Continuous Primary Beer Fermentation with Yeast Immobilized in Alginate–Chitosan Microcapsules with a Liquid Core
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Wort
2.3. Reagents
2.4. Immobilization Procedure
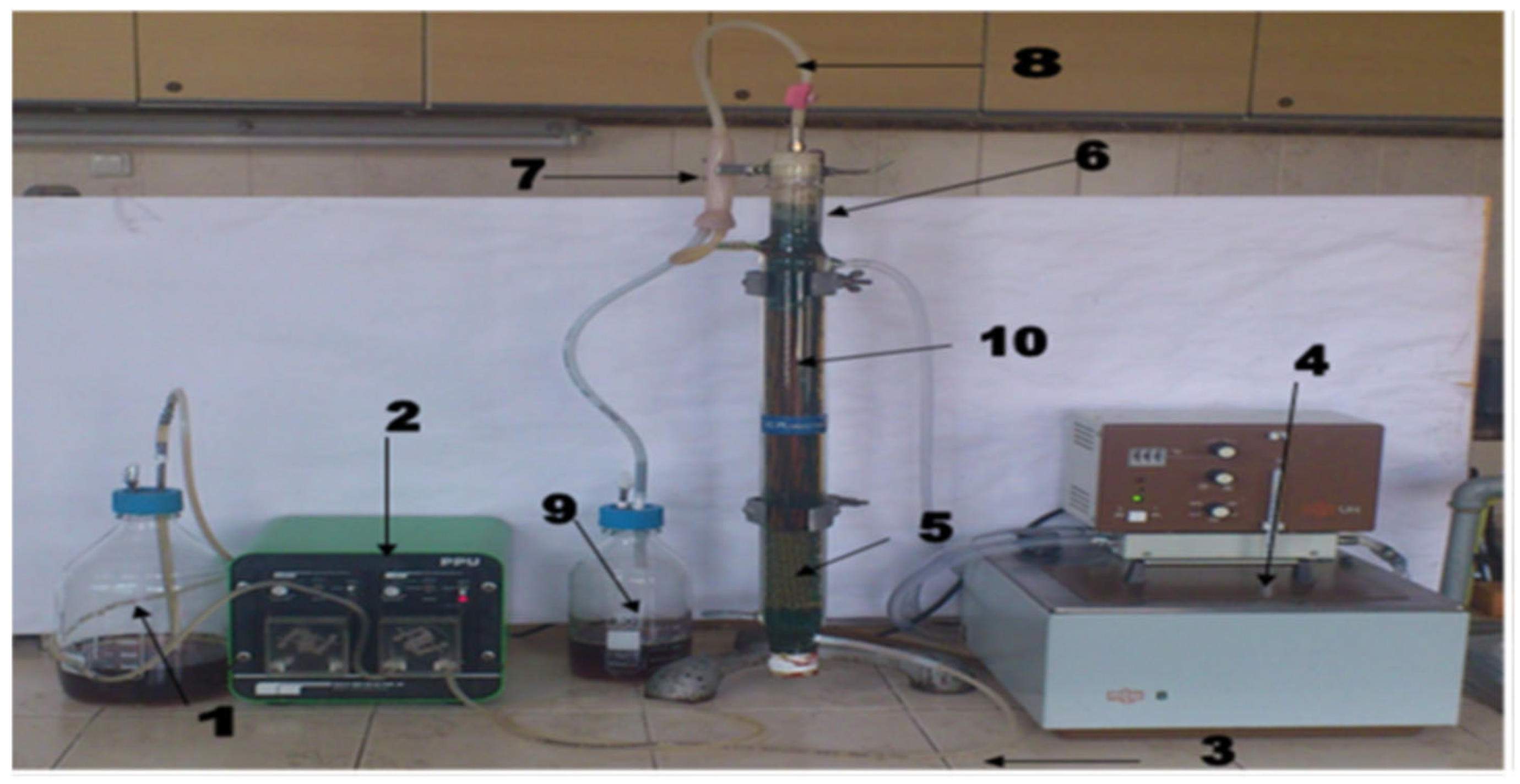
2.5. Immobilized Cells Bioreactor
2.6. The Start and Working of the Immobilized Cells Bioreactor
2.7. Analytical Methods
2.8. Statistical Analysis
3. Results and Discussion
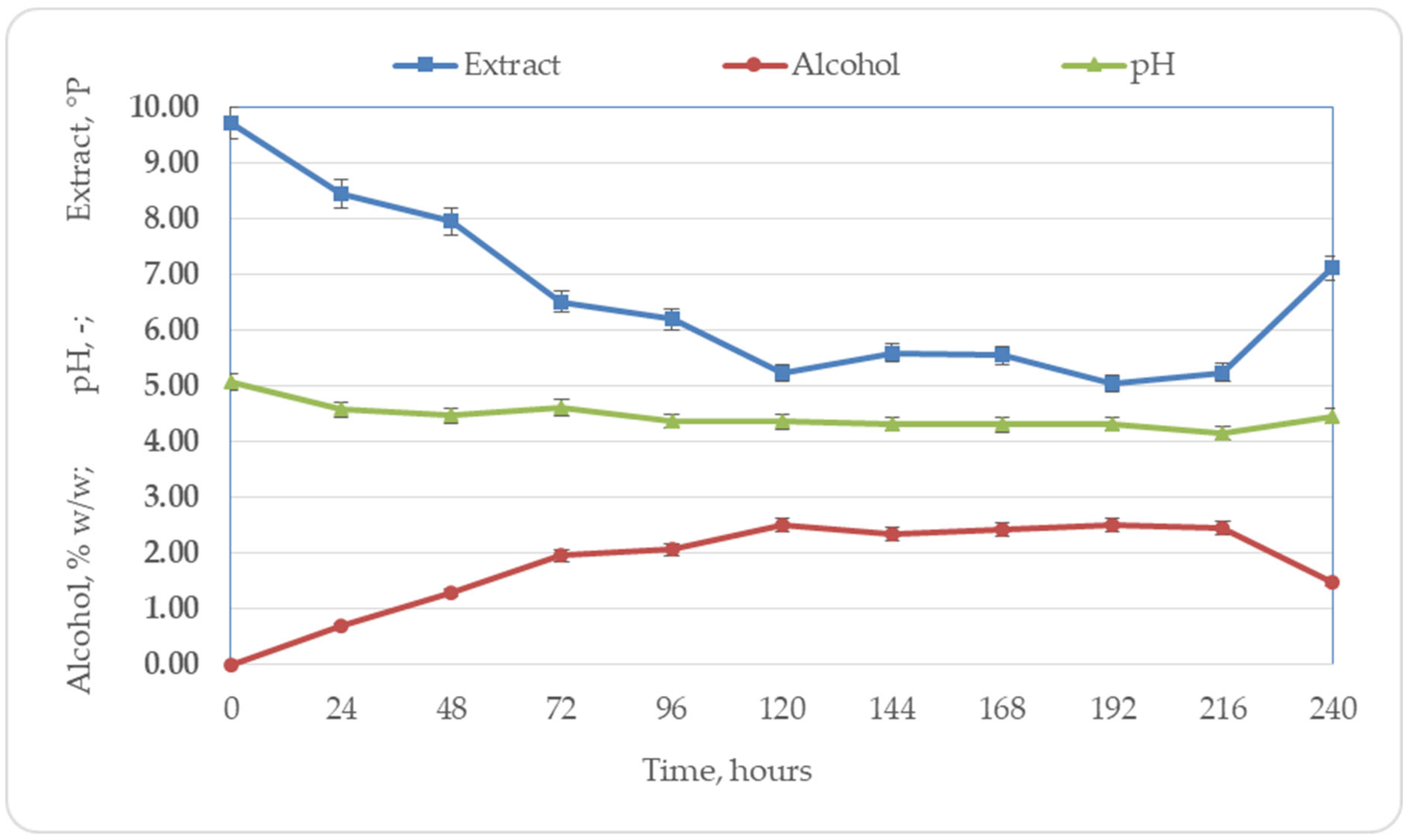
3.1. Basic Beer Parameter Changes during Fermentation
3.1.1. Changes in the Basic Beer Parameters during Primary Fermentation
3.1.2. Basic Beer Parameter Change during Secondary Fermentation
3.2. Secondary Yeast Metabolite Changes during Primary and Secondary Fermentation
3.3. A Comparison of the Volatile Profiles of the Beer Produced by Continuous Fermentation and Two Commercial Beers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno-García, J.; García-Martínez, T.; Mauricio, J.C.; Moreno, J. Yeast immobilization systems for alcoholic wine fermentations: Actual trends and future perspectives. Front. Microbiol. 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Verbelen, P.; Nedovic, V.; Manojlovic, V.; Delvaux, F.; Leskosek-Cukalovic, I.; Bugarski, B.; Willaert, R. Bioprocess intensification of beer fermentation using immobilised cells. In Encapsulation Technologies for Food Active Ingredients and Food Processing; Zuidam, N.-J., Nedovic, V.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 303–327. [Google Scholar]
- Nedović, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Benucci, I.; Cecchi, T.; Lombardelli, C.; Maresca, D.; Mauriello, G.; Esti, M. Novel microencapsulated yeast for the primary fermentation of green beer: Kinetic behavior, volatiles and sensory profile. Food Chem. 2021, 340, 127900. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.C.; Rodrigues, J.A.R.; Moran, P.J.; Valença, G.P.; Nunhez, J.R. Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, G.; Lystad, K.Q.; Levine, D.W.; Dyrset, N. Cell release from alginate immobilized Lactococcus lactis ssp. lactis in chitosan and alginate coated beads. J. Dairy Sci. 2001, 84, 1118–1127. [Google Scholar] [CrossRef]
- Liouni, M.; Drichoutis, P.; Nerantzis, E.T. Studies of the mechanical properties and the fermentation behavior of double layer alginate–chitosan beads, using Saccharomyces cerevisiae entrapped cells. World J. Microbiol. Biotechnol. 2008, 24, 281–288. [Google Scholar] [CrossRef]
- Naydenova, V.; Badova, M.; Vassilev, S.; Iliev, V.; Kaneva, M.; Kostov, G. Encapsulation of brewing yeast in alginate/chitosan matrix: Lab-scale optimization of lager beer fermentation. Biotechnol. Biotechnol. Equip. 2014, 28, 277–284. [Google Scholar] [CrossRef]
- Vassilev, S.; Shopska, V.; Iliev, V.; Kaneva, V.; Kostov, G.; Popova, S. Modeling of alcohol fermentation in brewing—Integrated approach for control of continuous alcohol fermentation. In Proceedings of the 29th European Conference on Modelling and Simulation, Albena, Bulgaria, 26–29 May 2015; pp. 286–291. [Google Scholar]
- Benucci, I.; Cerreti, M.; Maresca, D.; Mauriello, G.; Esti, M. Yeast cells in double layer calcium alginate–chitosan microcapsules for sparkling wine production. Food Chem. 2019, 300, 125174. [Google Scholar] [CrossRef]
- Vilela, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Reduction of volatile acidity of acidic wines by immobilized Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 2013, 97, 4991–5000. [Google Scholar] [CrossRef]
- Branyik, T.; Vicente, A.; Dostalek, P.; Teixeira, J.A. Continuous beer fermentation using immobilized yeast cell bioreactor systems. Biotechnol. Prog. 2005, 21, 653–663. [Google Scholar] [CrossRef]
- Ogawa, M.; Carmona-Jiménez, P.; García-Martínez, T.; Jorrín-Novo, J.V.; Moreno, J.; Rey, M.D.; Moreno-García, J. Use of yeast biocapsules as a fungal-based immobilized cell technology for Indian Pale Ale-type beer brewing. Appl. Microbiol. Biotechnol. 2022, 106, 7615–7625. [Google Scholar] [CrossRef] [PubMed]
- Brányik, T.; Vicente, A.A.; Teixeira, J.A. Continuous primary fermentation of beer—Yeast immobilization kinetics and product quality. Braz. J. Food Technol. 2005, 8, 74–79. [Google Scholar]
- Naydenova, V.; Vassilev, S.; Kaneva, M.; Kostov, G. Encapsulation of brewing yeast in alginate/chitosan matrix: Comparative study of beer fermentation with immobilized and free cells. Bulg. J. Agric. Sci. 2013, 19 (Suppl. S2), 8123–8127. [Google Scholar]
- EBC Analytica. Available online: https://brewup.eu/ebc-analytica/beer (accessed on 21 June 2024).
- Naydenova, V.; Badova, M.; Vassilev, S.; Murigneux, J.; Kostov, G. Encapsulation of brewing yeast in alginate/chitosan microcapsules: Production of pale lager beer. Sci. Work. UFT 2013, 60, 380–385. (In Bulgarian) [Google Scholar]
- Giannakou, K.; Visinoni, F.; Zhang, P.; Nathoo, N.; Jones, P.; Cotterrell, M.; Vrhovsek, U.; Delneri, D. Biotechnological exploitation of Saccharomyces jurei and its hybrids in craft beer fermentation uncovers new aroma combinations. Food Microbiol. 2021, 100, 103838. [Google Scholar] [CrossRef] [PubMed]
- Willaert, R. The Beer Brewing Process: Wort Production and Beer Fermentation. In Handbook of Food Products Manufacturing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Volume 1, pp. 443–506. [Google Scholar]
- Kunze, W. Technology Brewing & Malting; Hendel, O., Ed.; Westkreuz-Druckerei Ahrens KG: Berlin/Bonn, Germany, 2019. [Google Scholar]
- Ripari, V.; Tomassetti, M.; Cecchi, T.; Berardi, E. Recipe, volatiles profile, sensory analysis, physico-chemical and microbial characterization of acidic beers from both sourdough yeasts and lactic acid bacteria. Eur. Food Res. Technol. 2018, 244, 2027–2040. [Google Scholar] [CrossRef]
- Postigo, V.; García, M.; Cabellos, J.M.; Arroyo, T. Wine Saccharomyces Yeasts for Beer Fermentation. Fermentation 2021, 7, 290. [Google Scholar] [CrossRef]
- Stewart, G.G. The Production of Secondary Metabolites with Flavour Potential during Brewing and Distilling Wort Fermentations. Fermentation 2017, 3, 63. [Google Scholar] [CrossRef]
- Iorizzo, M.; Coppola, F.; Letizia, F.; Testa, B.; Sorrentino, E. Role of Yeasts in the Brewing Process: Tradition and Innovation. Processes 2021, 9, 839. [Google Scholar] [CrossRef]
- Branyik, T.; Vicente, A.; Dostalek, P.; Teixeira, J.A. A review of flavour formation in continuous beer fermentations. J. Inst. Brew. 2008, 114, 3–13. [Google Scholar] [CrossRef]
- Willaert, R.; Nedovic, V. Primary beer fermentation by immobilised yeast—A review on flavour formation and control strategies. J. Chem. Technol. Biotechnol. 2006, 81, 1353–1367. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Guido, L.F. Impact of Wort Amino Acids on Beer Flavour: A Review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Djordjevic, V.; Willaert, R.; Gibson, B.; Nedovic, V. Immobilized yeast cells and secondary metabolites. In Fungal Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2016; pp. 599–638. [Google Scholar]

| Sample, Taken at the Reactor Output after Hours | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Secondary Metabolites, mg/L | 24 | 48 | 72 | 96 | 120 | 144 | 168 | 192 | 216 | 240 | |
| Vicinal diketones | In “green” beer | 2.6 ± 0.3 | 1.1 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.1 | 0.6 ± 0.1 |
| In final beer | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.17 ± 0.06 | 0.12 ± 0.04 | 0.14 ± 0.06 | 0.05 ± 0.02 | 0.10 ± 0.03 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.12 ± 0.06 | |
| Acetaldehyde | In “green” beer | 11 ± 1 | 12 ± 1 | 13 ± 2 | 16 ± 2 | 15 ± 2 | 11 ± 1 | 10 ± 2 | 11 ± 1 | 8.1 ± 0.6 | 7.9 ± 0.6 |
| In final beer | 6.8 ± 0.8 | 5.9 ± 0.8 | 4.1 ± 0.4 | 4.8 ± 0.4 | 2.3 ± 0.2 | 5.3 ± 0.6 | 4.9 ± 0.6 | 4.3 ± 0.6 | 2.1 ± 0.3 | 6.0 ± 0.4 | |
| Ethyl acetate | In “green” beer | 11 ± 1 | 3.1 ± 0.8 | 4.2 ± 0.8 | 5.2 ± 0.6 | 9.6 ± 0.7 | 3.4 ± 0.4 | 3.6 ± 0.4 | 3.3 ± 0.4 | 6.2 ± 0.4 | 9.1 ± 0.8 |
| In final beer | 10 ± 1 | 13 ± 2 | 8 ± 1 | 13 ± 2 | 26 ± 2 | 14 ± 2 | 13 ± 2 | 11 ± 2 | 14 ± 2 | 11 ± 2 | |
| 1-propanol | In “green” beer | 2.3 ± 0.6 | 2.2 ± 0.6 | 2.4 ± 0.4 | 2.8 ± 0.4 | 4.5 ± 0.5 | n.d. * | 1.1 ± 0.2 | n.d. | n.d. | 3.4 ± 0.3 |
| In final beer | 14 ± 2 | 13 ± 1 | 4.8 ± 0.7 | 4.2 ± 0.8 | 3.8 ± 0.6 | 12 ± 1 | 13 ± 1 | 10 ± 1 | 9.1 ± 0.8 | 10 ± 2 | |
| Isobutanol | In “green” beer | 4.6 ± 0.5 | 3.9 ± 0.4 | 5.0 ± 0.5 | 6.6 ± 0.7 | 6.3 ± 0.7 | 1.6 ± 0.7 | 5.5 ± 0.3 | 2.0 ± 0.2 | n.d. | 4.5 ± 0.3 |
| In final beer | 16 ± 1 | 13.3 ± 0.9 | 5.7 ± 0.6 | 6.5 ± 0.4 | n.d. | 9.5 ± 0.8 | 6.6 ± 0.7 | 7.7 ± 0.8 | 10.4 ± 0.8 | 11 ± 1 | |
| Isoamyl alcohol | In “green” beer | 25 ± 1 | 27 ± 1 | 41 ± 1 | 40 ± 2 | 65 ± 2 | 33 ± 1 | 37 ± 1 | 29 ± 2 | 32 ± 2 | 35 ± 1 |
| In final beer | 73 ± 2 | 78 ± 3 | 46 ± 2 | 55 ± 2 | 65 ± 2 | 66 ± 2 | 65 ± 2 | 66 ± 2 | 78 ± 2 | 61 ± 2 | |
| Amyl alcohol | In “green” beer | 3.3 ± 0.1 | 7 ± 2 | 13 ± 2 | 15 ± 1 | 38 ± 2 | 14 ± 1 | 9.4 ± 0.8 | 11 ± 1 | 12 ± 1 | 10.1 ± 0.6 |
| In final beer | 17.2 ± 0.1 | 20.9 ± 0.6 | 13.9 ± 0.4 | 15.7 ± 0.4 | 17.1 ± 0.2 | 15.9 ± 0.3 | 16.2 ± 0.3 | 17.9 ± 0.2 | 18.9 ± 0.2 | 15.0 ± 0.2 | |
| Metabolites, mg/L | Laboratory Beer | Commercial Beer 1 | Commercial Beer 2 |
|---|---|---|---|
| Vicinal diketones | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.02 ± 0.01 |
| Acetaldehyde | 4.2 ± 0.1 | 3.7 ± 0.1 | 3.0 ± 0.1 |
| Ethyl acetate | 13.7 ± 0.1 | 5.0 ± 0.2 | 4.5 ± 0.2 |
| 1-propanol | 8.4 ± 0.2 | 4.5 ± 0.1 | 6.8 ± 0.1 |
| Isobutanol | 8.2 ± 0.5 | 9.9 ± 0.5 | 13 ± 1 |
| Isoamyl alcohol | 63 ± 2 | 68 ± 2 | 83 ± 2 |
| Amyl alcohol | 16.3 ± 0.7 | 14.7 ± 0.7 | 17.8 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shopska, V.; Dzhivoderova-Zarcheva, M.; Kostov, G. Continuous Primary Beer Fermentation with Yeast Immobilized in Alginate–Chitosan Microcapsules with a Liquid Core. Beverages 2024, 10, 87. https://doi.org/10.3390/beverages10030087
Shopska V, Dzhivoderova-Zarcheva M, Kostov G. Continuous Primary Beer Fermentation with Yeast Immobilized in Alginate–Chitosan Microcapsules with a Liquid Core. Beverages. 2024; 10(3):87. https://doi.org/10.3390/beverages10030087
Chicago/Turabian StyleShopska, Vesela, Mina Dzhivoderova-Zarcheva, and Georgi Kostov. 2024. "Continuous Primary Beer Fermentation with Yeast Immobilized in Alginate–Chitosan Microcapsules with a Liquid Core" Beverages 10, no. 3: 87. https://doi.org/10.3390/beverages10030087
APA StyleShopska, V., Dzhivoderova-Zarcheva, M., & Kostov, G. (2024). Continuous Primary Beer Fermentation with Yeast Immobilized in Alginate–Chitosan Microcapsules with a Liquid Core. Beverages, 10(3), 87. https://doi.org/10.3390/beverages10030087






