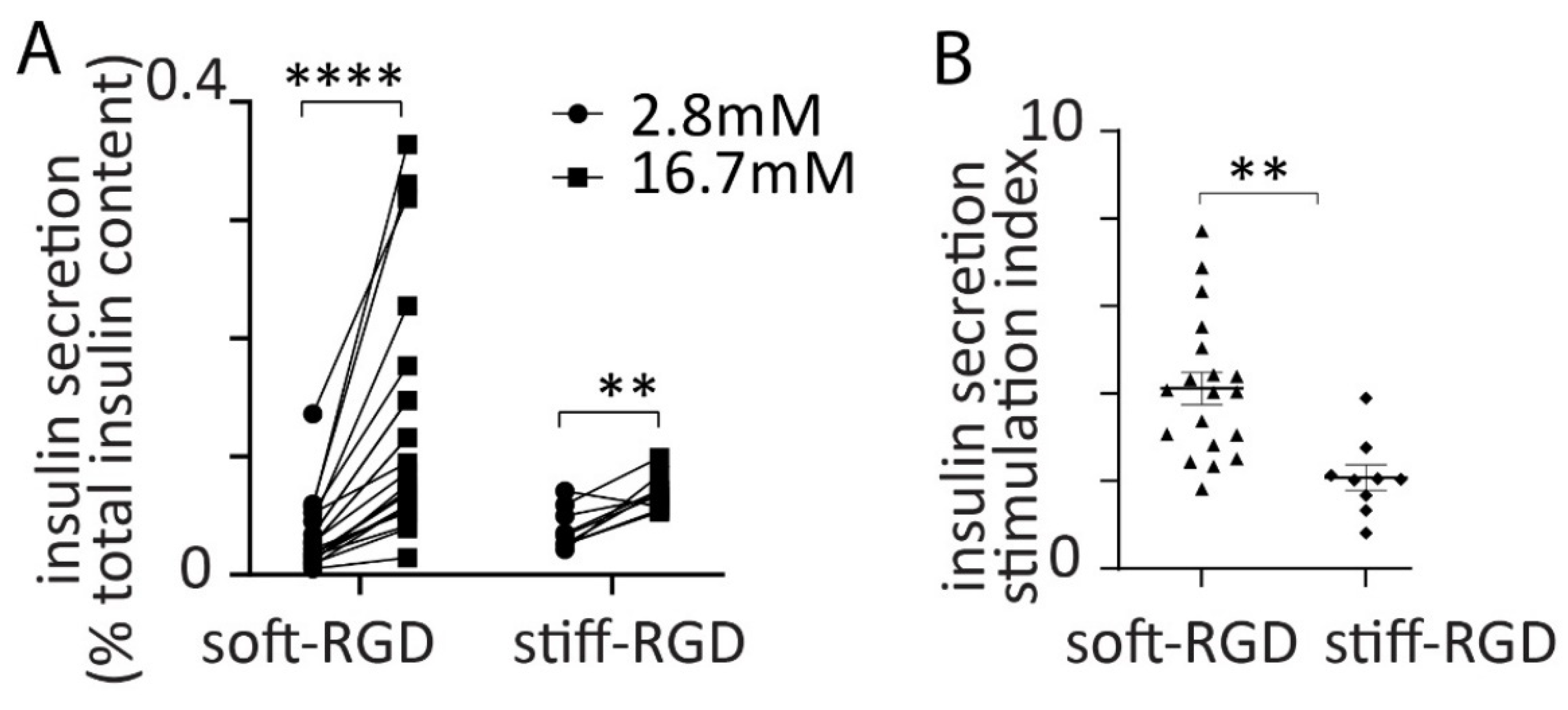
3.4. Effect of Alginate Stiffness on Glucose-Dependent Insulin Secretion
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Pagliuca, F.W.; Millman, J.R.; Gürtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic beta cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Hering, B.J.; Kandaswamy, R.; Ansite, J.D.; Eckman, P.M.; Nakano, M.; Sawada, T.; Matsumoto, I.; Ihm, S.H.; Zhang, H.J.; Parkey, J.; et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA 2005, 293, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Tomei, A.A.; Manzoli, V.; Fraker, C.A.; Giraldo, J.; Velluto, D.; Najjar, M.; Pileggi, A.; Molano, R.D.; Ricordi, C.; Stabler, C.L.; et al. Device design and materials optimization of conformal coating for islets of Langerhans. Proc. Natl. Acad. Sci. USA 2014, 111, 10514. [Google Scholar] [CrossRef] [PubMed]
- Vegas, A.J.; Veiseh, O.; Gurtler, M.; Millman, J.R.; Pagliuca, F.W.; Bader, A.R.; Doloff, J.C.; Li, J.; Chen, M.; Olejnik, K.; et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016, 22, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.; Shea, L.D. Advances in islet encapsulation technologies. Nat. Rev. Drug Discov. 2017, 16, 338–350. [Google Scholar] [CrossRef]
- Scharp, D.W.; Marchetti, P. Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution. Adv. Drug Deliv. Rev. 2014, 67–68, 35–73. [Google Scholar] [CrossRef]
- Lammert, E.; Thorn, P. The Role of the Islet Niche on Beta Cell Structure and Function. J. Mol. Biol. 2020, 432, 1407–1418. [Google Scholar] [CrossRef]
- Jevon, D.; Deng, K.; Hallahan, N.; Kumar, K.; Tong, J.; Gan, W.J.; Tran, C.; Bilek, M.; Thorn, P. Local activation of focal adhesion kinase orchestrates the positioning of presynaptic scaffold proteins and Ca2+ signalling to control glucose-dependent insulin secretion. eLife 2022, 11, e76262. [Google Scholar] [CrossRef]
- Benninger, R.K.P.; Head, W.S.; Zhang, M.; Satin, L.S.; Piston, D.W. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J. Physiol. 2011, 589, 5453–5466. [Google Scholar] [CrossRef]
- Diaferia, G.R.; Jimenez-Caliani, A.J.; Ranjitkar, P.; Yang, W.; Hardiman, G.; Rhodes, C.J.; Crisa, L.; Cirulli, V. beta1 integrin is a crucial regulator of pancreatic beta-cell expansion. Development 2013, 140, 3360–3372. [Google Scholar] [CrossRef]
- Parnaud, G.; Hammar, E.; Rouiller, D.G.; Armanet, M.; Halban, P.A.; Bosco, D. Blockade of beta1 integrin-laminin-5 interaction affects spreading and insulin secretion of rat beta-cells attached on extracellular matrix. Diabetes 2006, 55, 1413–1420. [Google Scholar] [CrossRef][Green Version]
- Gan, W.J.; Do, O.H.; Cottle, L.; Ma, W.; Kosobrodova, E.; Cooper-White, J.; Bilek, M.; Thorn, P. Local Integrin Activation in Pancreatic β Cells Targets Insulin Secretion to the Vasculature. Cell Rep. 2018, 24, 2819–2826.e2813. [Google Scholar] [CrossRef]
- Weber, L.M.; Hayda, K.N.; Anseth, K.S. Cell–Matrix Interactions Improve β-Cell Survival and Insulin Secretion in Three-Dimensional Culture. Tissue Eng. Part A 2008, 14, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Nyitray, C.E.; Chavez, M.G.; Desai, T.A. Compliant 3D microenvironment improves β-cell cluster insulin expression through mechanosensing and β-catenin signaling. Tissue Eng. Part A 2014, 20, 1888–1895. [Google Scholar] [CrossRef]
- Llacua, A.; de Haan, B.J.; Smink, S.A.; de Vos, P. Extracellular matrix components supporting human islet function in alginate-based immunoprotective microcapsules for treatment of diabetes. J. Biomed. Mater. Res. Part A 2016, 104, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.M.; Anseth, K.S. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 2008, 27, 667–673. [Google Scholar] [CrossRef]
- Jiang, K.; Chaimov, D.; Patel, S.N.; Liang, J.P.; Wiggins, S.C.; Samojlik, M.M.; Rubiano, A.; Simmons, C.S.; Stabler, C.L. 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials 2018, 198, 37–48. [Google Scholar] [CrossRef]
- Weaver, J.D.; Headen, D.M.; Hunckler, M.D.; Coronel, M.M.; Stabler, C.L.; García, A.J. Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials 2018, 172, 54–65. [Google Scholar] [CrossRef]
- Tremmel, D.M.; Sackett, S.D.; Feeney, A.K.; Mitchell, S.A.; Schaid, M.D.; Polyak, E.; Chlebeck, P.J.; Gupta, S.; Kimple, M.E.; Fernandez, L.A.; et al. A human pancreatic ECM hydrogel optimized for 3-D modeling of the islet microenvironment. Sci. Rep. 2022, 12, 7188. [Google Scholar] [CrossRef]
- Singh, R.; Cottle, L.; Loudovaris, T.; Xiao, D.; Yang, P.; Thomas, H.E.; Kebede, M.A.; Thorn, P. Enhanced structure and function of human pluripotent stem cell-derived beta-cells cultured on extracellular matrix. Stem Cells Transl. Med. 2021, 10, 492–505. [Google Scholar] [CrossRef]
- Matyash, M.; Despang, F.; Mandal, R.; Fiore, D.; Gelinsky, M.; Ikonomidou, C. Novel Soft Alginate Hydrogel Strongly Supports Neurite Growth and Protects Neurons Against Oxidative Stress. Tissue Eng. Part A 2011, 18, 55–66. [Google Scholar] [CrossRef]
- Kang, S.-W.; Cha, B.-H.; Park, H.; Park, K.-S.; Lee, K.Y.; Lee, S.-H. The Effect of Conjugating RGD into 3D Alginate Hydrogels on Adipogenic Differentiation of Human Adipose-Derived Stromal Cells. Macromol. Biosci. 2011, 11, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Halban, P.A.; Wollheim, C.B.; Blondel, B.; Meda, P.; Niesor, E.N.; Mintz, D.H. The possible importance of contact between pancreatic-islet cells for the control of insulin release. Endocrinology 1982, 111, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Parnaud, G.; Lavallard, V.; Bedat, B.; Matthey-Doret, D.; Morel, P.; Berney, T.; Bosco, D. Cadherin engagement improves insulin secretion of single human beta-cells. Diabetes 2015, 64, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Sasson, A.; Rachi, E.; Sakhneny, L.; Baer, D.; Lisnyansky, M.; Epshtein, A.; Landsman, L. Islet Pericytes Are Required for beta-Cell Maturity. Diabetes 2016, 65, 3008–3014. [Google Scholar] [CrossRef]
- Gan, W.J.; Zavortink, M.; Ludick, C.; Templin, R.; Webb, R.; Webb, R.; Ma, W.; Poronnik, P.; Parton, R.G.; Gaisano, H.Y.; et al. Cell polarity defines three distinct domains in pancreatic beta-cells. J. Cell Sci. 2017, 130, 143–151. [Google Scholar] [CrossRef]
- Low, J.T.; Zavortink, M.; Mitchell, J.M.; Gan, W.J.; Do, O.H.; Schwiening, C.J.; Gaisano, H.Y.; Thorn, P. Insulin secretion from beta cells in intact mouse islets is targeted towards the vasculature. Diabetologia 2014, 57, 1655–1663. [Google Scholar] [CrossRef]
- Talavera-Adame, D.; Woolcott, O.O.; Ignatius-Irudayam, J.; Arumugaswami, V.; Geller, D.H.; Dafoe, D.C. Effective endothelial cell and human pluripotent stem cell interactions generate functional insulin-producing beta cells. Diabetologia 2016, 59, 2378–2386. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, M.L.; Deng, K.; Tran, H.A.; Singh, R.; Rnjak-Kovacina, J.; Thorn, P. Glucose-Dependent Insulin Secretion from β Cell Spheroids Is Enhanced by Embedding into Softer Alginate Hydrogels Functionalised with RGD Peptide. Bioengineering 2022, 9, 722. https://doi.org/10.3390/bioengineering9120722
Amin ML, Deng K, Tran HA, Singh R, Rnjak-Kovacina J, Thorn P. Glucose-Dependent Insulin Secretion from β Cell Spheroids Is Enhanced by Embedding into Softer Alginate Hydrogels Functionalised with RGD Peptide. Bioengineering. 2022; 9(12):722. https://doi.org/10.3390/bioengineering9120722
Chicago/Turabian StyleAmin, Md Lutful, Kylie Deng, Hien A. Tran, Reena Singh, Jelena Rnjak-Kovacina, and Peter Thorn. 2022. "Glucose-Dependent Insulin Secretion from β Cell Spheroids Is Enhanced by Embedding into Softer Alginate Hydrogels Functionalised with RGD Peptide" Bioengineering 9, no. 12: 722. https://doi.org/10.3390/bioengineering9120722
APA StyleAmin, M. L., Deng, K., Tran, H. A., Singh, R., Rnjak-Kovacina, J., & Thorn, P. (2022). Glucose-Dependent Insulin Secretion from β Cell Spheroids Is Enhanced by Embedding into Softer Alginate Hydrogels Functionalised with RGD Peptide. Bioengineering, 9(12), 722. https://doi.org/10.3390/bioengineering9120722









