The Impacts of Frozen Material-Other-Than-Grapes (MOG) on Aroma Compounds of Cabernet Franc and Cabernet Sauvignon
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Commercial Wines
2.2. Gas Chromatography-Mass Spectrometry
2.3. GC-MS Conditions; Conditioning of Material
2.4. Calibration Compounds and Odor Activity Values
2.5. Conventional Analysis
2.6. Analysis of Controlled Fermentations
2.7. Sensory Analysis
2.7.1. Commercial Samples
2.7.2. Controlled Fermentations
2.8. Statistical Analysis
3. Results
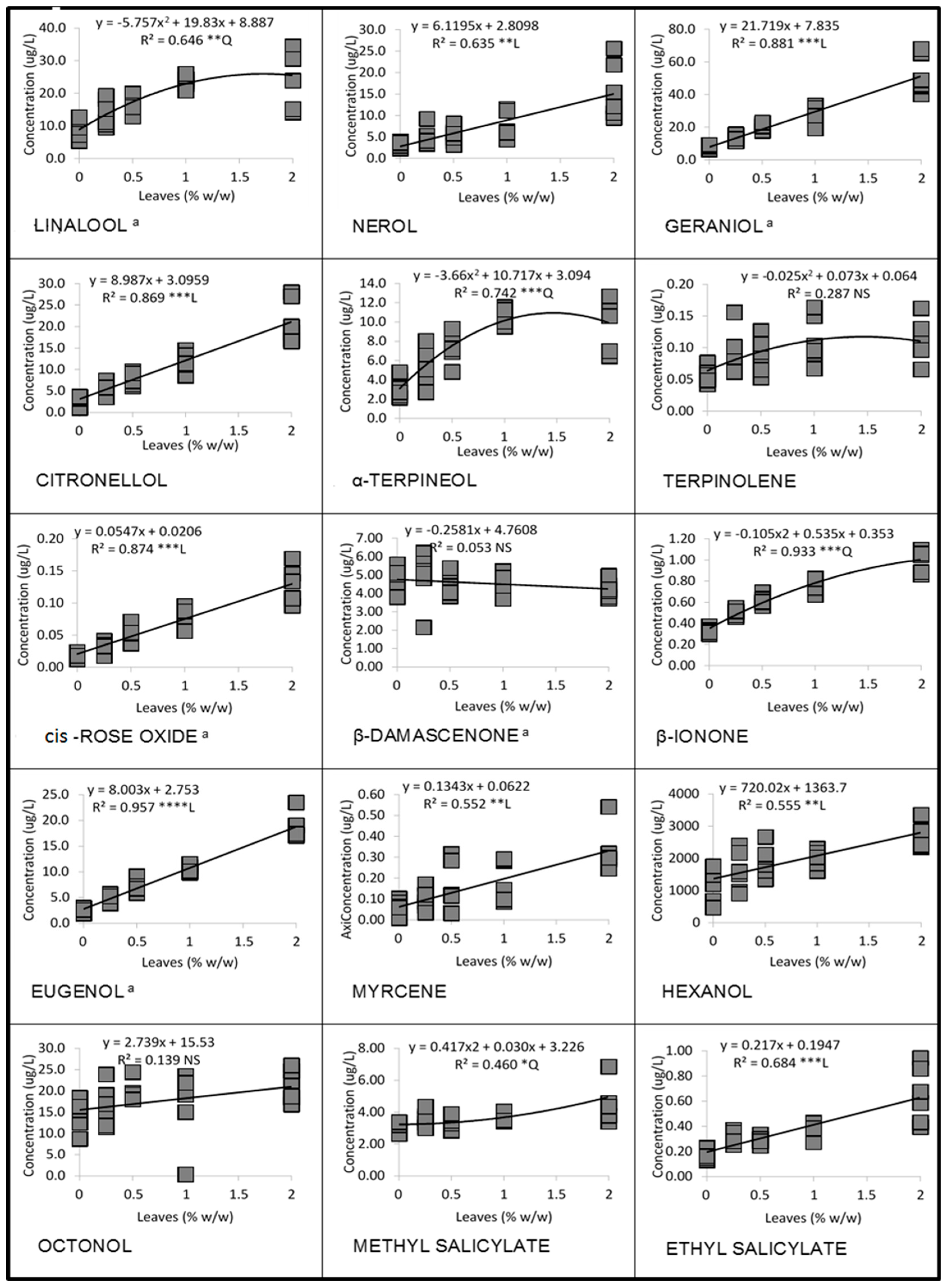
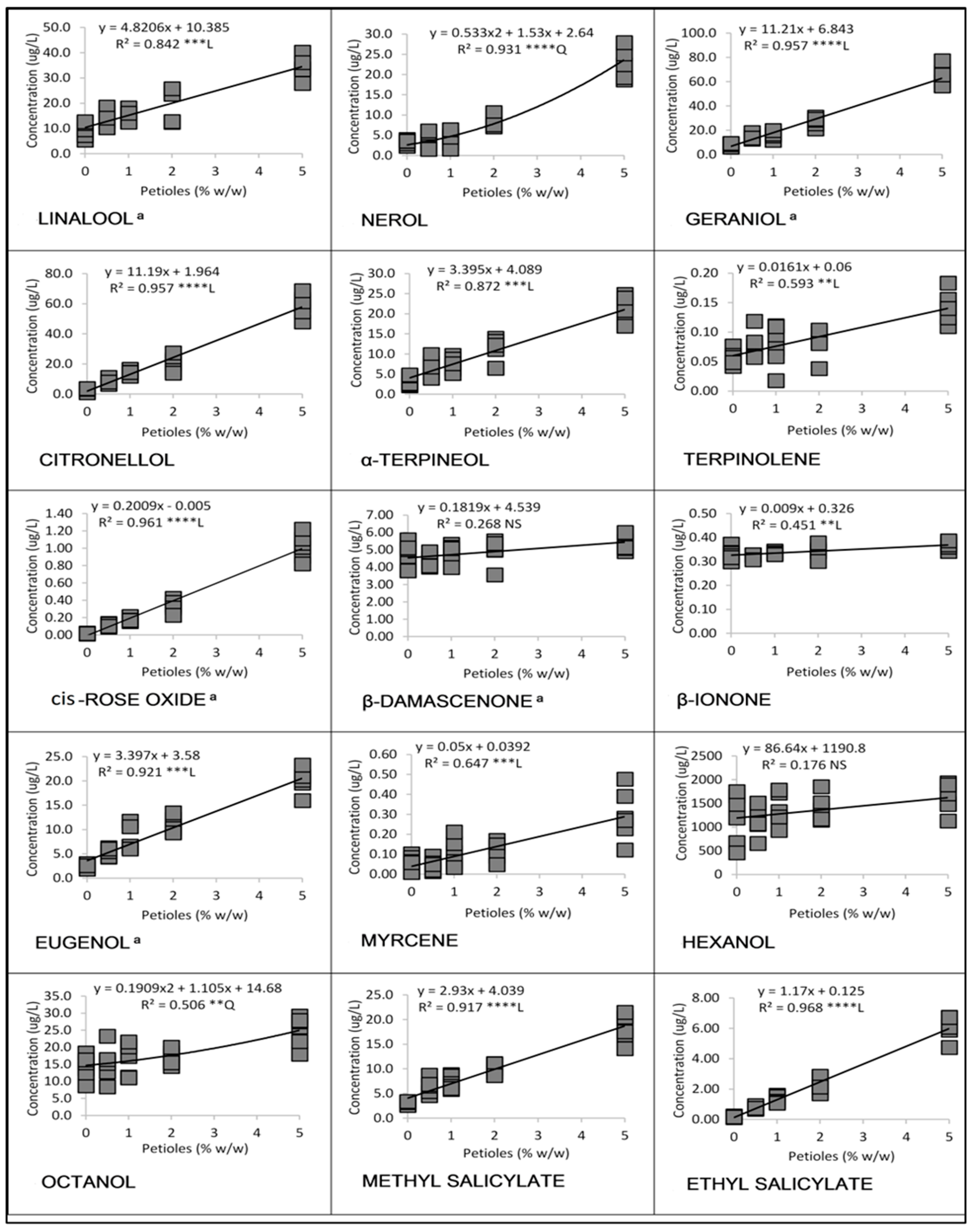
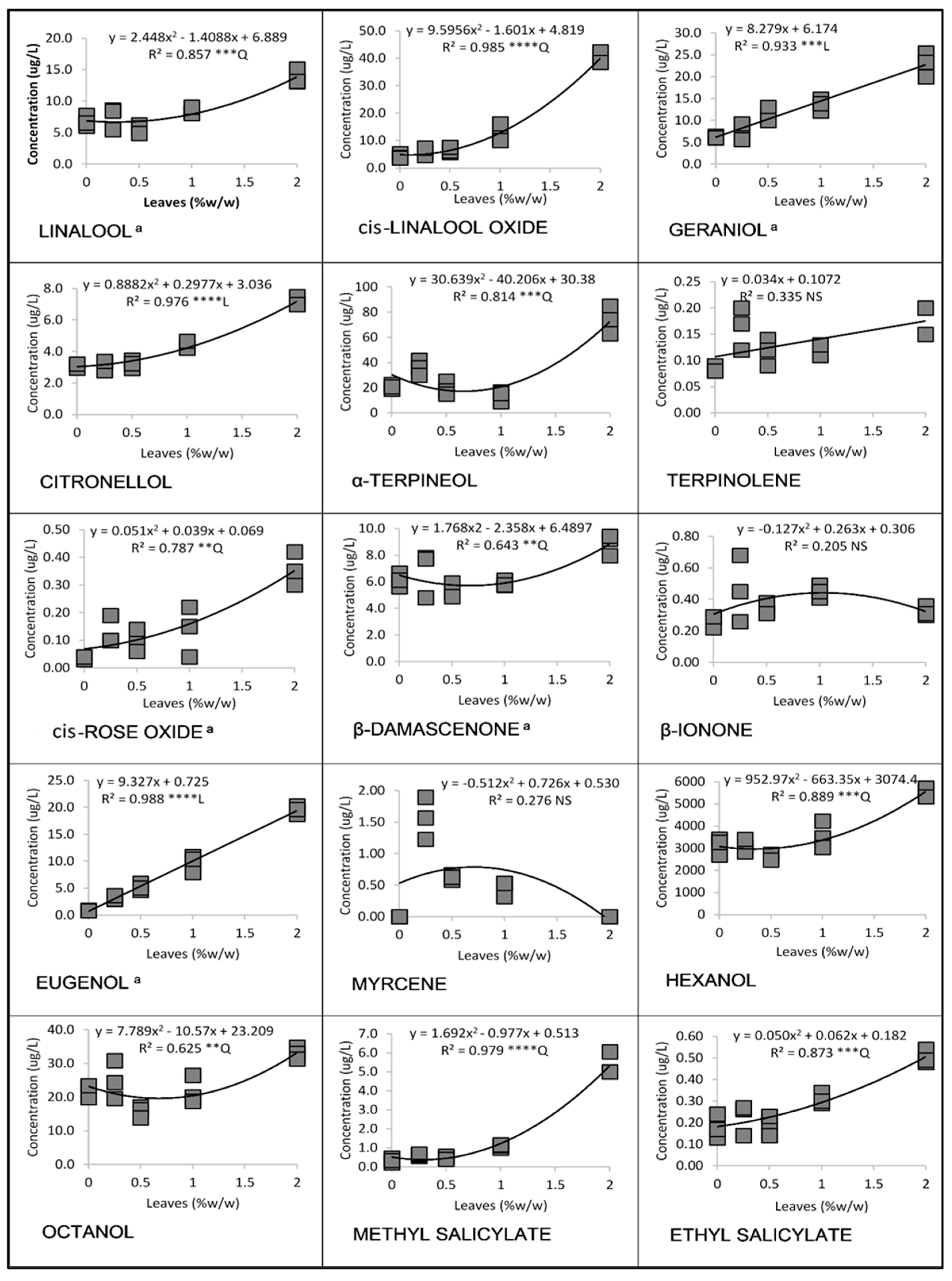
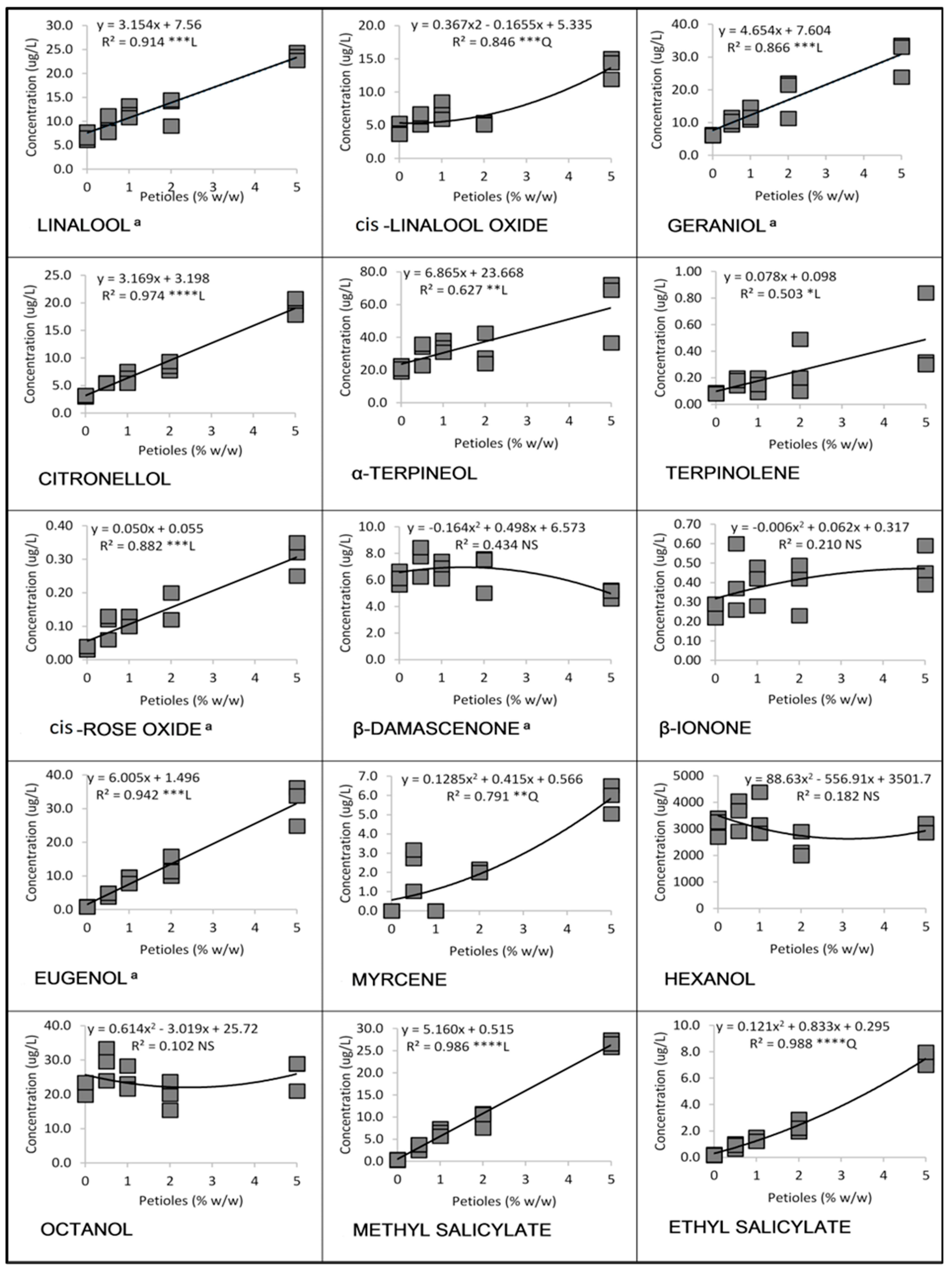
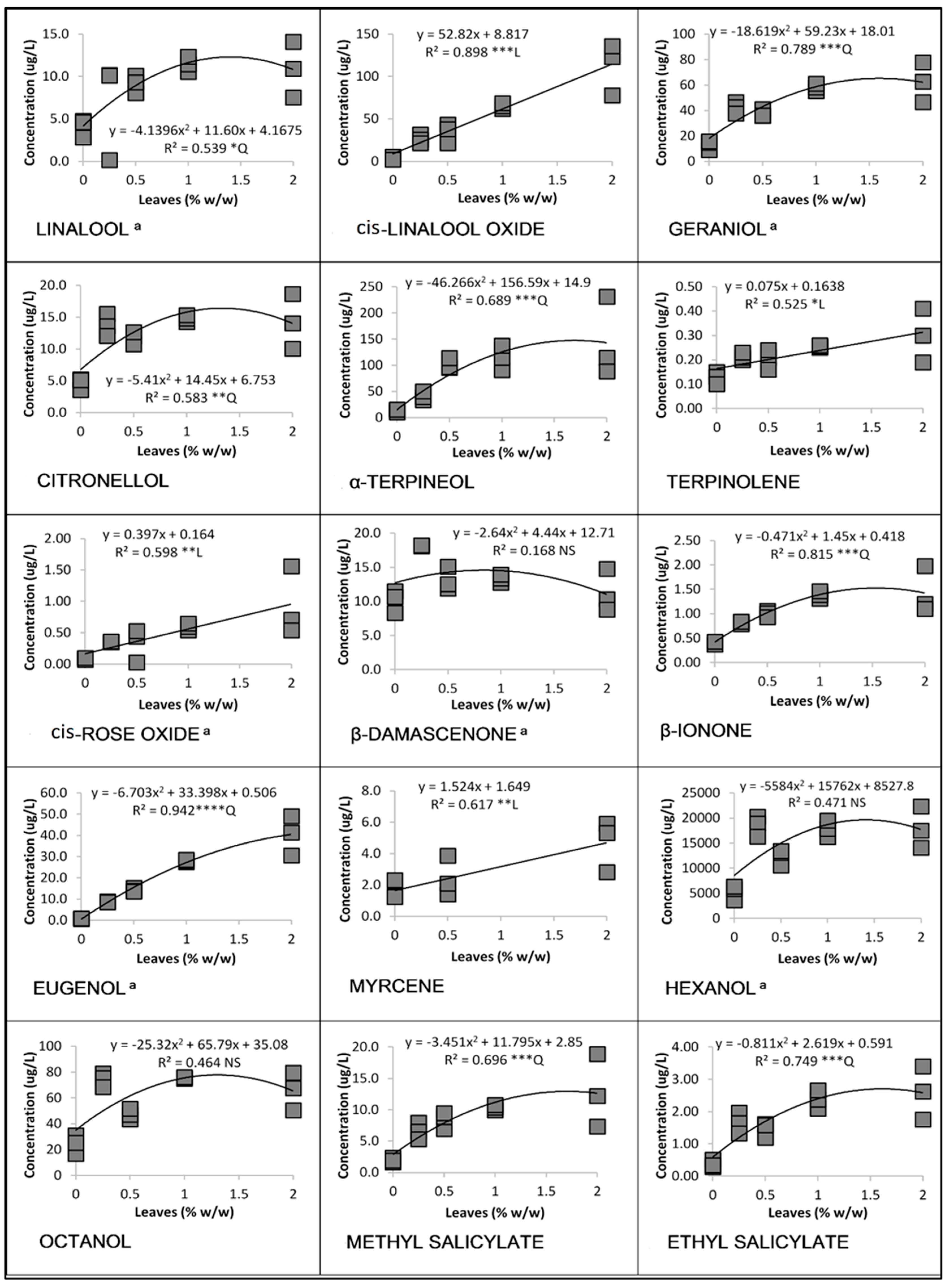
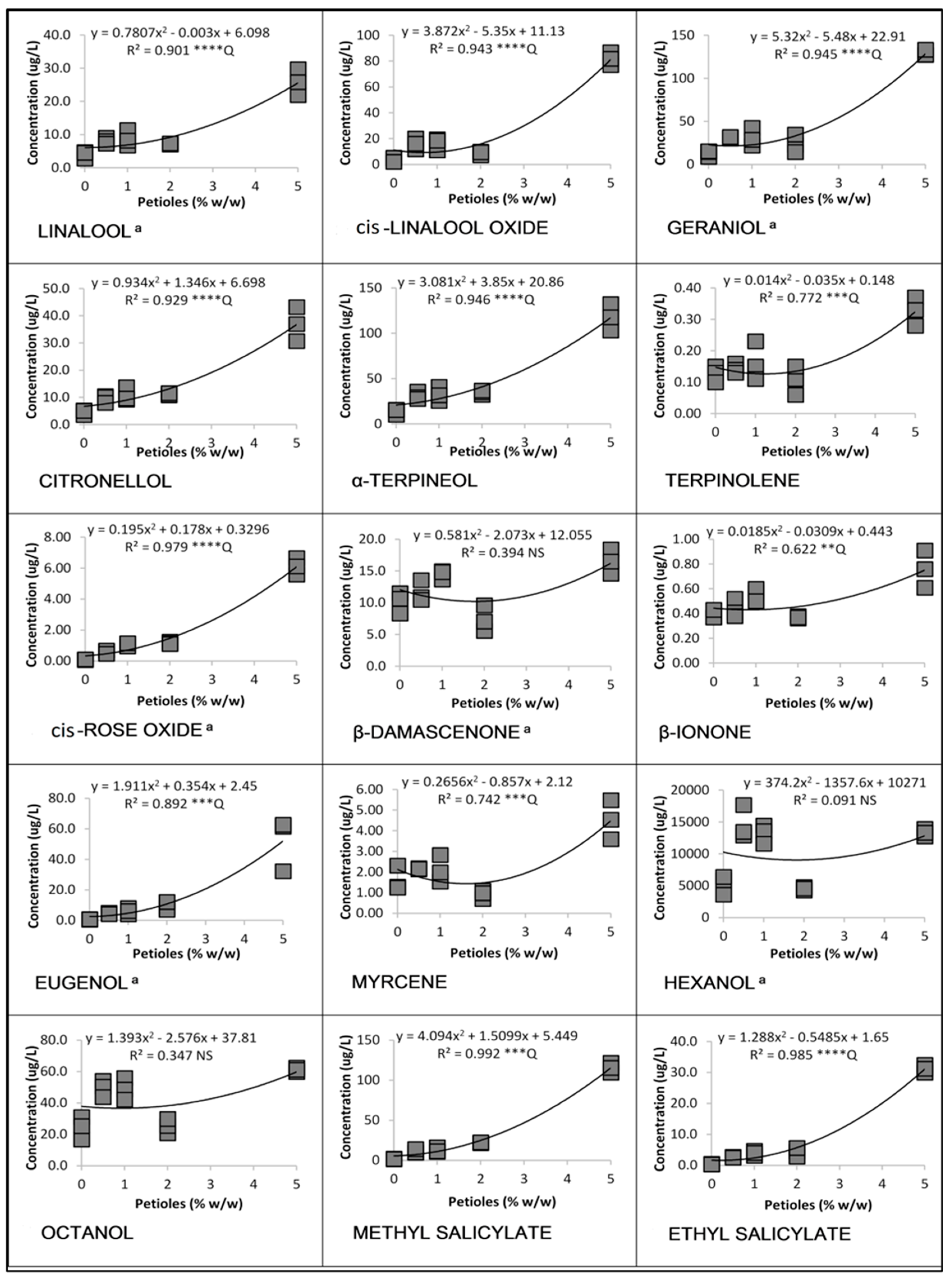
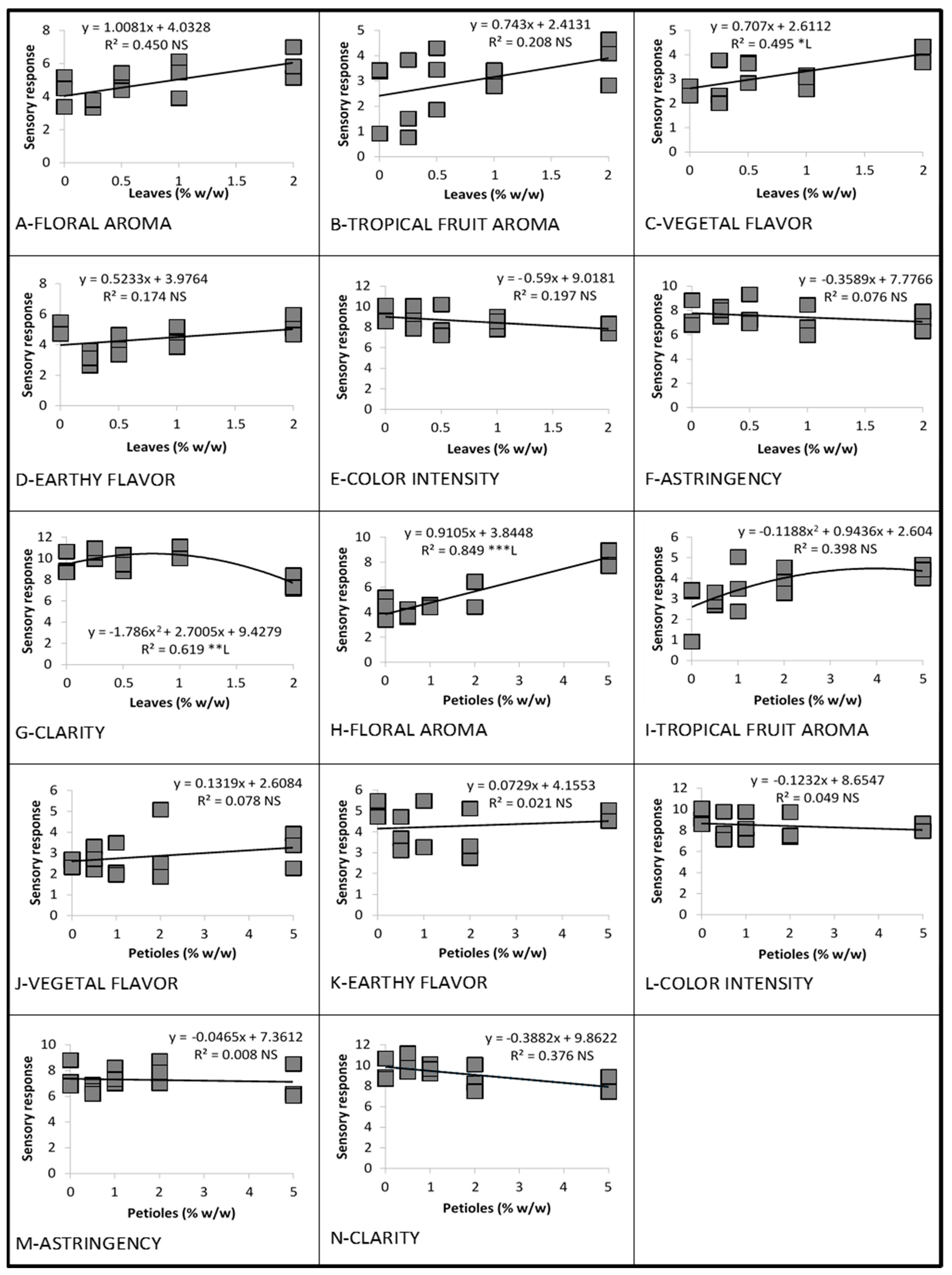
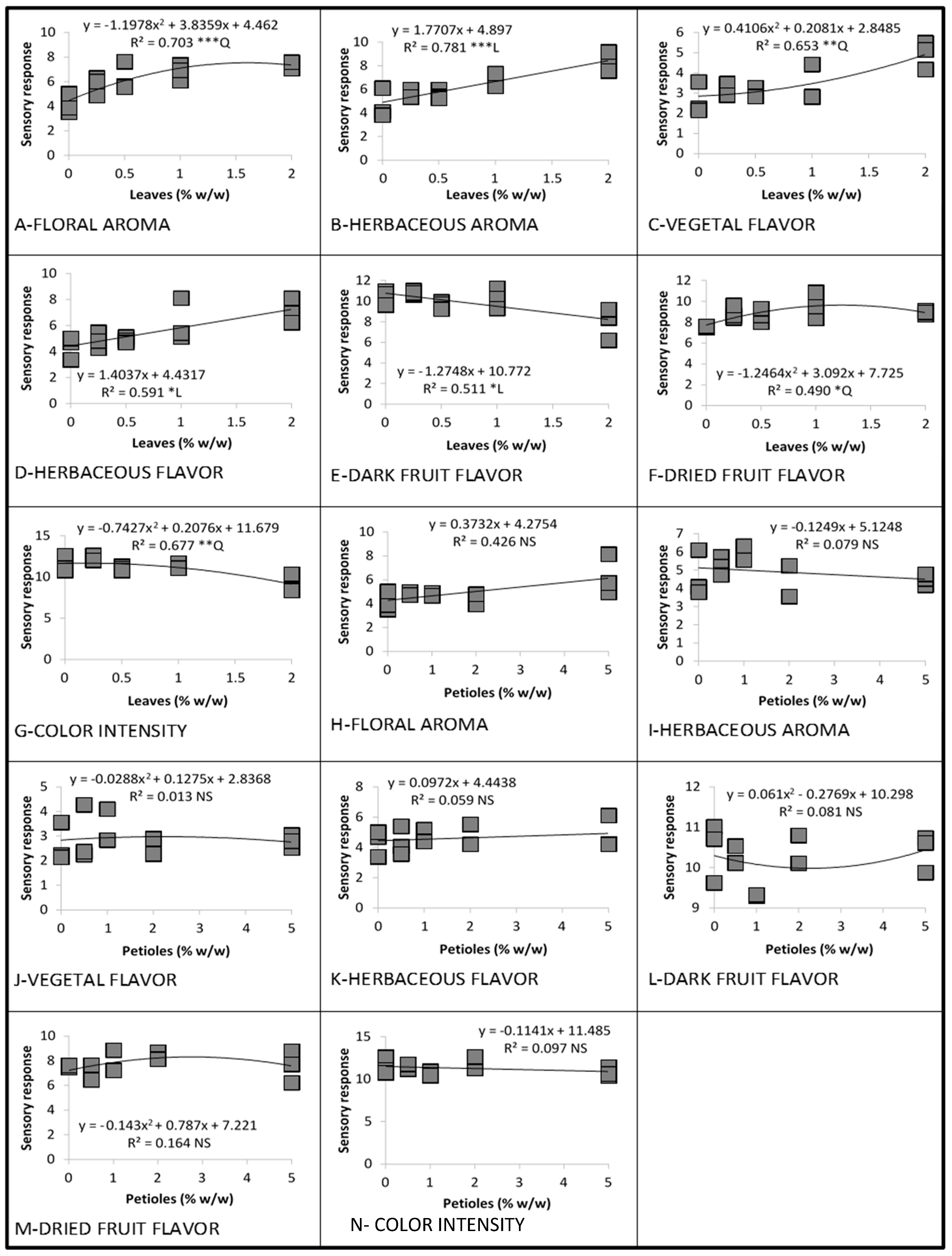
3.1. Linear and Quadratic Relationships
3.1.1. Cabernet Franc in 2016
3.1.2. Cabernet Sauvignon in 2016
3.1.3. Cabernet Franc in 2017
3.1.4. Cabernet Sauvignon in 2017
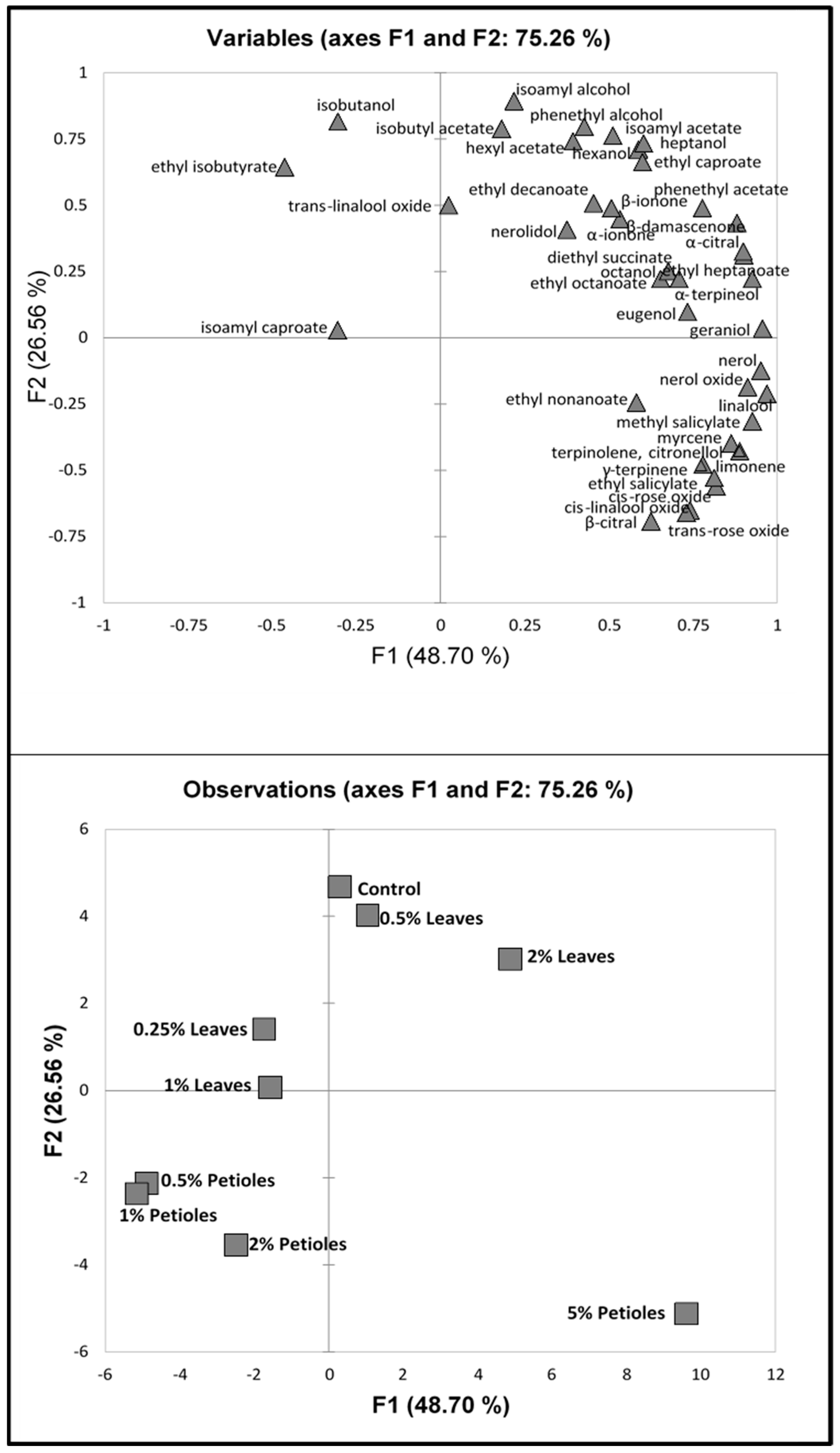
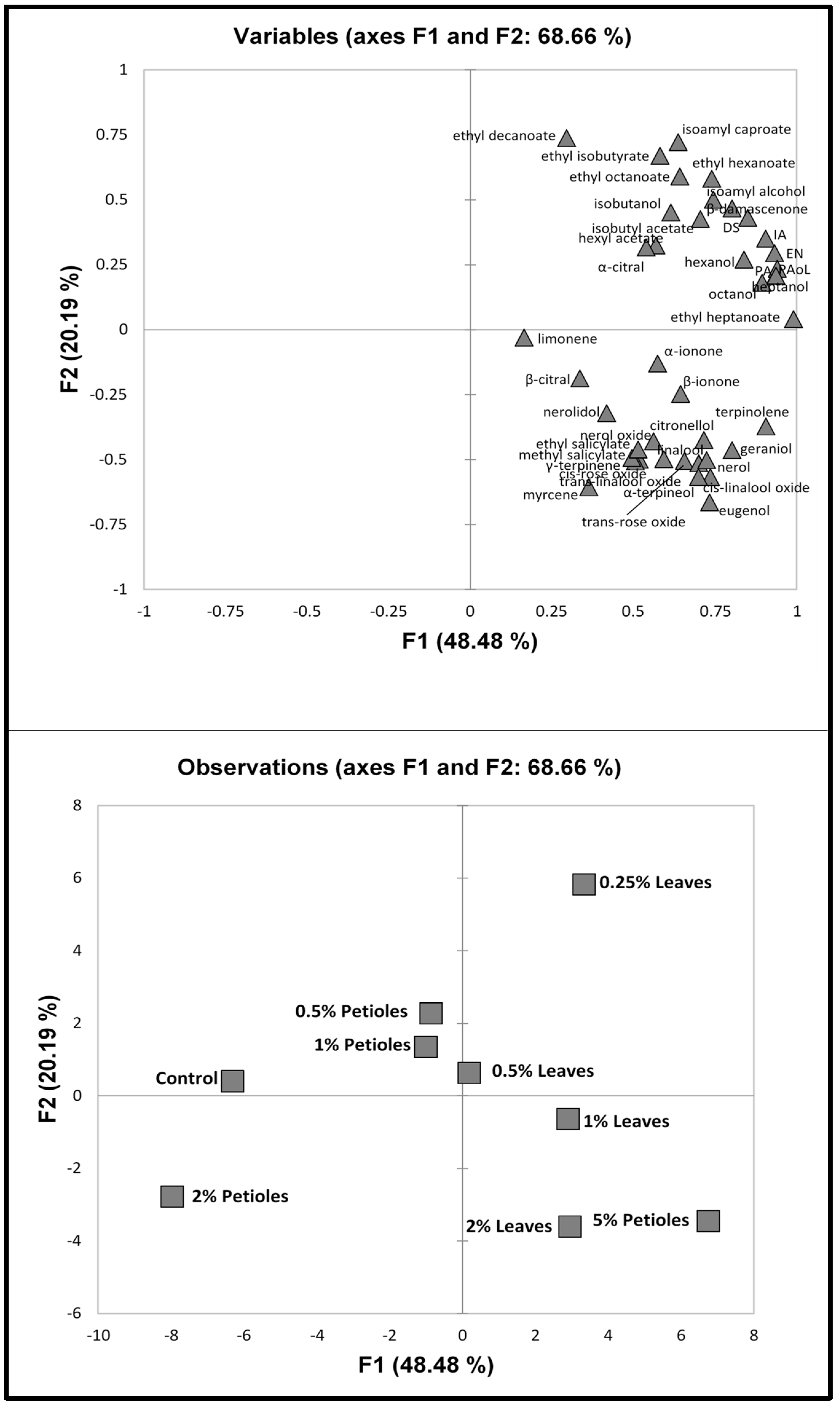
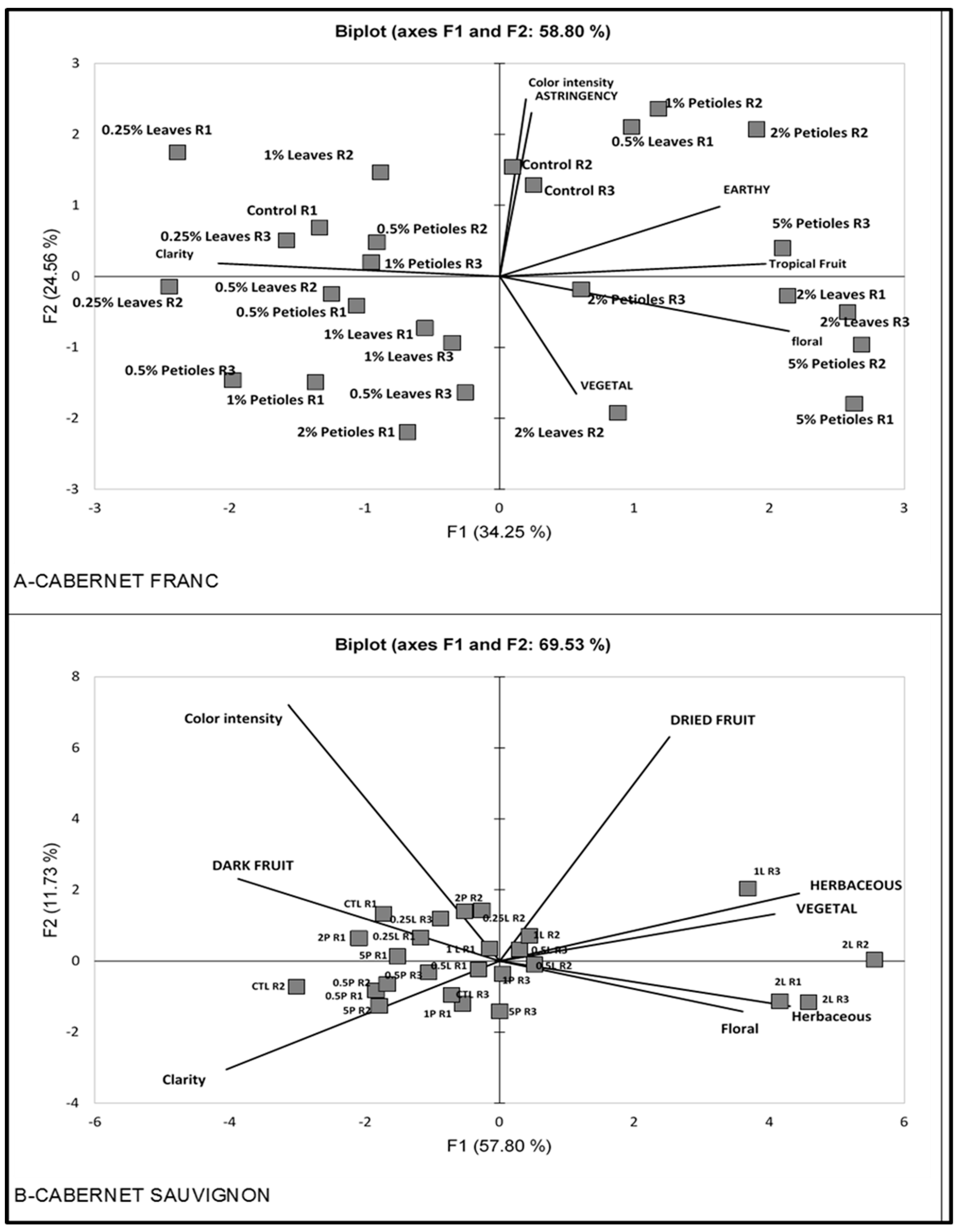
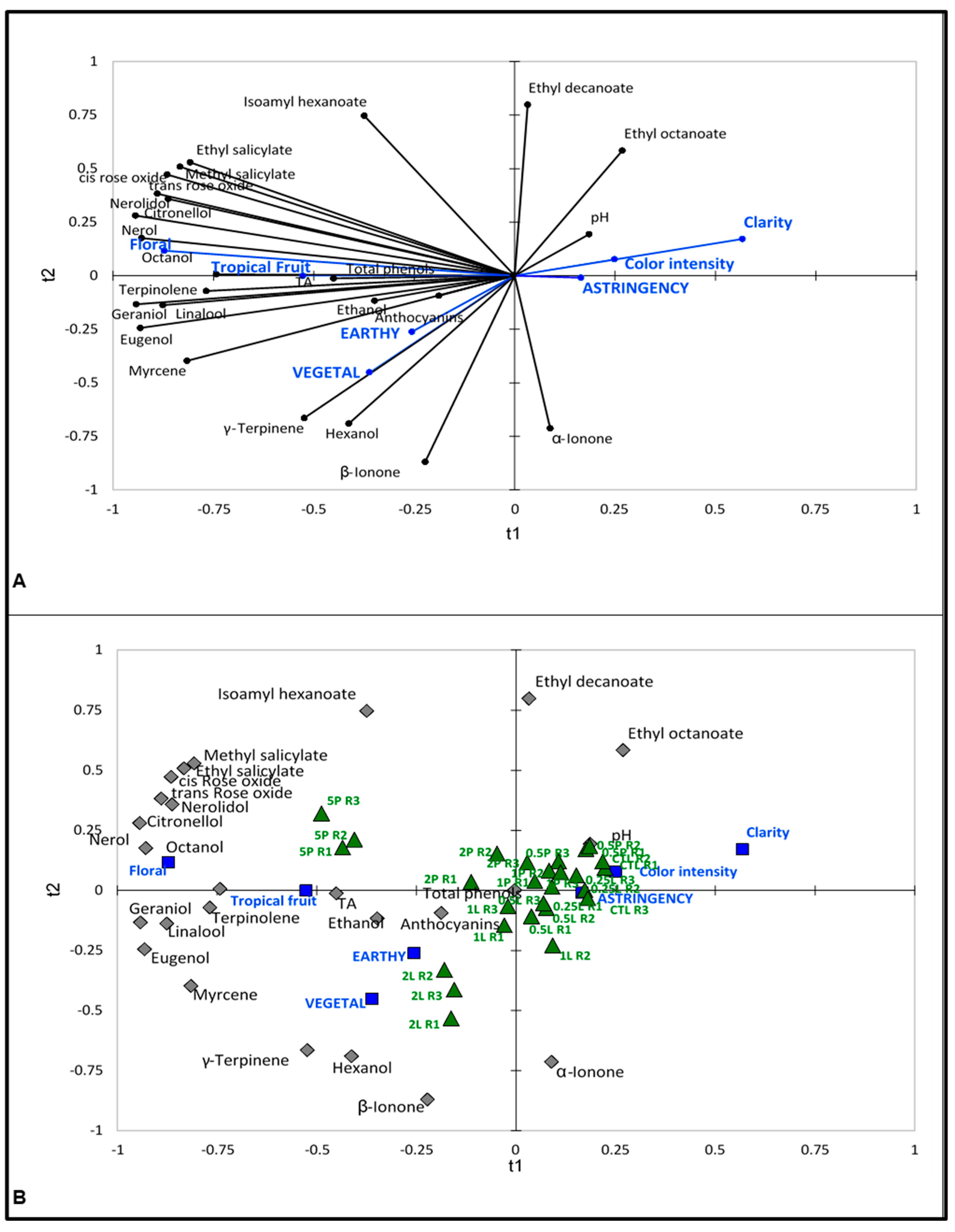
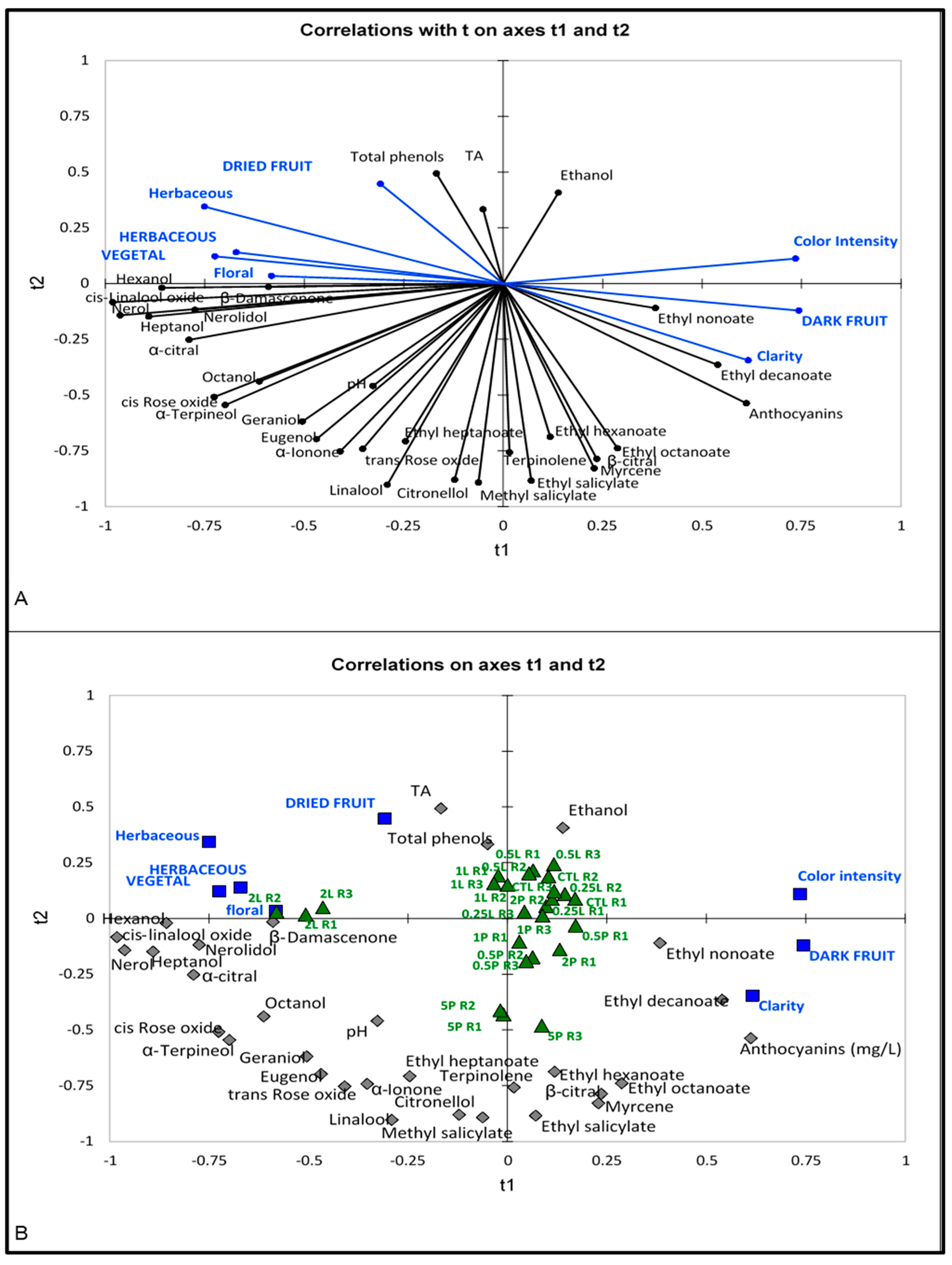
3.2. Multivariate Statistics
3.2.1. 2016 Vintage
3.2.2. 2017 Vintage
3.3. Sensory Analysis
3.3.1. Linear and Quadratic Relationships
3.3.2. Multivariate Statistics
4. Discussion
4.1. Chemical Composition
4.2. Sensory Profiles
4.3. Correlation between Sensory Attributes, Chemical Profile and MOG Content
4.3.1. Correlation of Sensory Attributes among Wines
4.3.2. Correlation of Sensory Attributes, Aroma Compounds and Basic Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arfelli, G.; Sartini, E.; Bordini, F.; Caprara, C.; Pezzi, F. Mechanical harvesting optimization and postharvest treatments to improve wine quality. OENO One 2010, 44, 101–115. [Google Scholar] [CrossRef]
- Petrucci, V.E.; Siegfried, R. The extraneous matter in mechanically harvested wine grapes. Am. J. Enol. Viticult. 1976, 27, 40–41. [Google Scholar] [CrossRef]
- Morris, J.R. Influence of mechanical harvesting on quality of small fruits and grapes. HortScience 1983, 18, 412–417. [Google Scholar] [CrossRef]
- Clary, C.D.; Steinhauer, R.E.; Frisinger, J.E.; Peffer, T.E. Evaluation of machine-vs. hand-harvested Chardonnay. Am. J. Enol. Viticult. 1990, 41, 176–181. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Oberholster, A. Grape harvesting and effects on wine composition. In Managing Wine Quality; Woodhead Publishing: Cambridge, MA USA, 2022; pp. 705–726. [Google Scholar]
- Christensen, L.P.; Kasimatis, A.; Kissler, J.J.; Jensen, F.; Luvisi, D. Mechanical Harvesting of Grapes for the Winery; University of California: Berkeley, CA, USA, 1973; p. 403. [Google Scholar]
- Parenti, A.; Spugnoli, P.; Masella, P.; Guerrini, L.; Benedettelli, S.; Blasi, S.D. Comparison of grape harvesting and sorting methods on factors affecting the must quality. J. Agric. Eng. Res. 2015, 46, 19–22. [Google Scholar] [CrossRef]
- Hendrickson, D.A.; Lerno, L.A.; Hjelmeland, A.K.; Ebeler, S.E.; Heymann, H.; Hopfer, H.; Block, K.L.; Brenneman, C.A.; Oberholster, A. Impact of mechanical harvesting and optical berry sorting on grape and wine composition. Am. J. Enol. Viticult. 2016, 67, 385–397. [Google Scholar] [CrossRef]
- Noble, A.C.; Ough, C.S.; Kasimatis, A.N. Effect of leaf content and mechanical harvest on wine “quality”. Am. J. Enol. Viticult. 1975, 26, 158–163. [Google Scholar] [CrossRef]
- Huang, P.-J.D.; Cash, J.N.; Santerre, C.R. Influence of stems, petioles and leaves on the phenolic content of Concord and Aurora blanc juice and wine. J. Food Sci. 1988, 53, 173–175. [Google Scholar] [CrossRef]
- Guerrini, L.; Masella, P.; Angeloni, G.; Calamai, L.; Spinelli, S.; Di Blasi, S.; Parenti, A. Harvest of Sangiovese grapes: The influence of material other than grape and unripe berries on wine quality. Eur. Food Res. Technol. 2018, 244, 1487–1496. [Google Scholar] [CrossRef]
- Wang, J.; Aubie, E.; Lan, Y.; Crombleholme, M.; Reynolds, A. The impacts of frozen material-other-than-grapes (MOG) on aroma compounds of red wine varieties. Internet J. Vitic. Enol. 2020, 9, 1–9. [Google Scholar]
- Lan, Y.; Wang, J.; Aubie, E.; Crombleholme, M.; Reynolds, A.G. Effects of frozen materials other than grapes on red wine volatiles. mitigation of floral taints by yeast strains. Am. J. Enol. Viticult. 2022, 73, 117–133. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, J.; Aubie, E.; Crombleholme, M.; Reynolds, A.G. Effects of frozen materials-other-than-grapes on red wine aroma compounds. Impacts of harvest technologies. Am. J. Enol. Viticult. 2022, 73, 134–147. [Google Scholar] [CrossRef]
- Frost, S.C.; Fox, D.J.; Keller, M.; Collins, T.S.; Harbertson, J.F. Frozen leaf material causes “Frost Taint” in Cabernet Sauvignon. Am. J. Enol. Viticult. 2023, 74, 0740005. [Google Scholar] [CrossRef]
- Wildenradt, H.L.; Christensen, E.N.; Stackler, B.; Caputi, A.; Slinkard, K.; Scutt, K. Volatile constituents of grape leaves. I. Vitis vinifera variety ‘Chenin blanc’. Am. J. Enol. Viticult. 1975, 26, 148–153. [Google Scholar] [CrossRef]
- Ward, S.C.; Petrie, P.R.; Johnson, T.E.; Boss, P.K.; Bastian, S.E.P. Unripe berries and petioles in Vitis vinifera cv. Cabernet Sauvignon fermentations affect sensory and chemical profiles. Am. J. Enol. Viticult. 2015, 66, 435–443. [Google Scholar] [CrossRef]
- Capone, D.L.; Barker, A.; Pearson, W.; Francis, I.L. Influence of inclusion of grapevine leaves, rachis and peduncles during fermentation on the flavour and volatile composition of Vitis vinifera cv. Shiraz wine. Aust. J. Grape Wine R. 2021, 27, 348–359. [Google Scholar] [CrossRef]
- Matarese, F.; Cuzzola, A.; Scalabrelli, G.; D’Onofrio, C. Expression of terpene synthase genes associated with the formation of volatiles in different organs of Vitis vinifera. Phytochemistry 2014, 105, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.; Herbst-Johnstone, M.; Girault, M.; Butler, P.; Logan, G.; Jouanneau, S.; Nicolau, L.; Kilmartin, P.A. Influence of grape-harvesting steps on varietal thiol aromas in Sauvignon blanc wines. J. Agric. Food Chem. 2011, 59, 10641–10650. [Google Scholar] [CrossRef] [PubMed]
- Herbst-Johnstone, M.; Araujo, L.D.; Allen, T.A.; Logan, G.; Nicolau, L.; Kilmartin, P.A. Effects of mechanical harvesting on ‘Sauvignon blanc’ aroma. Acta Hortic. 2012, 978, 179–186. [Google Scholar] [CrossRef]
- Wirth, J.; Guo, W.; Baumes, R.; Günata, Z. Volatile compounds released by enzymatic hydrolysis of glycoconjugates of leaves and grape berries from Vitis vinifera Muscat of Alexandria and Shiraz cultivars. J. Agric. Food. Chem. 2001, 49, 2917–2923. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. Changes in free and bound fractions of aromatic components in vine leaves during development of muscat grapes. Phytochemistry 1986, 25, 943–946. [Google Scholar] [CrossRef]
- Suriano, S.; Alba, V.; Di Gennaro, D.; Basile, T.; Tamborra, M.; Tarricone, L. Major phenolic and volatile compounds and their influence on sensorial aspects in stem-contact fermentation winemaking of Primitivo red wines. Int. J. Food Sci. Technol. 2016, 53, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, K.; Samuta, T. Green odorants of grape cluster stem and their ability to cause a wine stemmy flavor. J. Agric. Food. Chem. 1997, 45, 1333–1337. [Google Scholar] [CrossRef]
- Joslin, W.S.; Ough, C.S. Cause and fate of certain C6 compounds formed enzymatically in macerated grape leaves during harvest and wine fermentation. Am. J. Enol. Vitic. 1978, 29, 11–17. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Moreno Luna, L.H.; Reynolds, A.G.; di Profio, F.A.; Zhang, L.; Kotsaki, E. Crop level and harvest date impact on four Ontario wine grape cultivars. II. Wine aroma compounds and sensory analysis. S. Afric. J. Enol. Vitic. 2018, 39, 246–270. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Odor Potency of aroma compounds in Riesling and Vidal blanc table wines and icewines by gas chromatography–olfactometry–mass spectrometry. J. Agric. Food. Chem. 2012, 60, 2874–2883. [Google Scholar] [CrossRef]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food. Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Buttery, R.G.; Seifert, R.M.; Ling, L.C.; Soderstrom, E.L.; Ogawa, J.M.; Turnbaugh, J.G. Additional aroma components of honeydew melon. J. Agric. Food. Chem. 1982, 30, 1208–1211. [Google Scholar] [CrossRef]
- Buttery, R.G.; Seifert, R.M.; Guadagni, D.G.; Black, D.R.; Ling, L. Characterization of some volatile constituents of carrots. J. Agric. Food. Chem. 1968, 16, 1009–1015. [Google Scholar] [CrossRef]
- Ruth, J.H. Odor thresholds and irritation levels of several chemical substances: A review. Am. Ind. Hyg. Assoc. J. 1986, 47, A-142–A-151. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.R.; Flath, R.A.; Mon, T.R.; Teranishi, R.; Guentert, M. Volatile constituents of apricot (Prunus armeniaca). J. Agric. Food. Chem. 1990, 38, 471–477. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food. Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Dunlevy, J.D.; Kalua, C.M.; Keyzers, R.A.; Boss, P.K. The production of flavour & aroma compounds in grape berries. In Grapevine Molecular Physiology & Biotechnology; Springer: Dordrecht, The Netherlands, 2009; pp. 293–340. [Google Scholar]
- Reynolds, A.G.; Wardle, D.A. Influence of fruit microclimate on monoterpene levels of Gewürztraminer. Am. J. Enol. Vitic. 1989, 40, 149–154. [Google Scholar] [CrossRef]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, phenolics, and color of Cabernet franc, Merlot, and Pinot noir wines from British Columbia. J. Agric. Food. Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Fuleki, T.; Francis, F. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanin in cranberries. J. Food Sci. 1968, 33, 73–83. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1964, 16, 144–158. [Google Scholar] [CrossRef]
- Waterhouse, A. Determination of Total Phenolics. Curr. Protoc. Food Analyt. Chem. 2002, 6, 11.1. [Google Scholar]
- Reynolds, A.G.; Baker, L.T.; Zhang, L.; Jasinski, M.; Profio, F.D.; Kögel, S.; Pickering, G.J. Impacts of natural yield variances on wine composition and sensory attributes of Vitis vinifera cultivars Riesling and Cabernet Franc. Can. J. Plant. Sci. 2018, 98, 851–880. [Google Scholar] [CrossRef]
- Carrau, F.M.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, P.A. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pascual, O.; González-Royo, E.; Gil, M.; Gómez-Alonso, S.; García-Romero, E.; Canals, J.M.; Hermosín-Gutíerrez, I.; Zamora, F. Influence of grape seeds and stems on wine composition and astringency. J. Agric. Food. Chem. 2016, 64, 6555–6566. [Google Scholar] [CrossRef] [PubMed]
- Spranger, I.; Cristina Clímaco, M.; Sun, B.; Eiriz, N.; Fortunato, C.; Nunes, A.; Conceição Leandro, M.; Luísa Avelar, M.; Pedro Belchior, A. Differentiation of red winemaking technologies by phenolic and volatile composition. Anal. Chim. Acta 2004, 513, 151–161. [Google Scholar] [CrossRef]
| Sample | α-Ionone | β-Ionone | β-Damascenone | Citronellol | Geraniol | cis-Rose Oxide | trans-Rose Oxide | γ-Terpinene | Limonene |
|---|---|---|---|---|---|---|---|---|---|
| Threshold | 2.6 | 0.09 | 0.05 | 100 | 30 | 0.2 | 450 | 3260 | 15 |
| Mean low a | 2.95 | 0.175 | 2.47 | 1.08 | 5.14 | 0.027 | 0.007 | 0.100 | 0.185 |
| Range low a | 1.929–4.859 | 0.152–0.224 | 2.06–2.83 | 0.849–1.413 | 3.893–7.347 | 0.016–0.036 | 0.000–0.014 | 0.044–0.206 | 0.145–0.222 |
| Mean med-high b | 1.40 | 0.257 | 3.43 | 1.20 | 6.16 | 0.082 | 0.031 | 0.097 | 0.160 |
| Range med-high b | 0.662–1.869 | 0.232–0.291 | 2.18–5.17 | 0.922–2.088 | 4.708–7.716 | 0.049–0.217 | 0.007–0.068 | 0.065–0.138 | 0.115–0.215 |
| Significance c | NS | ** | ** | * | * | * | ** | NS | NS |
| OAV d | 0.538 | 2.86 | 68.6 | 0.012 | 0.205 | 0.410 | 0.155 | 0.00003 | 0.011 |
| Sample | Ethyl hexanoate | Ethyl heptanoate | Ethyl octanoate | Ethyl nonanoate | Ethyl decanoate | Phenylethyl acetate | Heptanol | Octanol | Phenylethyl alcohol |
| Threshold | 5 | 2.2 | 5 | --- | 200 | 250 | 3 | 110 | 10000 |
| Mean low a | 176.8 | 1.17 | 202.8 | 0.52 | 47.1 | 39.2 | 33.2 | 13.56 | 42310 |
| Range low a | 130.8–204.0 | 1.07–1.32 | 112.9–284.8 | 0.394–0.770 | 21.5–69.9 | 25.58–53.69 | 29.7–35.7 | 12.14–15.85 | 34148–47142 |
| Mean med-high b | 229.9 | 1.61 | 332.5 | 0.93 | 100.5 | 41.6 | 39.7 | 15.75 | 45902 |
| Range med-high b | 203.6–251.4 | 1.41–1.79 | 227.4–399.3 | 0.661–1.84 | 60.6–143.0 | 27.26–55.74 | 35.5–44.0 | 13.31–19.09 | 38734–60569 |
| Significance c | ** | * | ** | * | ** | * | * | * | * |
| OAV d | 45.98 | 0.73 | 66.5 | --- | 0.5 | 0.167 | 13.2 | 0.143 | 4.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.; Xu, X.; Wang, J.; Aubie, E.; Crombleholme, M.; Reynolds, A. The Impacts of Frozen Material-Other-Than-Grapes (MOG) on Aroma Compounds of Cabernet Franc and Cabernet Sauvignon. Beverages 2024, 10, 68. https://doi.org/10.3390/beverages10030068
Lan Y, Xu X, Wang J, Aubie E, Crombleholme M, Reynolds A. The Impacts of Frozen Material-Other-Than-Grapes (MOG) on Aroma Compounds of Cabernet Franc and Cabernet Sauvignon. Beverages. 2024; 10(3):68. https://doi.org/10.3390/beverages10030068
Chicago/Turabian StyleLan, Yibin, Xiaoyu Xu, Jiaming Wang, Emily Aubie, Marnie Crombleholme, and Andrew Reynolds. 2024. "The Impacts of Frozen Material-Other-Than-Grapes (MOG) on Aroma Compounds of Cabernet Franc and Cabernet Sauvignon" Beverages 10, no. 3: 68. https://doi.org/10.3390/beverages10030068
APA StyleLan, Y., Xu, X., Wang, J., Aubie, E., Crombleholme, M., & Reynolds, A. (2024). The Impacts of Frozen Material-Other-Than-Grapes (MOG) on Aroma Compounds of Cabernet Franc and Cabernet Sauvignon. Beverages, 10(3), 68. https://doi.org/10.3390/beverages10030068








