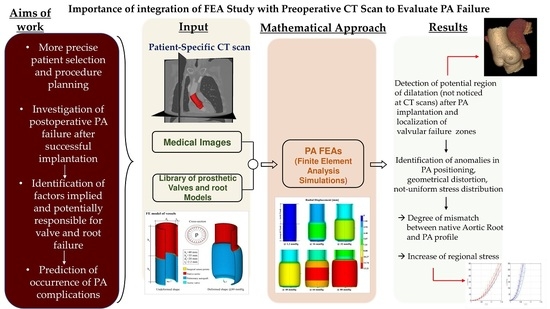
Biomechanics of Pulmonary Autograft as Living Tissue: A Systematic Review
Abstract
:1. Introduction
Pulmonary Autograft, a Biological Entity for a Modern Clinical Challenge
2. Methods
2.1. Search Strategy
2.2. Data Extraction
3. Results
3.1. Proposed Advantages of Pulmonary Autografts as Valve Substitutes: The Living Aortic Root
3.2. Insights on Adaptative Remodelling of the Pulmonary Autograft
3.3. Hemodynamic Performance of Pulmonary Autograft
4. Evidence from Deploying Mechanical Testing: Pulmonary Autograft Targets and Mechanisms of Action in Growing Tissue
FE Simulations
- The constitutive modeling of the explanted PA and pulmonary roots were assumed to be incompressible and nonlinear hyperelastic materials,
- Planar forces calculated by load cells during deformation were metamorphosed to Cauchy stresses in the principal longitudinal and transversal directions,
- A nonlinear regression Levenberg–Marquardt least-squares algorithm in MATLAB (version 7.0.1, Natick, MA, USA) was used to adapt experimentally gained stresses to the corresponding theoretically measured stresses for explanted autograft and pulmonary roots.
5. Remodeling Induced with the Use of Polyester: Crosstalk between Biophysical Features and Clinical Prosthetic Use
5.1. Specific Characteristics of Polydioxanone
5.2. Biodegradation Molecular Mechanisms
5.3. The Use of Polydioxanone as Crosslinked Prosthetics: When and How
6. Biomechanics of Pulmonary Autograft Leaflet and Root: Clinical Application
7. Limitations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC/AHA | American College of Cardiology/American Heart Association |
| ESC | European Society of Cardiology |
| FEA | finite element analysis |
| IC | inclusion cylinder |
| kPa | kilopascal |
| PA | pulmonary autocraft |
| polidioxanone | |
| RK | Ross-Konno |
| RR | root reiforgement |
| SC | subcoronary |
References
- Ross, D.N. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet 1967, 2, 956–958. [Google Scholar] [CrossRef]
- Lower, R.R.; Stofer, R.C.; Shumway, N.E. Autotransplantation of the pulmonic valve into the aorta. J. Thorac. Cardiovasc. Surg. 1960, 39, 680–687. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef] [PubMed]
- Kouchoukos, N.T.; Davila-Roman, V.G.; Spray, T.L.; Murphy, S.F.; Perrillo, J.B. Replacement of the aortic root with a pulmonary autograft in children and young adults with aortic-valve disease. N. Engl. J. Med. 1994, 330, 1–6. [Google Scholar] [CrossRef]
- Yacoub, M.H.; El-Hamamsy, I.; Sievers, H.H.; Carabello, B.A.; Bonow, R.O.; Stelzer, P.; da Costa, F.D.A.; Schäfers, H.J.; Skillington, P.; Charitos, E.I.; et al. Under-use of the Ross operation—A lost opportunity. Lancet 2014, 384, 559–560. [Google Scholar] [CrossRef]
- Nappi, F.; Avtaar Singh, S.S.; Spadaccio, C.; Acar, C. Ross operation 23 years after surgery: It should not be a “forgotten” option. J. Card. Surg. 2020, 35, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Acar, C.; El-Hamamsy, I. Lights and Shadows on the Ross Procedure: Biological Solutions for Biological Problems. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.H.; Stierle, U.; Petersen, M.; Klotz, S.; Richardt, D.; Diwoky, M.; Charitos, E.I. Valve performance classification in 630 subcoronary Ross patients over 22 years. J. Thorac. Cardiovasc. Surg. 2018, 156, 79–86.e2. [Google Scholar] [CrossRef] [PubMed]
- Mazine, A.; David, T.E.; Rao, V.; Hickey, E.J.; Christie, S.; Manlhiot, C.; Ouzounian, M. Long-Term Outcomes of the Ross Procedure Versus Mechanical Aortic Valve Replacement: Propensity-Matched Cohort Study. Circulation 2016, 134, 576–585. [Google Scholar] [CrossRef]
- Mazine, A.; El-Hamamsy, I.; Verma, S.; Peterson, M.D.; Bonow, R.O.; Yacoub, M.H.; David, T.E.; Bhatt, D.L. Ross Procedure in Adults for Cardiologists and Cardiac Surgeons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2761–2777. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Chello, M.; Acar, C. The Ross procedure: Underuse or under-comprehension ? J. Thorac. Cardiovasc. Surg. 2015, 149, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Takkenberg, J.J.; Klieverik, L.M.; Schoof, P.H.; van Suylen, R.J.; van Herwerden, L.A.; Zondervan, P.E.; Roos-Hesselink, J.W.; Eijkemans, M.J.; Yacoub, M.H.; Bogers, A.J. The Ross procedure: A systematic review and meta-analysis. Circulation 2009, 119, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Elkins, R.C.; Thompson, D.M.; Lane, M.M.; Elkins, C.C.; Peyton, M.D. Ross operation:16-year experience. J. Thorac. Cardiovasc. Surg. 2008, 136, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.H.; Stierle, U.; Charitos, E.I.; Hanke, T.; Misfeld, M.; Matthias Bechtel, J.F.; Gorski, A.; Franke, U.F.; Graf, B.; Robinson, D.R.; et al. German-Dutch Ross Registry Major adverse cardiac and cerebrovascular events after the Ross procedure: A report from the German-Dutch Ross Registry. Circulation 2010, 122 (Suppl. 11), S216–S223. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F. Biomechanics of Ross Operation: Still So Much to Learn. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 827–828. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Chester, A.H.; Yacoub, M.H. Cellular regulation of the structure and function of aortic valves. J. Adv. Res. 2010, 1, 5–12. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Balachandran, K.; Yacoub, M.H.; Stevens, L.M.; Sarathchandra, P.; Taylor, P.M.; Yoganathan, A.P.; Chester, A.H. Endothelium-dependent regulation of the mechanical properties of aortic valve cusps. J. Am. Coll. Cardiol. 2009, 53, 1448–1455. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Yacoub, M.H.; Chester, A.H. Neuronal regulation of aortic valve cusps. Curr. Vasc. Pharmacol. 2009, 7, 40–46. [Google Scholar] [CrossRef]
- Rabkin-Aikawa, E.; Aikawa, M.; Farber, M.; Kratz, J.R.; Garcia-Cardena, G.; Kouchoukos, N.T.; Mitchell, M.B.; Jonas, R.A.; Schoen, F.J. Clinical pulmonary autograft valves: Pathologic evidence of adaptive remodeling in the aortic site. J. Thorac. Cardiovasc. Surg. 2004, 128, 552–561. [Google Scholar] [CrossRef]
- Gorczynski, A.; Trenkner, M.; Anisimowicz, L.; Gutkowski, R.; Drapella, A.; Kwiatkowska, E.; Dobke, M. Biomechanics of the pulmonary autograft valve in the aortic position. Thorax 1982, 37, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Nappi, F.; Spadaccio, C.; Al-Attar, N.; Acar, C. The Ross procedure at the crossroads: Lessons from biology: Is Dr Ross’s dream concluded ? Int. J. Cardiol. 2015, 178, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Montagnani, S.; Acar, C.; Nappi, F. Introducing bioresorbable scaffolds into the show. A potential adjunct to resuscitate Ross procedure. Int. J. Cardiol. 2015, 190, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Fraldi, M.; Montagnani, S.; Fouret, P.; Chachques, J.C.; Acar, C. A composite semiresorbable armoured scaffold stabilizes pulmonary autograft after the Ross operation: Mr Ross’s dream fulfilled. J. Thorac. Cardiovasc. Surg. 2016, 151, 155–164.e1. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Fouret, P.; Hammoudi, N.; Chachques, J.C.; Chello, M.; Acar, C. An experimental model of the Ross operation: Development of resorbable reinforcements for pulmonary autografts. J. Thorac. Cardiovasc. Surg. 2015, 149, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Carotenuto, A.R.; Di Vito, D.; Spadaccio, C.; Acar, C.; Fraldi, M. Stress-shielding, growth and remodeling of pulmonary artery reinforced with copolymer scaffold and transposed into aortic position. Biomech. Model. Mechanobiol. 2016, 15, 1141–1157. [Google Scholar] [CrossRef]
- Nappi, F.; Fraldi, M.; Spadaccio, C.; Carotenuto, A.R.; Montagnani, S.; Castaldo, C.; Chachques, J.C.; Acar, C. Biomechanics drive histological wall remodeling of neoaortic root: A mathematical model to study the expression levels of ki 67, metalloprotease, and apoptosis transition. J. Biomed. Mater. Res. A 2016, 104, 2785–2793. [Google Scholar] [CrossRef]
- Spadaccio, C.; Rainer, A.; Mozetic, P.; Trombetta, M.; Dion, R.A.; Barbato, R.; Nappi, F.; Chello, M. The role of extracellular matrix in age-related conduction disorders: A forgotten player ? J. Geriatr. Cardiol. 2015, 12, 76–82. [Google Scholar] [CrossRef]
- Nappi, F.; Nenna, A.; Larobina, D.; Carotenuto, A.R.; Jarraya, M.; Spadaccio, C.; Fraldi, M.; Chello, M.; Acar, C.; Carrel, T. Simulating the ideal geometrical and biomechanical parameters of the pulmonary autograft to prevent failure in the Ross operation. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 269–276. [Google Scholar] [CrossRef]
- Nappi, F.; Carotenuto, A.R.; Cutolo, A.; Fouret, P.; Acar, C.; Chachques, J.C.; Fraldi, M. Compliance mismatch and compressive wall stresses drive anomalous remodelling of pulmonary trunks reinforced with Dacron grafts. J. Mech. Behav. Biomed. Mater. 2016, 63, 287–302. [Google Scholar] [CrossRef]
- Carr-White, G.S.; Afoke, A.; Birks, E.J.; Hughes, S.; O’Halloran, A.; Glennen, S.; Edwards, S.; Eastwood, M.; Yacoub, M.H. Aortic root characteristics of human pulmonary autografts. Circulation 2000, 102, Iii15–Iii21. [Google Scholar] [CrossRef]
- Chambers, J.C.; Somerville, J.; Stone, S.; Ross, D.N. Pulmonary autograft procedure for aortic valve disease: Long-term results of the pioneer series. Circulation 1997, 96, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Elkins, R.C.; Lane, M.M.; McCue, C. Ross operation in children: Late results. J. Heart Valve Dis. 2001, 10, 736–741. [Google Scholar] [PubMed]
- Schoof, P.H.; Cromme-Dijkhuis, A.H.; Bogers, J.J.; Bogers, J.J.; Thijssen, E.J.; Witsenburg, M.; Hess, J.; Bos, E. Aortic root replacement with pulmonary autograft in children. J. Thorac. Cardiovasc. Surg. 1994, 107, 367–373. [Google Scholar] [CrossRef]
- Brown, J.W.; Ruzmetov, M.; Rodefeld, M.D.; Mahomed, Y.; Turrentine, M.W. Incidence of and risk factors for pulmonary autograft dilation after Ross aortic valve replacement. Ann. Thorac. Surg. 2007, 83, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Hörer, J.; Hanke, T.; Stierle, U.; Takkenberg, J.J.; Bogers, A.J.; Hemmer, W.; Rein, J.G.; Hetzer, R.; Hübler, M.; Robinson, D.R.; et al. Neoaortic root diameters and aortic regurgitation in children after the Ross operation. Ann. Thorac. Surg. 2009, 88, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Charitos, E.I.; Hanke, T.; Stierle, U.; Robinson, D.R.; Bogers, A.J.; Hemmer, W.; Bechtel, M.; Misfeld, M.; Gorski, A.; Boehm, J.O.; et al. Autograft reinforcement to preserve autograft function after the ross procedure: A report from the german-dutch ross registry. Circulation 2009, 120, S146–S154. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.H.; Stierle, U.; Charitos, E.I.; Takkenberg, J.J.; Hörer, J.; Lange, R.; Franke, U.; Albert, M.; Gorski, A.; Leyh, R.G.; et al. A multicentre evaluation of the autograft procedure for young patients undergoing aortic valve replacement: Update on the German Ross Registry. Eur. J. Cardiothorac. Surg. 2016, 49, 212–218. [Google Scholar] [CrossRef]
- Charitos, E.I.; Takkenberg, J.J.; Hanke, T.; Gorski, A.; Botha, C.; Franke, U.; Dodge-Khatami, A.; Hoerer, J.; Lange, R.; Moritz, A.; et al. Reoperations on the pulmonary autograft and pulmonary homograft after the Ross procedure: An update on the German Dutch Ross Registry. J. Thorac. Cardiovasc. Surg. 2012, 144, 813–821. [Google Scholar] [CrossRef]
- Mokhles, M.M.; Rizopoulos, D.; Andrinopoulou, E.R.; Bekkers, J.A.; Roos-Hesselink, J.W.; Lesaffre, E.; Bogers, A.J.; Takkenberg, J.J. Autograft and pulmonary allograft performance in the second post-operative decade after the Ross procedure: Insights from the Rotterdam Prospective Cohort Study. Eur. Heart, J. 2012, 33, 2213–2224. [Google Scholar] [CrossRef]
- Stulak, J.M.; Burkhart, H.M.; Sundt, T.M., 3rd; Connolly, H.M.; Suri, R.M.; Schaff, H.V.; Dearani, J.A. Spectrum and outcome of reoperations after the Ross procedure. Circulation 2010, 122, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Sharabiani, M.T.; Dorobantu, D.M.; Mahani, A.S.; Turner, M.; Peter Tometzki, A.J.; Angelini, G.D.; Parry, A.J.; Caputo, M.; Stoica, S.C. Aortic Valve Replacement and the Ross Operation in Children and Young Adults. J. Am. Coll. Cardiol. 2016, 67, 2858–2870. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Mohammadi, S.; Jacques, F.; Kalavrouziotis, D.; Voisine, P.; Doyle, D.; Perron, J. Clinical Outcomes Following the Ross Procedure in Adults: A 25-Year Longitudinal Study. J. Am. Coll. Cardiol. 2017, 70, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Mastrobuoni, S.; de Kerchove, L.; Solari, S.; Astarci, P.; Poncelet, A.; Noirhomme, P.; Rubay, J.; El Khoury, G. The Ross procedure in young adults: Over 20 years of experience in our Institution. Eur. J. Cardiothorac. Surg. 2016, 49, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.W.; Putter, H.; Klautz, R.J.M.; Bruggemans, E.F.; Holman, E.R.; Bökenkamp, R.; Hazekamp, M.G. Long-Term Follow-Up After the Ross Procedure: A Single Center 22-Year Experience. Ann. Thorac. Surg. 2017, 103, 1976–1983. [Google Scholar] [CrossRef]
- Skillington, P.D.; Mokhles, M.M.; Takkenberg, J.J.; Larobina, M.; O’Keefe, M.; Wynne, R.; Tatoulis, J. The Ross procedure using autologous support of the pulmonary autograft: Techniques and late results. J. Thorac. Cardiovasc. Surg. 2015, 149, S46–S52. [Google Scholar] [CrossRef]
- da Costa, F.D.; Takkenberg, J.J.; Fornazari, D.; Balbi Filho, E.M.; Colatusso, C.; Mokhles, M.M.; da Costa, A.B.; Sagrado, A.G.; Ferreira, A.D.; Fernandes, T.; et al. Long-term results of the Ross operation: An 18-year single institutional experience. Eur. J. Cardiothorac. Surg. 2014, 46, 415–422. [Google Scholar] [CrossRef]
- Andreas, M.; Seebacher, G.; Reida, E.; Wiedemann, D.; Pees, C.; Rosenhek, R.; Heinze, G.; Moritz, A.; Kocher, A.; Laufer, G. A single-center experience with the ross procedure over 20 years. Ann. Thorac. Surg. 2014, 97, 182–188. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Eryigit, Z.; Stevens, L.M.; Sarang, Z.; George, R.; Clark, L.; Melina, G.; Takkenberg, J.J.; Yacoub, M.H. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: A randomised controlled trial. Lancet 2010, 376, 524–531. [Google Scholar] [CrossRef]
- Buratto, E.; Shi, W.Y.; Wynne, R.; Poh, C.L.; Larobina, M.; O’Keefe, M.; Goldblatt, J.; Tatoulis, J.; Skillington, P.D. Improved Survival After the Ross Procedure Compared With Mechanical Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1337–1344. [Google Scholar] [CrossRef]
- David, T.E.; David, C.; Woo, A.; Manlhiot, C. The Ross procedure: Outcomes at 20 years. J. Thorac. Cardiovasc. Surg. 2014, 147, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Mookhoek, A.; Krishnan, K.; Chitsaz, S.; Kuang, H.; Ge, L.; Schoof, P.H.; Bogers, A.J.J.C.; Takkenberg, J.J.M.; Tseng, E.E. Biomechanics of Failed Pulmonary Autografts Compared to Native Aortic Roots. Ann. Thorac. Surg. 2017, 103, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Mookhoek, A.; Krishnan, K.; Chitsaz, S.; Kuang, H.; Ge, L.; Schoof, P.H.; Bogers, A.J.; Takkenberg, J.J.; Tseng, E.E. Biomechanics of Failed Pulmonary Autografts Compared With Normal Pulmonary Roots. Ann. Thorac. Surg. 2016, 102, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Avtaar Singh, S.S.; Acar, C. Biomechanical future of the growing pulmonary autograft in Ross operation. Transl. Pediatr. 2020, 9, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Nenna, A.; Spadaccio, C.; Chello, M. Pulmonary autograft in aortic position: Is everything known? Transl. Pediatr. 2017, 6, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Nappi, F.; De Marco, F.; Sedati, P.; Taffon, C.; Nenna, A.; Crescenzi, A.; Chello, M.; Trombetta, M.; Gambardella, I.; et al. Implantation of a Poly-L.-Lactide G.C.SF-Functionalized Scaffold in a Model of Chronic Myocardial Infarction. J. Cardiovasc. Transl. Res. 2017, 10, 47–65. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Lauer, M.S.; Blackstone, E.H.; Young, J.B.; Topol, E.J. Cause of death in clinical research: Time for a reassessment ? J. Am. Coll. Cardiol. 1999, 34, 618–620. [Google Scholar] [CrossRef]
- Zanolla, L.; Zardini, P. Selection of endpoints for heart failure clinical trials. Eur. J. Heart Fail. 2003, 5, 717–723. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/ EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Aboud, A.; Charitos, E.I.; Fujita, B.; Stierle, U.; Reil, J.C.; Voth, V.; Liebrich, M.; Andreas, M.; Holubec, T.; Bening, C.; et al. Long-Term Outcomes of Patients Undergoing the Ross Procedure. J. Am. Coll. Cardiol. 2021, 77, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Ruzmetov, M.; Geiss, D.M.; Shah, J.J.; Fortuna, R.S. Autograft or allograft aortic root replacement in children and young adults with aortic valve disease: A single-center comparison. Ann. Thorac. Surg. 2012, 94, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Böhm, J.O.; Hemmer, W.; Rein, J.G.; Horke, A.; Roser, D.; Blumenstock, G.; Botha, C.A. A single-institution experience with the Ross operation over 11 years. Ann. Thorac. Surg. 2009, 87, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Klieverik, L.M.; Bekkers, J.A.; Roos, J.W.; Eijkemans, M.J.; Raap, G.B.; Bogers, A.J.; Takkenberg, J.J. Autograft or allograft aortic valve replacement in young adult patients with congenital aortic valve disease. Eur. Heart J. 2008, 29, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Klieverik, L.M.; Takkenberg, J.J.; Bekkers, J.A.; Roos-Hesselink, J.W.; Witsenburg, M.; Bogers, A.J. The Ross operation: A Trojan horse ? Eur. Heart J. 2007, 28, 1993–2000. [Google Scholar] [CrossRef]
- Chiappini, B.; Absil, B.; Rubay, J.; Noirhomme, P.; Funken, J.C.; Verhelst, R.; Poncelet, A.; El Khoury, G. The Ross procedure: Clinical and echocardiographic follow-up in 219 consecutive patients. Ann. Thorac. Surg. 2007, 83, 1285–1289. [Google Scholar] [CrossRef]
- Pasquali, S.K.; Shera, D.; Wernovsky, G.; Cohen, M.S.; Tabbutt, S.; Nicolson, S.; Spray, T.L.; Marino, B.S. Midterm outcomes and predictors of reintervention after the Ross procedure in infants, children, and young adults. J. Thorac. Cardiovasc. Surg. 2007, 133, 893–899. [Google Scholar] [CrossRef]
- Kumar, A.S.; Talwar, S.; Mohapatra, R.; Saxena, A.; Singh, R. Aortic valve replacement with the pulmonary autograft: Mid-term results. Ann. Thorac. Surg. 2005, 80, 488–494. [Google Scholar] [CrossRef]
- Kumar, A.S.; Talwar, S.; Saxena, A.; Singh, R. Ross procedure in rheumatic aortic valve disease. Eur. J. Cardiothorac. Surg. 2006, 29, 156–161. [Google Scholar] [CrossRef]
- Kouchoukos, N.T.; Masetti, P.; Nickerson, N.J.; Castner, C.F.; Shannon, W.D.; Dávila-Román, V.G. The Ross procedure: Long-term clinical and echocardiographic follow-up. Ann. Thorac. Surg. 2004, 78, 773–781. [Google Scholar] [CrossRef]
- Luciani, G.B.; Lucchese, G.; De Rita, F.; Puppini, G.; Faggian, G.; Mazzucco, A. Reparative surgery of the pulmonary autograft: Experience with Ross reoperations. Eur. J. Cardiothorac. Surg. 2012, 41, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.G.; Pozzi, M. Ross operation in children and young adults: The Alder Hey case series. BMC Cardiovasc. Disord. 2004, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Alphonso, N.; Baghai, M.; Dhital, K.; Mood, G.; Tulloh, R.; Austin, C.; Anderson, D. Midterm results of the Ross procedure. Eur. J. Cardiothorac. Surg. 2004, 25, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Elkins, R.C.; Lane, M.M.; McCue, C. Effect of prior aortic valve intervention on results of the Ross operation. J. Heart Valve Dis. 2003, 12, 423–429. [Google Scholar] [PubMed]
- Concha, M.; Pradas, G.; Juffe, A.; Caffarena, J.M.; Montero, A.; Aranda, P.J. Comprehensive experience with the Ross operation in Spain. Eur. J. Cardiothorac. Surg. 2003, 24, 521–526. [Google Scholar] [CrossRef]
- Takkenberg, J.J.; Dossche, K.M.; Hazekamp, M.G.; Nijveld, A.; Jansen, E.W.; Waterbolk, T.W.; Bogers, A.J.; Dutch Ross Study Group. Report of the Dutch experience with the Ross procedure in 343 patients. Eur. J. Cardiothorac. Surg. 2002, 22, 70–77. [Google Scholar] [CrossRef]
- Pessotto, R.; Wells, W.J.; Baker, C.J.; Luna, C.; Starnes, V.A. Midterm results of the Ross procedure. Ann. Thorac. Surg. 2001, 71, S336–S339. [Google Scholar] [CrossRef]
- Laudito, A.; Brook, M.M.; Suleman, S.; Bleiweis, M.S.; Thompson, L.D.; Hanley, F.L.; Reddy, V.M. The Ross procedure in children and young adults: A word of caution. J. Thorac. Cardiovasc. Surg. 2001, 122, 147–153. [Google Scholar] [CrossRef]
- Sharoni, E.; Katz, J.; Dagan, O.; Lorber, A.; Hirsch, R.; Blieden, L.C.; Vidne, B.A.; Birk, E. The Ross operation: Initial Israeli experience. Isr. Med. Assoc. J. 2000, 2, 115–117. [Google Scholar] [PubMed]
- Moidl, R.; Simon, P.; Aschauer, C.; Chevtchik, O.; Kupilik, N.; Rödler, S.; Wolner, E.; Laufer, G. Does the Ross operation fulfill the objective performance criteria established for new prosthetic heart valves ? J. Heart Valve Dis. 2000, 9, 190–194. [Google Scholar]
- Matsuki, O.; Okita, Y.; Almeida, R.S.; McGoldrick, J.P.; Hooper, T.L.; Robles, A.; Ross, D.N. Two decades’ experience with aortic valve replacement with pulmonary autograft. J. Thorac. Cardiovasc. Surg. 1988, 95, 705–711. [Google Scholar] [CrossRef]
- Gula, G.; Wain, W.H.; Ross, D.N. Ten years’ experience with pulmonary autograft replacements for aortic valve disease. Ann. Thorac. Surg. 1979, 28, 392–396. [Google Scholar] [CrossRef]
- Somerville, J.; Saravalli, O.; Ross, D.; Stone, S. Long-term results of pulmonary autograft for aortic valve replacement. Br. Heart J. 1979, 42, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.D.; Backer, C.L.; Hillman, N.D.; Lundt, C.; Mavroudis, C. The Ross operation in children: Effects of aortic annuloplasty. Ann. Thorac. Surg. 2007, 84, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Kalavrouziotis, G.; Raja, S.; Ciotti, G.; Karunaratne, A.; Corno, A.F.; Pozzi, M. Medium-term results from pulmonary autografts after the Ross procedure in children and adolescents. Hellenic J. Cardiol. 2006, 47, 337–343. [Google Scholar]
- Bohm, J.O.; Botha, C.A.; Horke, A.; Hemmer, W.; Roser, D.; Blumenstock, G.; Uhlemann, F.; Rein, J.G. Is the Ross operation still an acceptable option in children and adolescents? Ann. Thorac. Surg. 2006, 82, 940–947. [Google Scholar] [CrossRef]
- Takkenberg, J.J.; Kappetein, A.P.; van Herwerden, L.A.; Witsenburg, M.; Van Osch-Gevers, L.; Bogers, A.J. Pediatric autograft aortic root replacement: A prospective follow-up study. Ann. Thorac. Surg. 2005, 80, 1628–1633. [Google Scholar] [CrossRef]
- Khwaja, S.; Nigro, J.J.; Starnes, V.A. The Ross procedure is an ideal aortic valve replacement operation for the teen patient. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2005, 8, 173–175. [Google Scholar] [CrossRef]
- Hazekamp, M.G.; Grotenhuis, H.B.; Schoof, P.H.; Rijlaarsdam, M.E.; Ottenkamp, J.; Dion, R.A. Results of the Ross operation in a pediatric population. Eur. J. Cardiothorac. Surg. 2005, 27, 975–979. [Google Scholar] [CrossRef]
- Hraska, V.; Krajci, M.; Haun, C.; Ntalakoura, K.; Razek, V.; Lacour-Gayet, F.; Weil, J.; Reichenspurner, H. Ross and Ross-Konno procedure in children and adolescents: Mid-term results. Eur. J. Cardiothorac. Surg. 2004, 25, 742–747. [Google Scholar] [CrossRef]
- Al-Halees, Z.; Pieters, F.; Qadoura, F.; Shahid, M.; Al-Amri, M.; Al-Fadley, F. The Ross procedure is the procedure of choice for congenital aortic valve disease. J. Thorac. Cardiovasc. Surg. 2002, 123, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, I.; Poirier, N.; Mazine, A.; Dore, A.; Mercier, L.A.; Leduc, L.; El-Hamamsy, I. Cardiac, obstetric, and fetal outcomes during pregnancy after biological or mechanical aortic valve replacement. Can. J. Cardiol. 2014, 30, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Dagum, P.; Green, G.R.; Nistal, F.J.; Daughters, G.T.; Timek, T.A.; Foppiano, L.E.; Bolger, A.F.; Ingels NBJr Miller, D.C. Deformational dynamics of the aortic root: Modes and physiologic determinants. Circulation 1999, 100, II54–II62. [Google Scholar] [CrossRef] [PubMed]
- Warnock, J.N.; Gamez, C.A.; Metzler, S.A.; Chen, J.; Elder, S.H.; Liao, J. Vasoactive agents alter the biomechanical properties of aortic heart valve leaflets in a time-dependent manner. J. Heart Valve Dis. 2010, 19, 86–95. [Google Scholar]
- Nappi, F.; Nenna, A.; Lemmo, F.; Chello, M.; Chachques, J.C.; Acar, C.; Larobina, D. Finite Element Analysis Investigate Pulmonary Autograft Root and Leaflet Stresses to Understand Late Durability of Ross Operation. Biomimetics 2020, 5, 37. [Google Scholar] [CrossRef]
- Chiarini, A.; Dal Prà, I.; Faggian, G.; Armato, U.; Luciani, G.B. Maladaptive remodeling of pulmonary artery root autografts after Ross procedure: A proteomic study. J. Thorac. Cardiovasc. Surg. 2020, 159, 621–632.e3. [Google Scholar] [CrossRef]
- Um, K.J.; Mc, C.G.; Belley-Cote, E.P.; Gupta, S.; Bouhout, I.; Lortie, H.; Alraddadi, H.; Alsagheir, A.; Bossard, M.; Mcintyre, W.F.; et al. Hemodynamic outcomes of the Ross procedure versus other aortic valve replacement: A systematic review and meta-analysis. J. Cardiovasc. Surg. 2018, 59, 462–470. [Google Scholar] [CrossRef]
- Chan, V.; Rubens, F.; Boodhwani, M.; Mesana, T.; Ruel, M. Determinants of persistent or recurrent congestive heart failure after contemporary surgical aortic valve replacement. J. Heart Valve Dis. 2014, 23, 665–670. [Google Scholar]
- Hauser, M.; Bengel, F.M.; Kuhn, A.; Sauer, U.; Zylla, S.; Braun, S.L.; Nekolla, S.G.; Oberhoffer, R.; Lange, R.; Schwaiger, M.; et al. Myocardial blood flow and flow reserve after coronary reimplantation in patients after arterial switch and ross operation. Circulation 2001, 103, 1875–1880. [Google Scholar] [CrossRef]
- Duebener, L.F.; Stierle, U.; Erasmi, A.; Bechtel, M.F.; Zurakowski, D.; Böhm, J.O.; Botha, C.A.; Hemmer, W.; Rein, J.G.; Sievers, H.H.; et al. Ross procedure and left ventricular mass regression. Circulation 2005, 112, I415–I422. [Google Scholar] [CrossRef]
- Sievers, H.H.; Hanke, T.; Stierle, U.; Bechtel, M.F.; Graf, B.; Robinson, D.R.; Ross, D.N. A critical reappraisal of the Ross operation: Renaissance of the subcoronary implantation technique? Circulation 2006, 114 (Suppl. 1), I504–I511. [Google Scholar] [CrossRef] [PubMed]
- Torii, R.; El-Hamamsy, I.; Donya, M.; Babu-Narayan, S.V.; Ibrahim, M.; Kilner, P.J.; Mohiaddin, R.H.; Xu, X.Y.; Yacoub, M.H. Integrated morphologic and functional assessment of the aortic root after different tissue valve root replacement procedures. J. Thorac. Cardiovasc. Surg. 2012, 143, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Nenna, A.; Petitti, T.; Spadaccio, C.; Gambardella, I.; Lusini, M.; Chello, M.; Acar, C. Long-term outcome of cryopreserved allograft for aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2018, 156, 1357–1365.e6. [Google Scholar] [CrossRef] [PubMed]
- Olivito, S.; Lalande, S.; Nappi, F.; Hammoudi, N.; D’Alessandro, C.; Fouret, P.; Acar, C. Structural deterioration of the cryopreserved mitral homograft valve. J. Thorac. Cardiovasc. Surg. 2012, 144, 313–320.e1. [Google Scholar] [CrossRef] [Green Version]
- Nappi, F.; Spadaccio, C.; Acar, C. Use of allogeneic tissue to treat infective valvular disease: Has everything been said? J. Thorac. Cardiovasc. Surg. 2017, 153, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Singh, S.S.A.; Bellomo, F.; Nappi, P.; Iervolino, A.; Acar, C. The Choice of Pulmonary Autograft in Aortic Valve Surgery: A State-of-the-Art Primer. Biomed. Res. Int. 2021, 2021, 5547342. [Google Scholar] [CrossRef]
- Nappi, F.; Singh, S.S.A.; Lusini, M.; Nenna, A.; Gambardella, I.; Chello, M. The use of allogenic and autologous tissue to treat aortic valve endocarditis. Ann. Transl. Med. 2019, 7, 491. [Google Scholar] [CrossRef]
- Doty, D.B. Aortic valve replacement with homograft and autograft. Semin. Thorac. Cardiovasc. Surg. 1996, 8, 249–258. [Google Scholar]
- Laforest, I.; Dumesnil, J.G.; Briand, M.; Cartier, P.C.; Pibarot, P. Hemodynamic performance at rest and during exercise after aortic valve replacement: Comparison of pulmonary autografts versus aortic homografts. Circulation 2002, 106, I57–I62. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Dumesnil, J.G.; Briand, M.; Laforest, I.; Cartier, P. Hemodynamic performance during maximum exercise in adult patients with the Ross operation and comparison with normal controls and patients with aortic bioprostheses. Am. J. Cardiol. 2000, 86, 982–988. [Google Scholar] [CrossRef]
- Puranik, R.; Tsang, V.T.; Broadley, A.; Nordmeyer, J.; Lurz, P.; Muthialu, N.; Derrick, G.; Walker, F.; Cullen, S.; de Leval, M.; et al. Functional outcomes after the Ross (pulmonary autograft) procedure assessed with magnetic resonance imaging and cardiopulmonary exercise testing. Heart 2010, 96, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Mookhoek, A.; de Heer, E.; Bogers, A.J.; Takkenberg, J.J.; Schoof, P.H. Pulmonary autograft valve explants show typical degeneration. J. Thorac. Cardiovasc. Surg. 2010, 139, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Azadani, A.N.; Chitsaz, S.; Matthews, P.B.; Nordmeyer, J.; Lurz, P.; Muthialu, N.; Derrick, G.; Walker, F.; Cullen, S.; de Leval, M.; et al. Regional mechanical properties of human pulmonary root used for the Ross operation. J. Heart Valve Dis. 2012, 21, 527–534. [Google Scholar] [PubMed]
- Nappi, F.; Spadaccio, C.; Castaldo, C.; Di Meglio, F.; Nurzynska, D.; Montagnani, S.; Chello, M.; Acar, C. Reinforcement of the pulmonary artery autograft with a polyglactin and polydioxanone mesh in the Ross operation: Experimental study in growing lamb. J. Heart Valve Dis. 2014, 23, 145–148. [Google Scholar]
- Spadaccio, C.; Nappi, F.; Al-Attar, N.; Sutherland, F.W.; Acar, C.; Nenna, A.; Trombetta, M.; Chello, M.; Rainer, A. Old Myths, New Concerns: The Long-Term Effects of Ascending Aorta Replacement with Dacron Grafts. Not All That Glitters Is Gold. J. Cardiovasc. Transl. Res. 2016, 9, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Mozetic, P.; Nappi, F.; Nenna, A.; Sutherland, F.; Trombetta, M.; Chello, M.; Rainer, A. Cells and extracellular matrix interplay in cardiac valve disease: Because age matters. Basic Res Cardiol. 2016, 111, 16. [Google Scholar] [CrossRef] [PubMed]
- Nataf, P.; Guettier, C.; Bourbon, A.; Nappi, F.; Lima, L.; Dorent, R.; Pavie, A.; Gandjbakhch, I. Influence of arterial allograft preparation techniques on chronic vascular rejection: A histological study. Transpl. Proc. 1996, 28, 2890–2892. [Google Scholar]
- Carrel, T.; Kadner, A. Long-Term Clinical and Imaging Follow-Up After Reinforced Pulmonary Autograft Ross Procedure. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2016, 19, 59–62. [Google Scholar] [CrossRef]
- Jacobsen, R.M.; Earing, M.G.; Hill, G.D.; Barnes, M.; Mitchell, M.E.; Woods, R.K.; Tweddell, J.S. The Externally Supported Ross Operation: Early Outcomes and Intermediate Follow-Up. Ann. Thorac. Surg. 2015, 100, 631–638. [Google Scholar] [CrossRef]
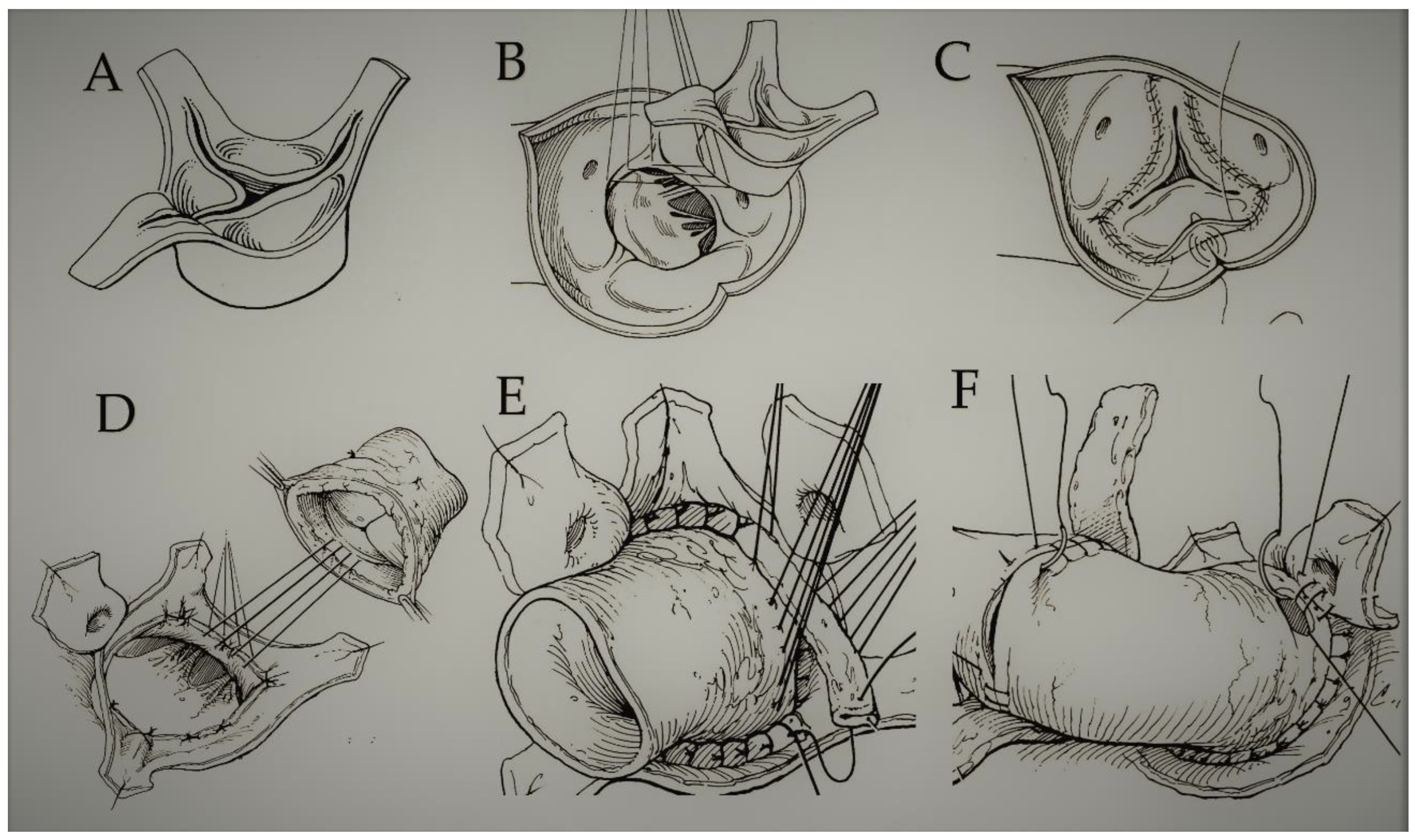
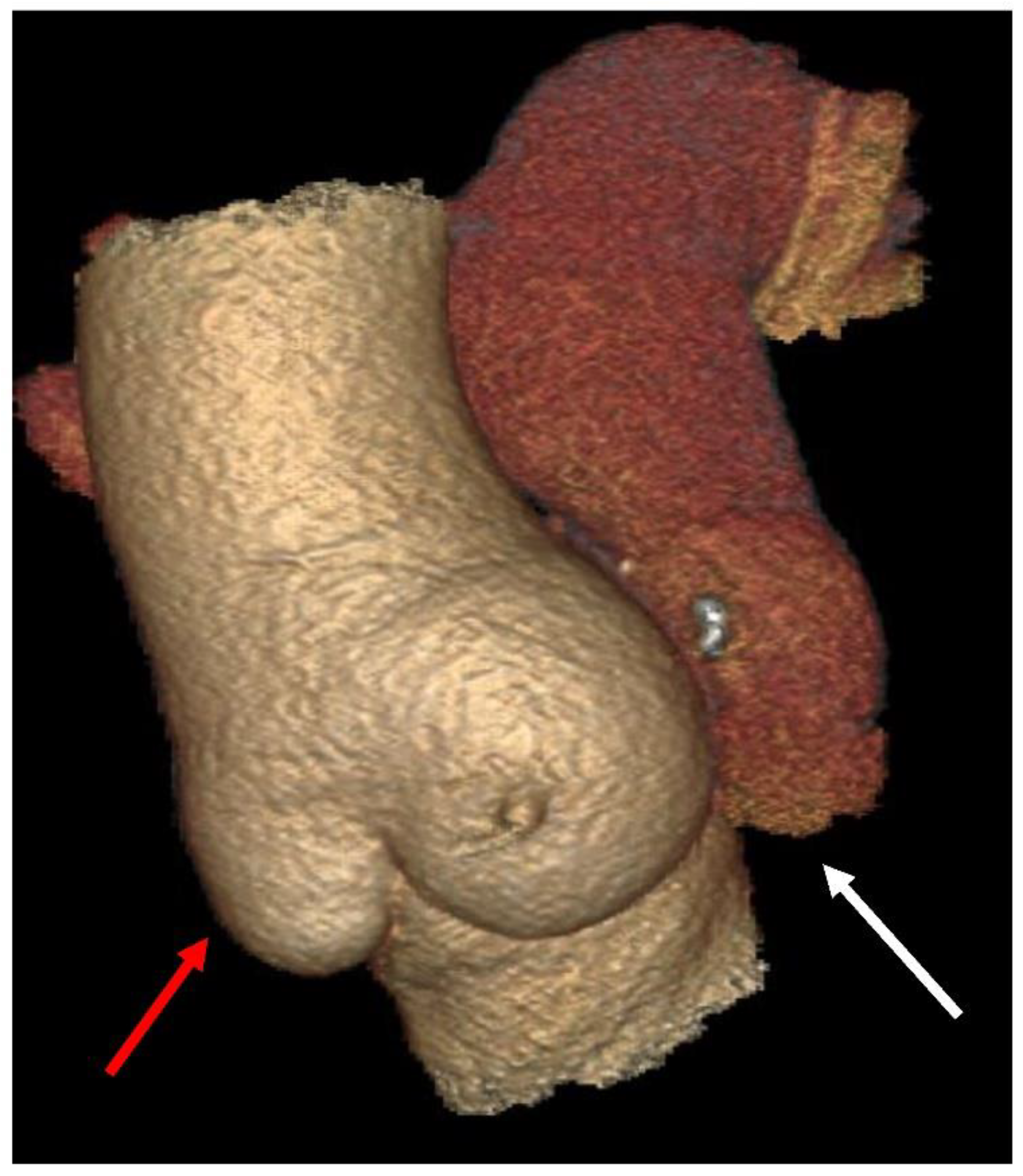
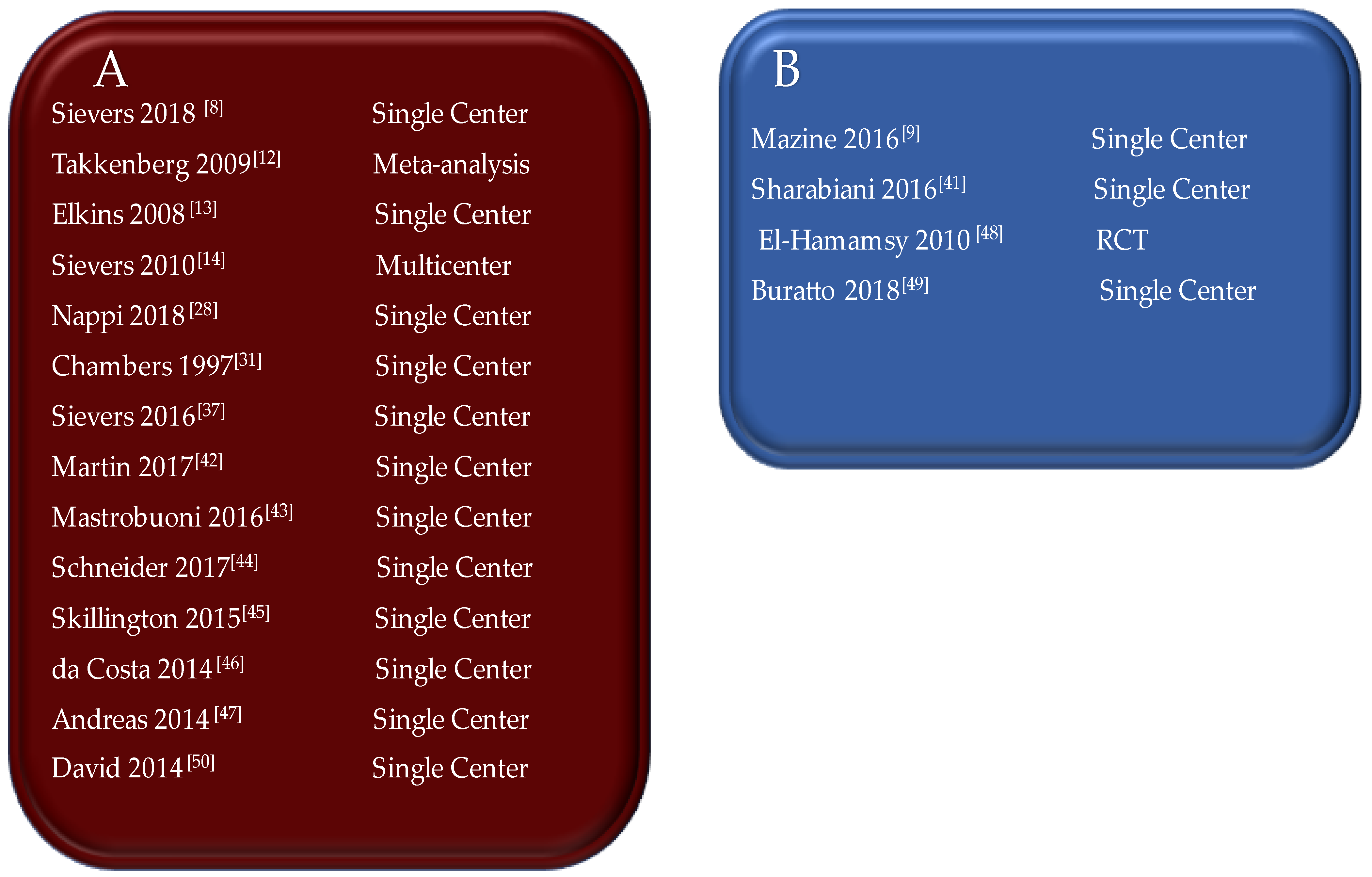

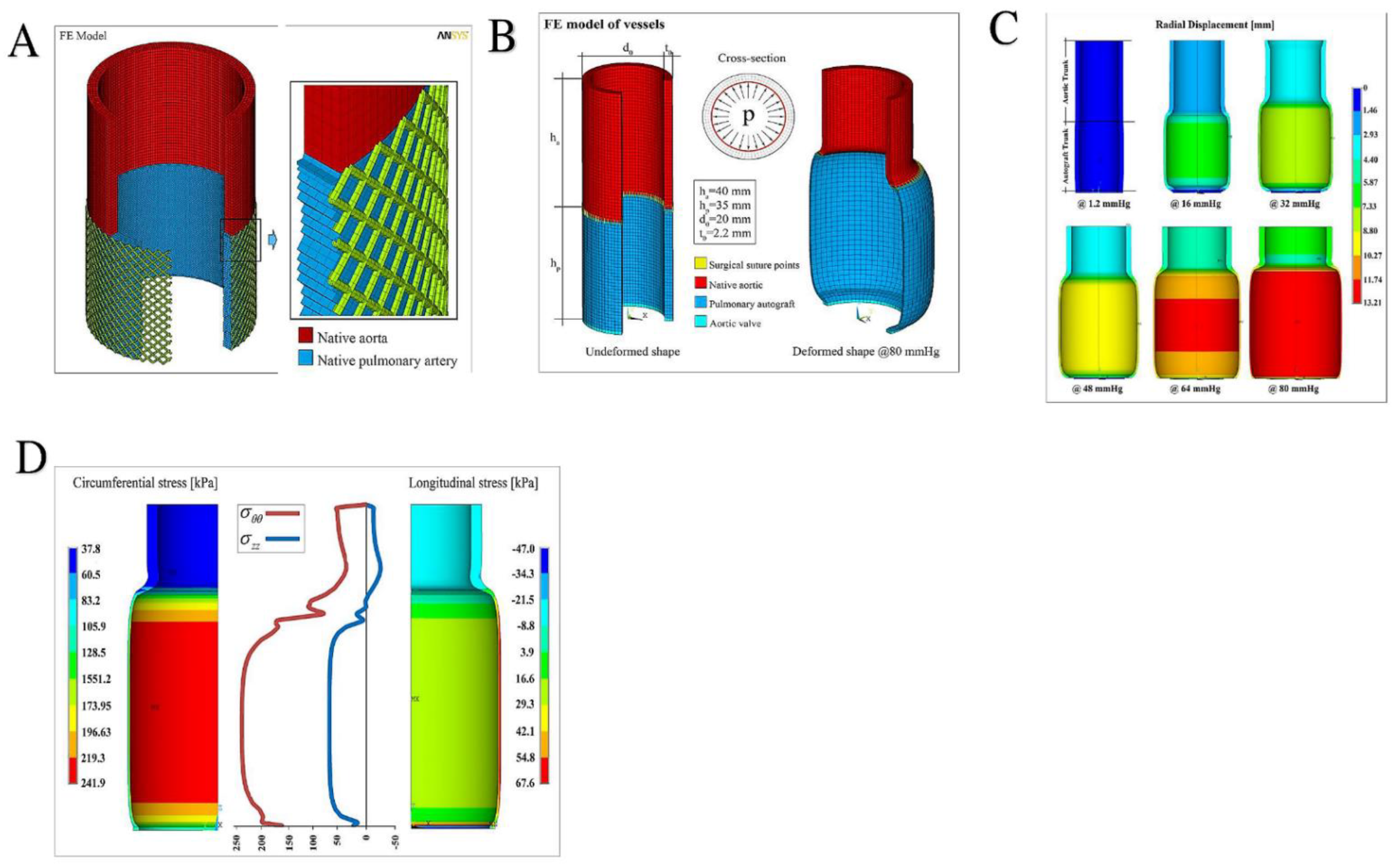
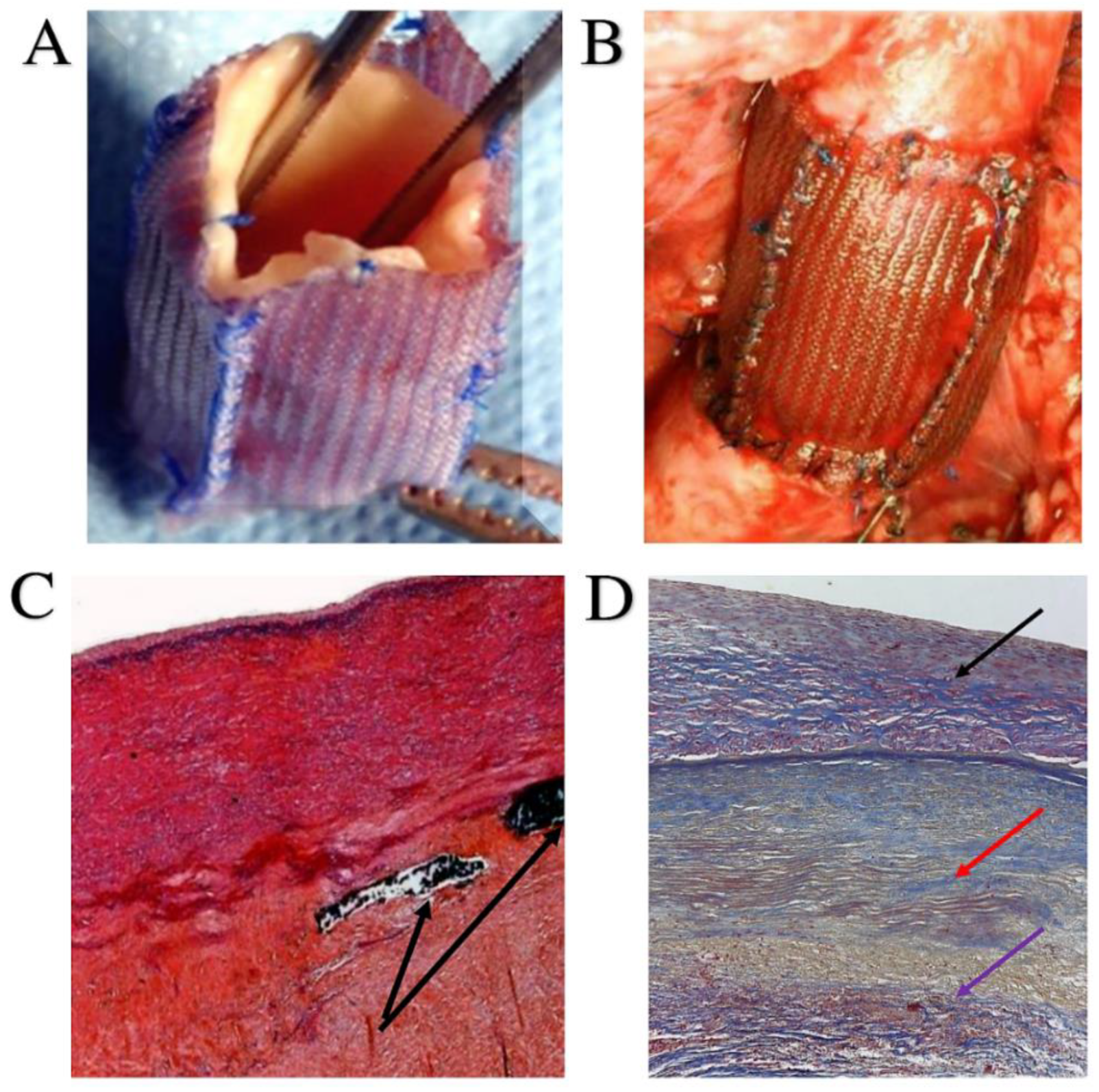
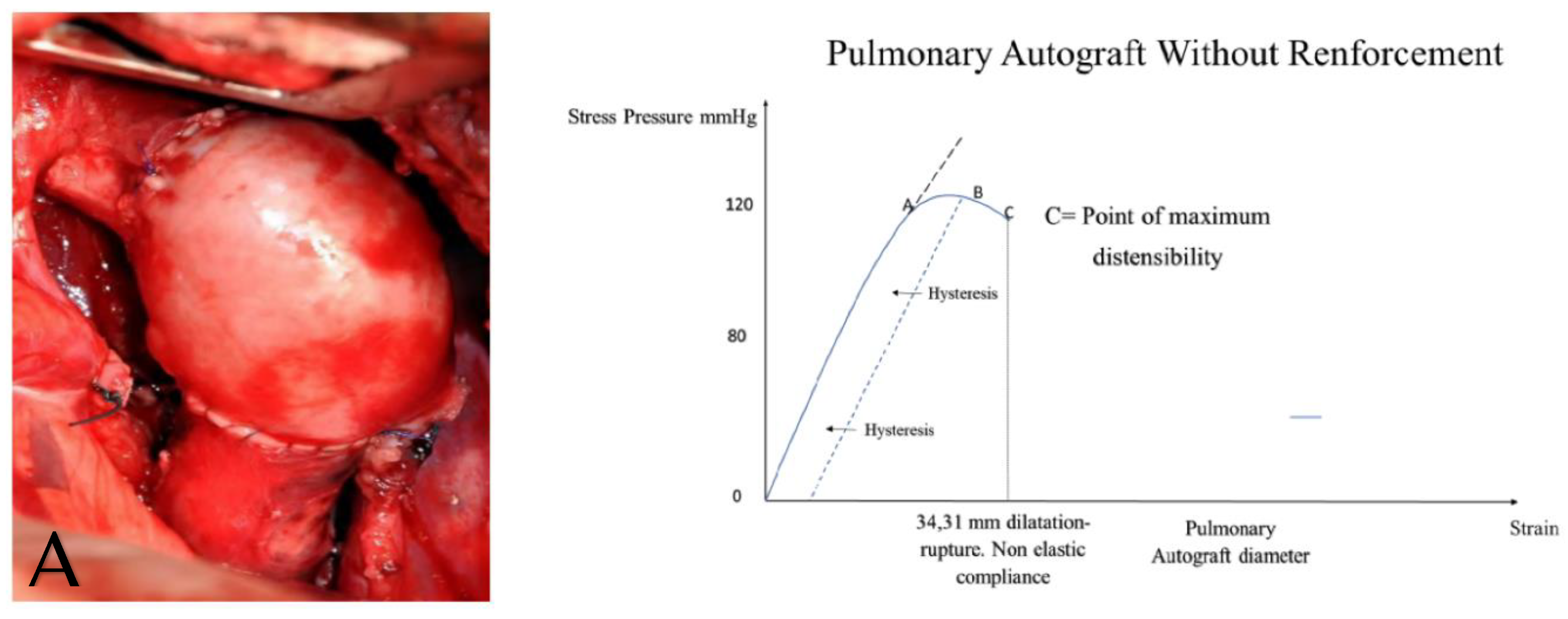
| First Author/Year of Publication (Ref.) | Study Type | Period of Surgery | Number of Patients (N) | Mean Age, y (Range) | Surgical Technique | Biomechanical Findings |
|---|---|---|---|---|---|---|
| Aboud 2021 [61] JACC Germany | Retrospective | 1988–2001 | 2244 | 33 (16–61) | RR Root SC | † At 25 yrs excellent biomechanical functioning in unreinforced PA root with slight dilatation. No dilatation in RR. Excellent biomechanical functioning of PA valve in SC implantation |
| Nappi 2018 [28] ICVTS France | Retrospective | 1998–2002 | 66 | 29 (16 mth–62) | RR Root SC | † At 22 yrs excellent biomechanical functioning in unreinforced PA root with slight dilatation. No dilatation in reinforced Ross. Excellent biomechanical functioning of PA valve in SC implantation |
| Sievers 2016 [37] EJCTS Germany | Retrospective/Prospective | 1990–2013 | 1779 569 (16–40 yrs) | 31 (16–40) | RR Root SC | † At 20 yrs Excellent biomechanical functioning in unreinforced PA root with slight dilatation. No dilatation in reinforced Ross. Excellent biomechanical functioning of PA valve in SC implantation |
| Andreas 2014 [47] Annals Germany | Retrospective | 1991–2011 | 246 | 25 (5–46) | RR Root | Slight dilatation in PA root implanted at STJ with excellent biomechanical performance |
| Da Costa 2014 [46] Eur J Cardiothorac Surg Brasil | Retrospective | 1995–2013 | 441 | 31 (5–56) | Root/IC/ SC | Slight dilatation in PA root implanted at STJ with excellent biomechanical performance. Excellent biomechanical performance of PA valve in SC implantation |
| Ruzmetov 2012 [62] Ann Thorac Surg. USA | Retrospective | 1990–2011 | 106 | 18 (1 mth–40) | Root | Slight dilatation in PA root implanted at STJ. Biomechanical performance of PA root guaranteed no failure |
| Bohm 2009 [63] Ann Thorac Surg. Germany | Retrospective | 1995-2006 | 467 | 41 (26–56) | Root/ SC | Slight dilatation in PA root with excellent biomechanical performance. No failure of PA valve with biomechanical performance in young adults with SC implantation |
| Elkins 2008 [13] JTCVS USA | Retrospective | 1986-2002 | 487 | 24 (2–62) | Root SC | Slight dilatation in PA root reinforced at level of STJ. Excellent biomechanical performance of PA root and valve |
| Klieverik 2008 [64] EHJ Holland | Prospective | 1987-2007 | 63 | 29 (16–52) | Root/IC | Excellent biomechanical functioning in reinforced PA root with IC procedure Slight dilatation of PA |
| Klieverik 2007 [65] EHJ Holland | Prospective | 1988–2005 | 146 | 22 (0.3–52) | Root/IC | Excellent biomechanical functioning in reinforced PA root with IC procedure Slight dilatation of PA |
| Chiappini 2007 [66] Ann Thorac Surg | Retrospective | 1991–2005 | 219 | 36 (0.5–64) | Root/IC/ Subcoronary | PA root reinforced with IC technique guaranteed slight dilatation and excellent biomechanical functioning |
| Pasquali 2007 [67] JTCVS | Retrospective | 1995–2004 | 121 | 8.2 (0–34) | RK Root | No PA root dilatation in RR. No histological studies have tested the detrimental effect of Dacron graft on biomechanics of RR |
| Brown 2007 [34] Ann Thorac Surg USA | Retrospective | 1993–2005 | 170 | 25 (0–61) | RK Root | No PA root dilatation No histological studies have tested the detrimental effect of Dacron graft on biomechanics in RR |
| Kumar 2005 [68] Ann Thorac Surg | Retrospective | 1993–2003 | 153 | 28 (0–65) | Root | Optimal biomechanical performance in PA root with slight dilatation. No use of external reinforcement |
| Kumar 2006 [69] Eur J Cardiothorac Surg. | Retrospective | 1993–2003 | 81 | 21 (0–51) | Root | Excellent biomechanical functioning in PA root with slight dilatation. No use of external reinforcement |
| Kouchoukos 2004 [70] Ann Thorac Surg USA | Retrospective | 1989–2002 | 119 | 31 (5–56) | Root | Optimal biomechanical functioning in PA root with slight dilatation. No use of external reinforcement in RR |
| Luciani 2012 [71] EJCTS Italy | Retrospective | 1994–2004 | 112 | 29 (6–49) | Root/IC/ SC | Slight dilatation in PA root implanted in IC. Biomechanical performance of PA root guaranteed no failure. Excellent biomechanical performance of PA valve in SC implantation and in IC technique |
| Raja 2004 [72] BMC Cardiovasc Disord UK | Retrospective | 1996–2003 | 38 | 13 (1–30) | Root | Optimal biomechanical performance in PA root with no dilatation. No use of external reinforcement in RR |
| Alphonso 2004 [73] Eur J Cardiothorac Surg. | Retrospective | 1991–2002 | 60 | 15 (0.5–67) | SC/IC | Very good biomechanical performance of PA valve in SC implantation using IC technique. |
| Sakaguchi 2003 [74] J Heart Valve Dis | Retrospective | 1986–2000 | 399 | 23 (0–59) | Root/IC/ SC | Optimal biomechanical performance of PA valve in SC implantation using IC technique. Slight expansion in PA root implant |
| Concha 2003 [75] Eur J Cardiothorac Surg | Prospective | 1991–2002 | 169 | 30 (0–54) | Root | Excellent biomechanical performance in PA root implant with slight expansion. No use of external reinforcement |
| Takkenberg 2002 [76] Eur J Cardiothorac Surg Holland. | Retrospective | 1988–2000 | 343 | 26 (0–58) | Root/IC/ SC | Excellent biomechanical performance in unreinforced PA root using the IC technique. Slight dilatation. Excellent biomechanical functioning of PA valve in SC implantation |
| Pessotto 2001 [77] Ann Thorac Surg. | Retrospective | 1992–1999 | 111 | 16 (0–67) | Root SC | No PA root expansion with optimal biomechanical performance in unreinforced root. No PA valve failure in SC implantation with excellent biomechanical functioning. |
| Laudito 2001 [78] JTCVS | Retrospective | 1993–2000 | 72 | 9 (0–40) | RK Root | Preserved biomechanical features of PA root |
| Sharoni 2000 [79] Isr Med Assoc J. Israel | Retrospective | 1996–1999 | 40 | 8 (0–41) | Root | Slight expansion of unreinforced PA root with preserved biomechanical features of PA root |
| Moidl 2000 [80] J Heart Valve Dis. | Prospective | 1991 | 109 | 32 (6–59) | Root Subcoronary | Slight expansion of unreinforced PA root with preserved biomechanical features. Optimal performance of PA valve in SC implantation |
| Chambers1997 [31] Circulation UK | Retrospective | 1967–1984 | 131 | 32 (11–52) | Root/SC | Slight expansion of unreinforced PA root with preserved biomechanical features. Optimal performance of PA valve in SC implantation |
| Matsuki 1988 [81] JTCVS Japan | Retrospective | 1967–1986 | 241 | (9–60) | SC | 25 yrs follow up optimal performance of PA valve in SC implantation without failure |
| Gula 1979 [82] Ann Thorac Surg Japan | Retrospective | 1967–1977 | 188 | 30 (9–64) | SC | Optimal performance of PA valve in SC implantation without failure |
| Somerville 1979 [83] Br Heart J UK | Retrospective | 1967–1972 | 85 | 30 (12–54) | SC | Optimal performance of PA valve in SC implantation without failure |
| First Author/Year of Publication/Location (Ref.) | Study Type | Period of Surgery | Number of Patients (N) | Mean Age, y (Range) | Surgical Technique | Biomechanical Findings |
|---|---|---|---|---|---|---|
| Stewart 2007 [84] Ann Thorac Surg. | Retrospective | 1994–2005 | 46 | 13 (1–21) | Root | Optimal biomechanics with slight dilatation in PA unreinforced root |
| Ruzmetov 2012 [62] Int J Cardiol USA | Retrospective | 1993-2005 | 81 | <18 yrs | Root/IC | No dilatation in PA root with IC. Optimal biomechanical performance without failure in PA valve and root |
| Kalavrouziotis 2006 [85] Hellenic J Cardiol. Greece | Retrospective | 1996–2004 | 35 | 10 (0.3–18) | Root | Optimal biomechanics of PA valve and root with slight dilatation of PA root |
| Bohm 2006 [86] Ann Thorac Surg. Germany | Retrospective | 1995–2004 | 60 | 12 (1–20) | Root | Slight dilatation in PA unreinforced root. Preserved biomechanics of PA valve and root |
| Takkenberg 2005 [87] Ann Thorac Surg. Holland | Prospective | 1988–2003 | 47 | 8 (0–15) | Root | Optimal biomechanics of PA valve and root in absence of IA. No dilatation in PA unreinforced root |
| Khwaja 2005 [88] Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. USA | Retrospective | 1992–2005 | 53 | 14 (10–21) | Root | Slight dilatation in PA unreinforced root. Preserved biomechanics of PA valve and root |
| Hazekamp 2005 [89] J Cardiothorac Surg. | Retrospective | 1994–2003 | 53 | 9 (0–18) | Root | Slight dilatation in PA unreinforced root. Optimal biomechanics of PA valve and root |
| Hraska 2004 [90] Eur J Cardiothorac Surg | Retrospective | 1997–2003 | 66 | 13 (0–23) | Root/RK | No dilatation in PA-RR with Dacron graft. Very good biomechanical performance without failure in PA valve and root |
| Al-Halees 2002 [91] J Thorac Cardiovasc Surg | Retrospective | 1990–2000 | 53 | 8 (0–18) | Root/IC | No dilatation in PA root with IC. Optimal biomechanical performance without failure in PA valve and root |
| Elkins 2001 (61) J Heart Valve Dis USA | Retrospective | 1986–2001 | 178 | 10 (0–18) | Root/IC | No dilatation in PA root with IC. Optimal biomechanical performance without failure in PA valve and root |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F.; Avtaar Singh, S.S. Biomechanics of Pulmonary Autograft as Living Tissue: A Systematic Review. Bioengineering 2022, 9, 456. https://doi.org/10.3390/bioengineering9090456
Nappi F, Avtaar Singh SS. Biomechanics of Pulmonary Autograft as Living Tissue: A Systematic Review. Bioengineering. 2022; 9(9):456. https://doi.org/10.3390/bioengineering9090456
Chicago/Turabian StyleNappi, Francesco, and Sanjeet Singh Avtaar Singh. 2022. "Biomechanics of Pulmonary Autograft as Living Tissue: A Systematic Review" Bioengineering 9, no. 9: 456. https://doi.org/10.3390/bioengineering9090456
APA StyleNappi, F., & Avtaar Singh, S. S. (2022). Biomechanics of Pulmonary Autograft as Living Tissue: A Systematic Review. Bioengineering, 9(9), 456. https://doi.org/10.3390/bioengineering9090456