Evaluation of Plan Robustness Using Hybrid Intensity-Modulated Radiotherapy (IMRT) and Volumetric Arc Modulation Radiotherapy (VMAT) for Left-Sided Breast Cancer
Abstract
:1. Introduction
2. Methods
2.1. Ethics Approval and Consent to Participate
2.2. Patient Selection and Delineation
2.3. The 7-Field Hybrid IMRT and VMAT Plans
2.4. Dosimetric Evaluation
2.5. TCP and NTCP
2.6. A Robustness Quantification Method
2.7. Statistical Analysis
3. Results
3.1. Dosimetric Parameters
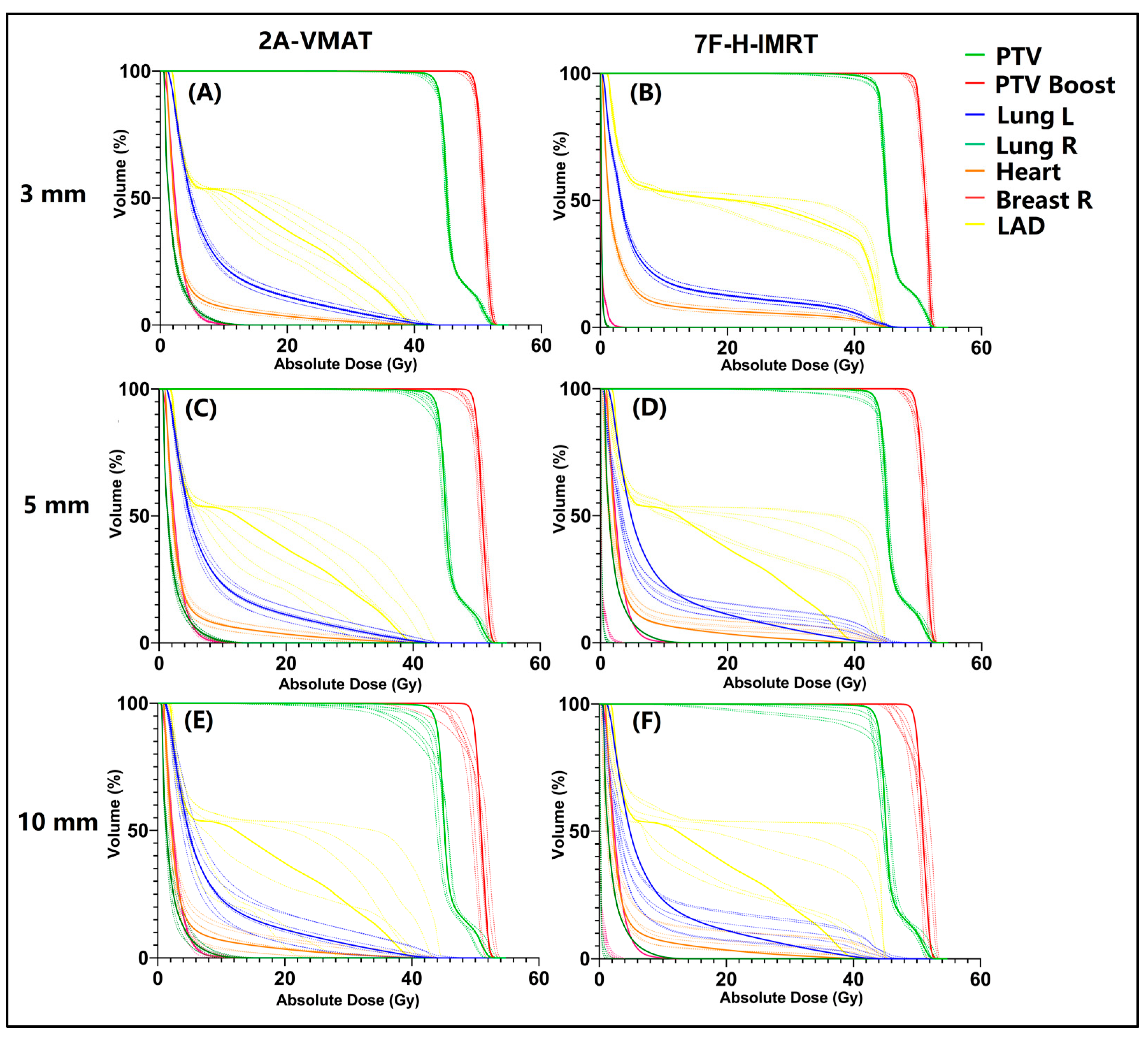
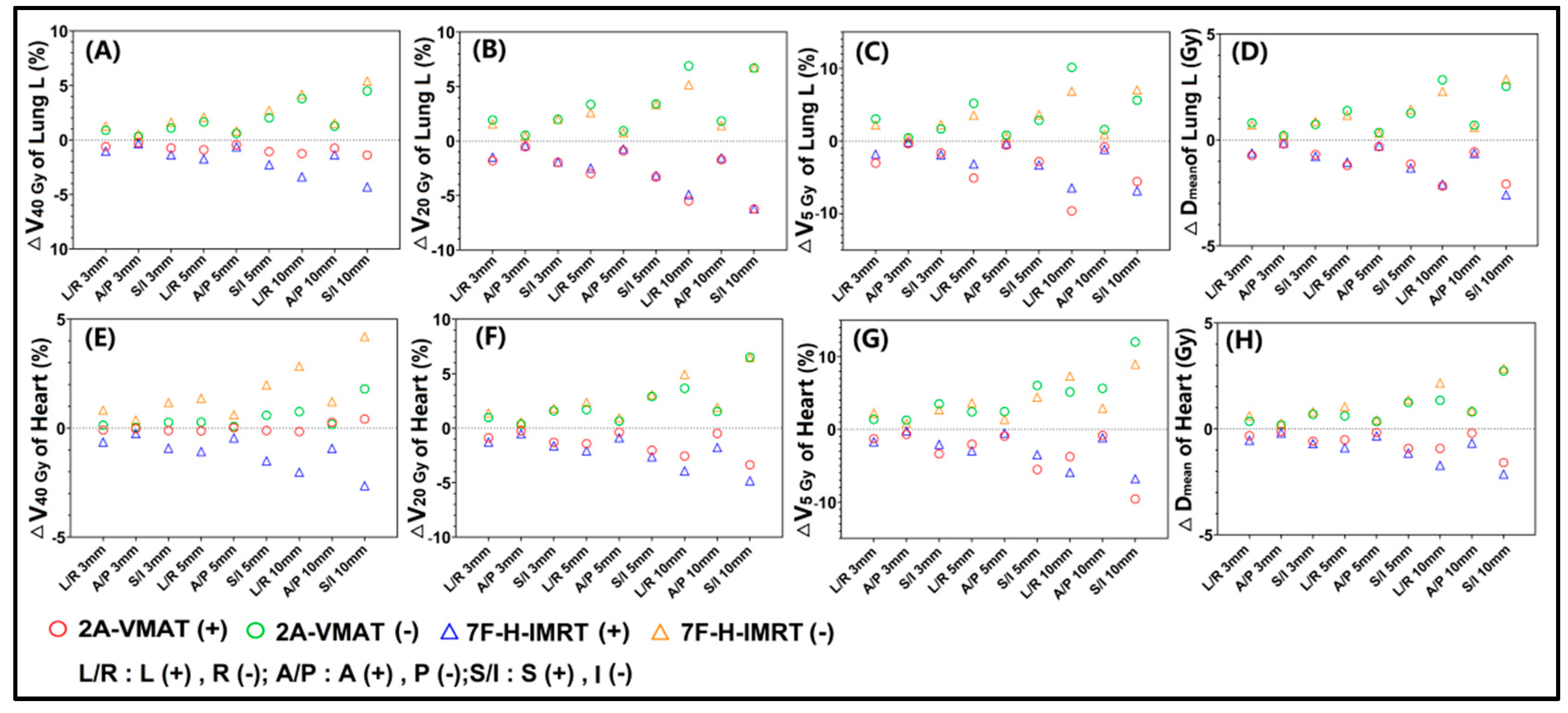
3.2. Plan Robustness Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Rose, F.; Fogliata, A.; Franceschini, D.; Iftode, C.; Navarria, P.; Comito, T.; Franzese, C.; Fernandes, B.; Masci, G.; Torrisi, R.; et al. Hypofractionation with simultaneous boost in breast cancer patients receiving adjuvant chemotherapy: A prospective evaluation of a case series and review of the literature. Breast 2018, 42, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef]
- Whelan, T.J.; Pignol, J.P.; Levine, M.N.; Julian, J.A.; MacKenzie, R.; Parpia, S.; Shelley, W.; Grimard, L.; Bowen, J.; Lukka, H.; et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 2010, 362, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickey, B.E.; James, M.L.; Lehman, M.; Hider, P.N.; Jeffery, M.; Francis, D.P.; See, A.M. Fraction size in radiation therapy for breast conservation in early breast cancer. Cochrane Database Syst. Rev. 2016, 7, CD003860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alford, S.L.; Prassas, G.N.; Vogelesang, C.R.; Leggett, H.J.; Hamilton, C.S. Adjuvant breast radiotherapy using a simultaneous integrated boost: Clinical and dosimetric perspectives. J. Med. Imaging Radiat. Oncol. 2013, 57, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Van Parijs, H.; Reynders, T.; Heuninckx, K.; Verellen, D.; Storme, G.; De Ridder, M. Breast conserving treatment for breast cancer: Dosimetric comparison of different non-invasive techniques for additional boost delivery. Radiat. Oncol. 2014, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- antema-Joppe, E.J.; Vredeveld, E.J.; de Bock, G.H.; Busz, D.M.; Woltman-van Iersel, M.; Dolsma, W.V.; van der Laan, H.P.; Langendijk, J.A.; Maduro, J.H. Five year outcomes of hypofractionated simultaneous integrated boost irradiation in breast conserving therapy; patterns of recurrence. Radiother. Oncol. 2013, 108, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Afifi, A.M.; Saad, A.M.; Al-Husseini, M.J.; Elmehrath, A.O.; Northfelt, D.W.; Sonbol, M.B. Causes of death after breast cancer diagnosis: A US population-based analysis. Cancer 2020, 126, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Jacobse, J.N.; Duane, F.K.; Boekel, N.B.; Schaapveld, M.; Hauptmann, M.; Hooning, M.J.; Seynaeve, C.M.; Baaijens, M.H.A.; Gietema, J.A.; Darby, S.C.; et al. Radiation Dose-Response for Risk of Myocardial Infarction in Breast Cancer Survivors. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 595–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, S.; Camilleri, J.; Derreumaux, S.; Walker, V.; Lairez, O.; Lapeyre, M.; Bruguière, E.; Pathak, A.; Bernier, M.O.; Laurier, D.; et al. Is mean heart dose a relevant surrogate parameter of left ventricle and coronary arteries exposure during breast cancer radiotherapy: A dosimetric evaluation based on individually-determined radiation dose (BACCARAT study). Radiat. Oncol. 2019, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Menon, G.; Wolfe, N.; Ploquin, N.; Trotter, T.; Pudney, D. IMRT for the breast: A comparison of tangential planning techniques. Phys. Med. Biol. 2010, 55, 1231–1241. [Google Scholar] [CrossRef]
- Fogliata, A.; Seppälä, J.; Reggiori, G.; Lobefalo, F.; Palumbo, V.; De Rose, F.; Franceschini, D.; Scorsetti, M.; Cozzi, L. Dosimetric trade-offs in breast treatment with VMAT technique. Br. J. Radiol. 2017, 90, 20160701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corradini, S.; Ballhausen, H.; Weingandt, H.; Freislederer, P.; Schönecker, S.; Niyazi, M.; Simonetto, C.; Eidemüller, M.; Ganswindt, U.; Belka, C. Left-sided breast cancer and risks of secondary lung cancer and ischemic heart disease: Effects of modern radiotherapy techniques. Strahlenther. Onkol. 2018, 194, 196–205. [Google Scholar] [CrossRef]
- Hernandez, V.; Hansen, C.R.; Widesott, L.; Bäck, A.; Canters, R.; Fusella, M.; Götstedt, J.; Jurado-Bruggeman, D.; Mukumoto, N.; Kaplan, L.P.; et al. What is plan quality in radiotherapy? The importance of evaluating dose metrics, complexity, and robustness of treatment plans. Radiother. Oncol. 2020, 153, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Teoh, S.; George, B.; Fiorini, F.; Vallis, K.A.; Van den Heuvel, F. Assessment of robustness against setup uncertainties using probabilistic scenarios in lung cancer: A comparison of proton with photon therapy. Br. J. Radiol. 2020, 93, 20190584. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Patel, S.H.; Shen, J.J.; Hu, Y.; Harrington, D.P.; Ding, X.; Halyard, M.Y.; Schild, S.E.; Wong, W.W.; Ezzell, G.A.; et al. Robustness quantification methods comparison in volumetric modulated arc therapy to treat head and neck cancer. Pract. Radiat. Oncol. 2016, 6, e269–e275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yock, A.D.; Mohan, R.; Flampouri, S.; Bosch, W.; Taylor, P.A.; Gladstone, D.; Kim, S.; Sohn, J.; Wallace, R.; Xiao, Y.; et al. Robustness Analysis for External Beam Radiation Therapy Treatment Plans: Describing Uncertainty Scenarios and Reporting Their Dosimetric Consequences. Pract. Radiat. Oncol. 2019, 9, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gay, H.A.; Niemierko, A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys. Med. 2007, 23, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Luxton, G.; Keall, P.J.; King, C.R. A new formula for normal tissue complication probability (NTCP) as a function of equivalent uniform dose (EUD). Phys. Med. Biol. 2008, 53, 23–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.S.; White, J.; Li, X.A. Is α/β for breast cancer really low? Radiother. Oncol. 2011, 100, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Wennstig, A.K.; Wadsten, C.; Garmo, H.; Fredriksson, I.; Blomqvist, C.; Holmberg, L.; Nilsson, G.; Sund, M. Long-term risk of ischemic heart disease after adjuvant radiotherapy in breast cancer: Results from a large population-based cohort. Breast Cancer Res. 2020, 22, 10. [Google Scholar] [CrossRef]
- Chung, S.Y.; Oh, J.; Chang, J.S.; Shin, J.; Kim, K.H.; Chun, K.H.; Keum, K.C.; Suh, C.O.; Kang, S.M.; Kim, Y.B. Risk of Cardiac Disease in Patients With Breast Cancer: Impact of Patient-Specific Factors and Individual Heart Dose From Three-Dimensional Radiation Therapy Planning. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 473–481. [Google Scholar] [CrossRef]
- Wennstig, A.K.; Garmo, H.; Isacsson, U.; Gagliardi, G.; Rintelä, N.; Lagerqvist, B.; Holmberg, L.; Blomqvist, C.; Sund, M.; Nilsson, G. The relationship between radiation doses to coronary arteries and location of coronary stenosis requiring intervention in breast cancer survivors. Radiat. Oncol. 2019, 14, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, C.; Cunha, G.; Monteiro-Grillo, I.; Vaz, P.; Teixeira, N. Comparison of different breast planning techniques and algorithms for radiation therapy treatment. Phys. Med. 2014, 30, 160–170. [Google Scholar] [CrossRef]
- Vieillevigne, L.; Khamphan, C.; Saez, J.; Hernandez, V. On the need for tuning the dosimetric leaf gap for stereotactic treatment plans in the Eclipse treatment planning system. J. Appl. Clin. Med. Phys. 2019, 20, 68–77. [Google Scholar] [CrossRef]
- Hubley, E.; Pierce, G. The influence of plan modulation on the interplay effect in VMAT liver SBRT treatments. Phys. Med. 2017, 40, 115–121. [Google Scholar] [CrossRef]
- McNiven, A.L.; Sharpe, M.B.; Purdie, T.G. A new metric for assessing IMRT modulation complexity and plan deliverability. Med. Phys. 2010, 37, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Younge, K.C.; Matuszak, M.M.; Moran, J.M.; McShan, D.L.; Fraass, B.A.; Roberts, D.A. Penalization of aperture complexity in inversely planned volumetric modulated arc therapy. Med. Phys. 2012, 39, 7160–7170. [Google Scholar] [CrossRef]
- Götstedt, J.; Karlsson Hauer, A.; Bäck, A. Development and evaluation of aperture-based complexity metrics using film and EPID measurements of static MLC openings. Med. Phys. 2015, 42, 3911–3921. [Google Scholar] [CrossRef]
- Kairn, T.; Crowe, S.B.; Kenny, J.; Knight, R.T.; Trapp, J.V. Predicting the likelihood of QA failure using treatment plan accuracy metrics. J. Phys. Conf. Ser. 2014, 489, 12051. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Rong, Y.; Morrill, S.; Fang, J.; Narayanasamy, G.; Galhardo, E.; Maraboyina, S.; Croft, C.; Xia, F.; Penagaricano, J. Robust optimization in lung treatment plans accounting for geometric uncertainty. J. Appl. Clin. Med. Phys. 2018, 19, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlop, A.; Colgan, R.; Kirby, A.; Ranger, A.; Blasiak-Wal, I. Evaluation of organ motion-based robust optimisation for VMAT planning for breast and internal mammary chain radiotherapy. Clin. Transl. Radiat. Oncol. 2019, 16, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Keall, P.J.; Mageras, G.S.; Balter, J.M.; Emery, R.S.; Forster, K.M.; Jiang, S.B.; Kapatoes, J.M.; Low, D.A.; Murphy, M.J.; Murray, B.R.; et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med. Phys. 2006, 33, 3874–3900. [Google Scholar] [CrossRef]
- Yoganathan, S.A.; Maria Das, K.J.; Agarwal, A.; Kumar, S. Magnitude, Impact, and Management of Respiration-induced Target Motion in Radiotherapy Treatment: A Comprehensive Review. J. Med. Phys. 2017, 42, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Diao, P.; Zhang, D.; Wu, J.; Xin, X.; Fontanarosa, D.; Liu, M.; Li, J.; Orlandini, L.C. Impact of Positioning Errors on the Dosimetry of Breath-Hold-Based Volumetric Arc Modulated and Tangential Field-in-Field Left-Sided Breast Treatments. Front. Oncol. 2020, 10, 554131. [Google Scholar] [CrossRef] [PubMed]
- Naumann, P.; Batista, V.; Farnia, B.; Fischer, J.; Liermann, J.; Tonndorf-Martini, E.; Rhein, B.; Debus, J. Feasibility of Optical Surface-Guidance for Position Verification and Monitoring of Stereotactic Body Radiotherapy in Deep-Inspiration Breath-Hold. Front. Oncol. 2020, 10, 573279. [Google Scholar] [CrossRef] [PubMed]
- Gaál, S.; Kahán, Z.; Paczona, V.; Kószó, R.; Drencsényi, R.; Szabó, J.; Rónai, R.; Antal, T.; Deák, B.; Varga, Z. Deep-inspirational breath-hold (DIBH) technique in left-sided breast cancer: Various aspects of clinical utility. Radiat. Oncol. 2021, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.; Lawrence, Y.R.; Appel, S.; Weiss, I.; Ben Ayun, M.; Akiva Ben-David, M.; Peled, N.; Goldstein, J.D.; Weizman, N.; Galper, S.; et al. Benefits of Continuous Positive Airway Pressure (CPAP) During Radiation Therapy: A Prospective Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1466–1472. [Google Scholar] [CrossRef]
- Van Herk, M. Errors and margins in radiotherapy. Semin. Radiat. Oncol. 2004, 14, 52–64. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, S.; Yang, Y.; Yang, D.; Xie, C.; Yin, L.; Zhang, H.; Liu, A. SU-E-J-19: How Should CTV to PTV Margin Be Created-Analysis of Set-Up Uncertainties of Different Body Parts Using Daily Image Guidance. Med. Phys. 2012, 39, 3656. [Google Scholar] [CrossRef]
- Boekhoff, M.R.; Defize, I.L.; Borggreve, A.S.; van Hillegersberg, R.; Kotte, A.N.T.J.; Lagendijk, J.J.W.; van Lier, A.L.H.M.W.; Ruurda, J.P.; Takahashi, N.; Mook, S.; et al. CTV-to-PTV margin assessment for esophageal cancer radiotherapy based on an accumulated dose analysis. Radiother. Oncol. 2021, 161, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Xu, Y.; Tian, Y.; Liu, Z.; Dai, J. A study of nonuniform CTV to PTV margin expansion incorporating both rotational and translational uncertainties. J. Appl. Clin. Med. Phys. 2019, 20, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Gordon, J.J.; Siebers, J.V. Coverage-based treatment planning: Optimizing the IMRT PTV to meet a CTV coverage criterion. Med. Phys. 2009, 36, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Engelsman, M.; Damen, E.M.; De Jaeger, K.; van Ingen, K.M.; Mijnheer, B.J. The effect of breathing and set-up errors on the cumulative dose to a lung tumor. Radiother. Oncol. 2001, 60, 95–105. [Google Scholar] [CrossRef]
- Karlsson, K.; Lax, I.; Lindbäck, E.; Grozman, V.; Lindberg, K.; Wersäll, P.; Poludniowski, G. Estimation of delivered dose to lung tumours considering setup uncertainties and breathing motion in a cohort of patients treated with stereotactic body radiation therapy. Phys. Med. 2021, 88, 53–64. [Google Scholar] [CrossRef] [PubMed]

| Patient Number | Age | Patient Anatomy | Stage | |
|---|---|---|---|---|
| PTV Volume (cm3) | PTV Boost Volume (cm3) | |||
| 1 | 53 | 737.3 | 81.4 | T1N0M0 |
| 2 | 59 | 1011.4 | 126.8 | T1N0M0 |
| 3 | 39 | 523.2 | 83 | T1N0M0 |
| 4 | 52 | 1600.9 | 125.4 | T1N0M0 |
| 5 | 57 | 321.7 | 59.6 | T2N0M0 |
| 6 | 62 | 524.3 | 74.5 | T1N0M0 |
| 7 | 47 | 874 | 156.7 | T1N0M0 |
| 8 | 55 | 503.6 | 137.8 | T1N0M0 |
| 9 | 64 | 693.3 | 98.2 | T1N0M0 |
| 10 | 48 | 408.9 | 55.2 | T1N0M0 |
| Mean | 54 | 719.9 | 99.9 | - |
| Median | 54 | 608.8 | 90.6 | - |
| Evaluated Items | 2A-VMAT(Gy) | 7F-H-IMRT(Gy) | p-Value | |
|---|---|---|---|---|
| PTV Boost | D2cc | 52.61 (51.98–53.30) | 52.41 (51.82–52.89) | 0.06 |
| D98% | 48.96 (48.76–49.15) | 49.08 (48.54–49.08) | 0.19 | |
| Dmean | 51.21 (50.75–51.93) | 51.00 (50.62–51.59) | 0.11 | |
| CTV Boost | D98% | 49.74 (49.18–50.08) | 49.75 (48.32–50.37) | 0.62 |
| D95% | 50.62 (49.96–50.18) | 50.07 (49.20–50.61) | 0.69 | |
| Dmean | 51.54 (40.91–52.37) | 51.28 (50.93–51.76) | 0.13 | |
| TCP | 97.74 | 97.66 | 0.11 | |
| PTV | D98% | 42.98 (41.97–46.39) | 42.39 (41.70–42.98) | 0.43 |
| D95% | 43.58 (43.36–44.35) | 43.81 (42.83–44.21) | 0.19 | |
| Dmean | 46.51 (45.76–48.10) | 46.40 (45.77–47.63) | 0.23 | |
| CTV | D98% | 43.64 (43.12–44.45) | 42.82 (41.53–42.82) | ** 0.002 |
| D95% | 44.24 (43.83–45.18) | 43.81 (42.83–44.21) | ** 0.002 | |
| Dmean | 46.96 (46.00–48.58) | 46.70 (45.96–47.60) | * 0.05 | |
| TCP | 95.44 | 95.24 | 0.13 |
| Evaluated Items | 2A-VMAT(%/Gy) | 7F-H-IMRT(%/Gy) | p-Value | |
|---|---|---|---|---|
| Lung L | V40 Gy | 1.49 (0.02–3.36) | 7.72 (1.04–11.79) | ** 0.002 |
| V20 Gy | 13.58 (8.83–20.56) | 16.86 (6.64–25.08) | ** 0.001 | |
| V5 Gy | 44.62 (28.15–66.84) | 38.45 (31.11–52.74) | * 0.05 | |
| Dmean | 8.72 (6.86–12.27) | 9.65 (7.31–12.69) | * 0.04 | |
| NTCP | 0.07 | 0.18 | 0.08 | |
| Lung R | V5 Gy | 10.96 (0.01–30.62) | 0.14 (0.00–0.72) | ** 0.002 |
| Dmean | 2.47 (1.28–4.11) | 0.39 (0.09–1.02) | ** 0.002 | |
| NTCP | 1.97 × 10−5 | 2.08 × 10−6 | ** 0.002 | |
| Heart | V40 Gy | 0.18 (0.00–0.98) | 3.76 (1.28–6.24) | ** 0.002 |
| V20 Gy | 5.15 (1.18–40.90) | 9.58 (4.43–17.35) | ** 0.002 | |
| V5 Gy | 22.37 (12.08–31.62) | 24.13 (14.22–41.43) | 0.43 | |
| Dmean | 4.95 (3.10–6.92) | 6.06 (4.06–9.66) | ** 0.001 | |
| NTCP | 1.89 × 10−11 | 5.63 × 10−7 | ** 0.002 | |
| Breast R | V5 Gy | 17.01 (4.48–33.45) | 0.67 (0.00–2.57) | ** 0.002 |
| Dmean | 3.37 (1.51–4.67) | 0.63 (0.26–1.01) | ** 0.002 | |
| LAD | Dmax | 40.01 (34.96–44.19) | 45.50 (42.89–48.50) | ** 0.002 |
| Dmean | 18.97 (8.33–29.84) | 26.34 (12.26–38.62) | ** 0.002 |
| Uncertainty | CTV | |||||
|---|---|---|---|---|---|---|
| ∆D98% | ∆D95% | ∆Dmean | ||||
| 2A-VMAT (Gy) | 7F-H-IMRT (Gy) | 2A-VMAT (Gy) | 7F-H-IMRT (Gy) | 2A-VMAT (Gy) | 7F-H-IMRT (Gy) | |
| L (3 mm) | 0.87 (0.18–2.11) | 0.12 (0.001–0.26) | 0.25 (0.14–0.37) | 0.11 (0.00–0.30) | 0.20 (0.9–0.31) | 0.19 (0.05–0.46) |
| R (3 mm) | 0.43 (0.00–0.90) | 0.13 (0.01–0.24) | 0.52 (0.28–0.89) | 0.15 (0.04–0.27) | 0.29 (0.15–0.47) | 0.20 (0.01–0.88) |
| A (3 mm) | 0.49 (0.01–1.22) | 0.07 (0.01–0.21) | 0.03 (0.00–0.09) | 0.07 (0.01–0.18) | 0.04 (0.00–0.10) | 0.09 (0.02–0.26) |
| P (3 mm) | 0.08 (0.00–0.25) | 0.09 (0.00–0.31) | 0.08 (0.01–0.14) | 0.10 (0.00–0.32) | 0.04 (0.01–0.10) | 0.10 (0.02–0.27) |
| S (3 mm) | 1.57 (0.12–3.69) | 0.10 (0.02–0.25) | 0.29 (0.12–0.40) | 0.11 (0.01–0.29) | 0.21 (0.08–0.38) | 0.18 (0.03–0.48) |
| I (3 mm) | 0.84 (0.07–2.71) | 0.12 (0.03–0.30) | 0.92 (0.41–2.32) | 0.18 (0.02–0.40) | 0.40 (0.27–0.53) | 0.12 (0.02–0.23) |
| L (5 mm) | 2.00 (0.13–4.64) | 0.15 (0.02–0.27) | 0.31 (0.14–0.57) | 0.14 (0.01–0.29) | 0.29 (0.15–0.48) | 0.26 (0.05–0.60) |
| R (5 mm) | 1.46 (0.28–3.79) | 0.17 (0.03–0.39) | 1.22 (0.53–2.13) | 0.25 (0.05–0.44) | 0.63 (0.28–0.95) | 0.46 (0.01–2.60) |
| A (5 mm) | 1.45 (0.07–4.64) | 0.05 (0.01–0.12) | 0.09 (0.00–0.25) | 0.07 (0.00–0.13) | 0.08 (0.00–0.20) | 0.11 (0.03–0.24) |
| P (5 mm) | 0.50 (0.03–1.22) | 0.10 (0.00–0.32) | 0.20 (0.06–0.38) | 0.11 (0.00–0.33) | 0.07 (0.01–0.18) | 0.11 (0.00–0.28) |
| S (5 mm) | 3.37 (0.10–7.94) | 0.21 (0.00–0.63) | 0.29 (0.12–0.51) | 0.15 (0.03–0.37) | 0.30 (0.04–0.57) | 0.24 (0.04–0.62) |
| I (5 mm) | 2.77 (0.81–6.17) | 0.17 (0.03–0.52) | 2.45 (0.97–5.33) | 0.34 (0.02–0.67) | 0.91 (0.53–1.35) | 0.22 (0.02–0.50) |
| L (10 mm) | 5.97 (40.52–43.57) | 0.60 (0.02–1.21) | 0.45 (0.06–1.29) | 0.33 (0.01–0.80) | 0.87 (0.07–4.71) | 0.39 (0.00–0.86) |
| R (10 mm) | 7.36 (31.74–39.51) | 0.45 (0.07–0.83) | 5.70 (2.13–8.84) | 0.63 (0.03–1.01) | 1.93 (0.88–3.59) | 1.02 (0.05–5.04) |
| A (10 mm) | 6.40 (40.64–43.42) | 0.38 (0.03–0.83) | 0.44 (0.14–1.00) | 0.29 (0.07–0.50) | 0.24 (0.04–0.54) | 0.14 (0.03–0.25) |
| P (10 mm) | 3.90 (40.55–43.43) | 0.14 (0.00–0.29) | 0.94 (0.46–1.61) | 0.15 (0.07–0.25) | 0.30 (0.06–0.55) | 0.24 (0.03–0.98) |
| S (10 mm) | 10.28 (33.43–42.70) | 1.80 (0.11–4.37) | 1.89 (0.30–6.10) | 0.82 (0.01–2.30) | 0.82 (0.19–2.02) | 0.51 (0.02–0.89) |
| I (10 mm) | 10.21 (27.46–35.46) | 0.64 (0.17–1.22) | 9.14 (4.97–14.22) | 0.89 (0.12–1.29) | 2.99 (1.63–5.91) | 0.60 (0.12–1.96) |
| Uncertainty | CTV Boost | |||||
|---|---|---|---|---|---|---|
| ∆D98% | ∆D95% | ∆Dmean | ||||
| 2A-VMAT (Gy) | 7F-H-IMRT (Gy) | 2A-VMAT (Gy) | 7F-H-IMRT (Gy) | 2A-VMAT (Gy) | 7F-H-IMRT (Gy) | |
| L (3 mm) | 0.83 (0.13–2.11) | 0.31 (0.06–0.61) | 0.36 (0.15–0.71) | 0.27 (0.00–0.64) | 0.22 (0.02–0.48) | 0.27 (0.00–0.56) |
| R (3 mm) | 0.42 (0.00–1.31) | 0.25 (0.02–0.63) | 0.42 (0.15–1.13) | 0.23 (0.03–0.60) | 0.27 (0.10–0.47) | 0.99 (0.07–7.15) |
| A (3 mm) | 0.38 (0.03–1.02) | 0.13 (0.01–0.25) | 0.07 (0.01–0.12) | 0.11 (0.02–0.34) | 0.07 (0.00–0.15) | 0.13 (0.01–0.47) |
| P (3 mm) | 0.06 (0.00–0.31) | 0.18 (0.01–0.59) | 0.11 (0.00–0.35) | 0.13 (0.00–0.40) | 0.06 (0.01–0.13) | 0.25 (0.00–1.23) |
| S (3 mm) | 1.33 (0.03–3.69) | 0.31 (0.11–0.61) | 0.37 (0.02–0.98) | 0.28 (0.05–0.53) | 0.22 (0.05–0.35) | 0.36 (0.03–1.34) |
| I (3 mm) | 0.41 (0.07–0.78) | 0.37 (0.03–0.65) | 0.58 (0.29–1.68) | 0.32 (0.02–0.55) | 0.34 (0.05–0.74) | 0.40 (0.02–1.83) |
| L (5 mm) | 1.67 (0.16–4.24) | 0.58 (0.02–1.26) | 0.57 (0.12–1.69) | 0.45 (0.05–1.04) | 0.32 (0.18–0.82) | 0.42 (0.01–0.74) |
| R (5 mm) | 1.36 (0.28–4.16) | 0.40 (0.06–1.18) | 1.09 (0.41–3.42) | 0.41 (0.17–0.97) | 0.82 (0.16–3.13) | 2.65 (0.08–18.15) |
| A (5 mm) | 0.97 (0.03–2.60) | 0.24 (0.05–0.50) | 0.11 (0.03–0.21) | 0.14 (0.05–0.31) | 0.10 (0.02–0.21) | 0.15 (0.01–0.51) |
| P (5 mm) | 0.35 (0.03–0.86) | 0.22 (0.07–0.63) | 0.24 (0.02–0.63) | 0.15 (0.00–0.33) | 0.17 (0.01–0.47) | 0.57 (0.02–3.71) |
| S (5 mm) | 2.78 (0.10–7.94) | 0.53 (0.15–1.15) | 0.62 (0.03–2.55) | 0.43 (0.12–0.71) | 0.36 (0.05–0.63) | 0.81 (0.05–3.01) |
| I (5 mm) | 1.57 (0.81–3.23) | 0.69 (0.24–1.14) | 1.38 (0.75–3.85) | 0.61 (0.08–1.02) | 0.74 (0.23–1.47) | 0.77 (0.10–3.67) |
| L (10 mm) | 5.55 (0.34–11.97) | 1.73 (0.61–3.21) | 1.68 (0.17–5.48) | 1.16 (0.06–2.35) | 1.94 (0.04–14.86) | 0.72 (0.02–1.42) |
| R (10 mm) | 6.67 (1.40–12.45) | 1.64 (0.40–3.88) | 4.53 (1.08–9.24) | 1.36 (0.33–2.75) | 2.31 (0.38–9.16) | 5.01 (0.51–28.10) |
| A (10 mm) | 5.21 (0.38–12.28) | 2.29 (0.84–4.01) | 1.09 (0.27–2.62) | 1.47 (0.22–2.73) | 0.32 (0.03–0.84) | 0.29 (0.01–0.65) |
| P (10 mm) | 3.41 (0.64–7.52) | 1.26 (0.01–2.84) | 1.54 (0.35–2.84) | 0.75 (0.06–2.16) | 0.89 (0.11–2.75) | 1.39 (0.16–8.49) |
| S (10 mm) | 8.13 (0.57–20.81) | 2.06 (0.63–4.29) | 1.98 (0.03–8.15) | 1.25 (0.01–2.68) | 1.15 (0.08–5.94) | 2.59 (0.22–7.84) |
| I (10 mm) | 7.18 (2.61–11.84) | 2.22 (0.76–4.65) | 5.24 (2.08–11.47) | 1.84 (0.56–3.70) | 2.44 (0.1.47–4.35) | 1.35 (0.00–5.14) |
| Shift | ∆TCP(%) | ∆NTCP(%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTV | CTV Boost | Lung L | Lung R | Heart | ||||||
| 2A-VMAT | 7F-H-IMRT | 2A-VMAT | 7F-H-IMRT | 2A-VMAT | 7F-H-IMRT | 2A-VMAT | 7F-H-IMRT | 2A-VMAT | 7F-H-IMRT | |
| L (3 mm) | 0.10 | 0.09 | 0.03 | 0.02 | 0.03 | 0.06 | 3.77 × 10−6 | 1.14 × 10−7 | 9.82 × 10−12 | 3.15 × 10−8 |
| R (3 mm) | −0.16 | 0.02 | −0.06 | 0.07 | 0.06 | 0.12 | 4.08 × 10−6 | 1.48 × 10−7 | 3.55 × 10−11 | 1.15 × 10−7 |
| A (3 mm) | −0.01 | 0.04 | 0.01 | 0.03 | 0.01 | 0.02 | 2.11 × 10−6 | 1.05 × 10−8 | 3.79 × 10−12 | 1.97 × 10−8 |
| P (3 mm) | −0.02 | 0.07 | −0.01 | 0.04 | 0.01 | 0.04 | 2.14 × 10−6 | 2.90 × 10−8 | 7.93 × 10−12 | 5.67 × 10−8 |
| S (3 mm) | 0.11 | 0.14 | 0.05 | 0.09 | 0.03 | 0.07 | 5.64 × 10−6 | 6.60 × 10−8 | 1.14 × 10−11 | 3.59 × 10−8 |
| I (3 mm) | −0.30 | −0.03 | −0.07 | −0.02 | 0.05 | 0.15 | 5.95 × 10−6 | 9.55 × 10−8 | 7.30 × 10−11 | 1.73 × 10−7 |
| L (5 mm) | 0.14 | 0.12 | 0.02 | 0.02 | 0.05 | 0.09 | 6.33 × 10−6 | 1.88 × 10−7 | 1.20 × 10−11 | 4.13 × 10−8 |
| R (5 mm) | −0.39 | 0.00 | −0.16 | 0.11 | 0.13 | 0.23 | 6.90 × 10−6 | 2.48 × 10−7 | 1.01 × 10−10 | 2.79 × 10−7 |
| A (5 mm) | −0.05 | 0.03 | 0.01 | 0.02 | 0.01 | 0.04 | 3.74 × 10−6 | 1.79 × 10−8 | 5.12 × 10−12 | 2.99 × 10−8 |
| P (5 mm) | −0.06 | 0.07 | −0.02 | 0.04 | 0.02 | 0.06 | 3.71 × 10−6 | 4.31 × 10−8 | 1.75 × 10−11 | 1.17 × 10−7 |
| S (5 mm) | 0.12 | 0.18 | 0.07 | 0.12 | 0.04 | 0.11 | 9.43 × 10−6 | 1.10 × 10−7 | 1.29 × 10−11 | 4.43 × 10−8 |
| I (5 mm) | −0.93 | −0.10 | −0.14 | −0.05 | 0.11 | 0.30 | 1.03 × 10−5 | 1.59 × 10−7 | 2.90 × 10−10 | 4.97 × 10−7 |
| L (10 mm) | 0.05 | 0.01 | −0.16 | −0.03 | 0.06 | 0.13 | 1.20 × 10−5 | 3.40 × 10−7 | 1.59 × 10−11 | 4.84 × 10−8 |
| R (10 mm) | −2.65 | −0.11 | −1.03 | 0.08 | 0.49 | 0.67 | 1.35 × 10−5 | 5.27 × 10−7 | 9.13 × 10−10 | 1.64 × 10−6 |
| A (10 mm) | −9.43 | −3.26 | −0.08 | −0.09 | 0.02 | 0.07 | 7.65 × 10−6 | 3.40 × 10−8 | 6.60 × 10−12 | 4.28 × 10−8 |
| P (10 mm) | −2.88 | 0.04 | −0.19 | −0.02 | 0.05 | 0.13 | 7.16 × 10−6 | 8.31 × 10−8 | 7.94 × 10−11 | 4.84 × 10−7 |
| S (10 mm) | −1.32 | −3.65 | 0.02 | 0.28 | 0.06 | 0.15 | 1.77 × 10−5 | 2.01 × 10−7 | 1.36 × 10−11 | 4.90 × 10−8 |
| I (10 mm) | −9.16 | −0.31 | −0.58 | −0.19 | 0.36 | 0.98 | 2.07 × 10−5 | 3.43 × 10−7 | 7.44 × 10−9 | 4.53 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Zeng, Q.; Kang, K.; Xu, M.; Xiang, X.; Liu, C. Evaluation of Plan Robustness Using Hybrid Intensity-Modulated Radiotherapy (IMRT) and Volumetric Arc Modulation Radiotherapy (VMAT) for Left-Sided Breast Cancer. Bioengineering 2022, 9, 131. https://doi.org/10.3390/bioengineering9040131
Ding Z, Zeng Q, Kang K, Xu M, Xiang X, Liu C. Evaluation of Plan Robustness Using Hybrid Intensity-Modulated Radiotherapy (IMRT) and Volumetric Arc Modulation Radiotherapy (VMAT) for Left-Sided Breast Cancer. Bioengineering. 2022; 9(4):131. https://doi.org/10.3390/bioengineering9040131
Chicago/Turabian StyleDing, Zhen, Qi Zeng, Kailian Kang, Meiling Xu, Xiaoyong Xiang, and Chenbin Liu. 2022. "Evaluation of Plan Robustness Using Hybrid Intensity-Modulated Radiotherapy (IMRT) and Volumetric Arc Modulation Radiotherapy (VMAT) for Left-Sided Breast Cancer" Bioengineering 9, no. 4: 131. https://doi.org/10.3390/bioengineering9040131
APA StyleDing, Z., Zeng, Q., Kang, K., Xu, M., Xiang, X., & Liu, C. (2022). Evaluation of Plan Robustness Using Hybrid Intensity-Modulated Radiotherapy (IMRT) and Volumetric Arc Modulation Radiotherapy (VMAT) for Left-Sided Breast Cancer. Bioengineering, 9(4), 131. https://doi.org/10.3390/bioengineering9040131






