Effect of Partial Condensation (Dephlegmation) in Fruit Brandy Distillation Equipment on the Composition of Apple Brandies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lab-Scale Experiments
2.1.1. Preparations of Solutions
2.1.2. Experimental Setup
2.1.3. Distillation of Solutions
2.1.4. Calculation of Relative Volatility (α)
2.2. Large-Scale Experiments
2.2.1. Apple Mash Preparation and Fermentation
2.2.2. Distillation Profile Parameters and Equipment
2.2.3. Sampling
2.2.4. Sensory Evaluation: Triangle Test
2.3. Gas Chromatography Headspace Analysis
2.3.1. GC Sample Preparations
2.3.2. Gas Chromatography Headspace Analysis Setup
2.4. Statistical Analysis
3. Results
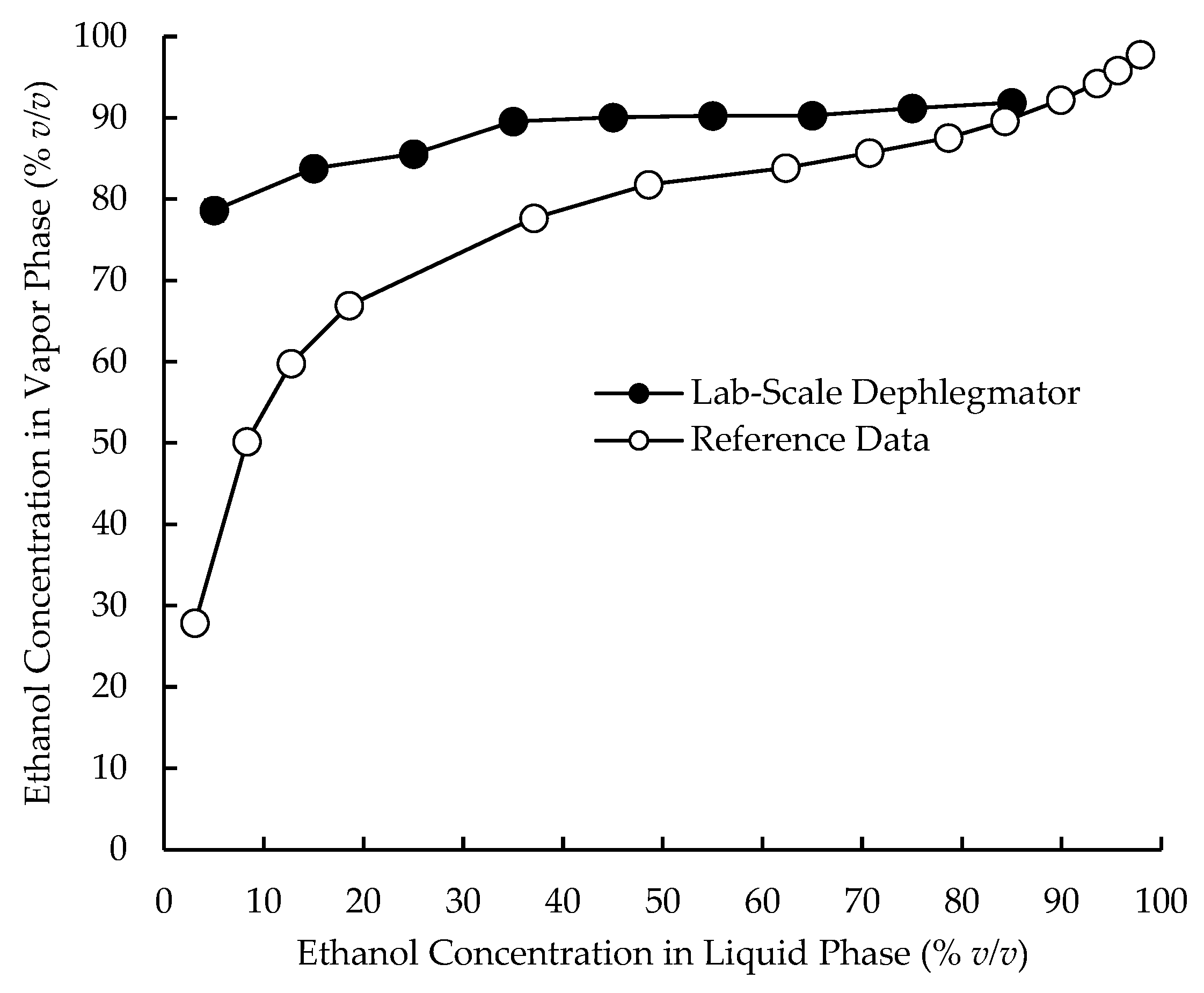
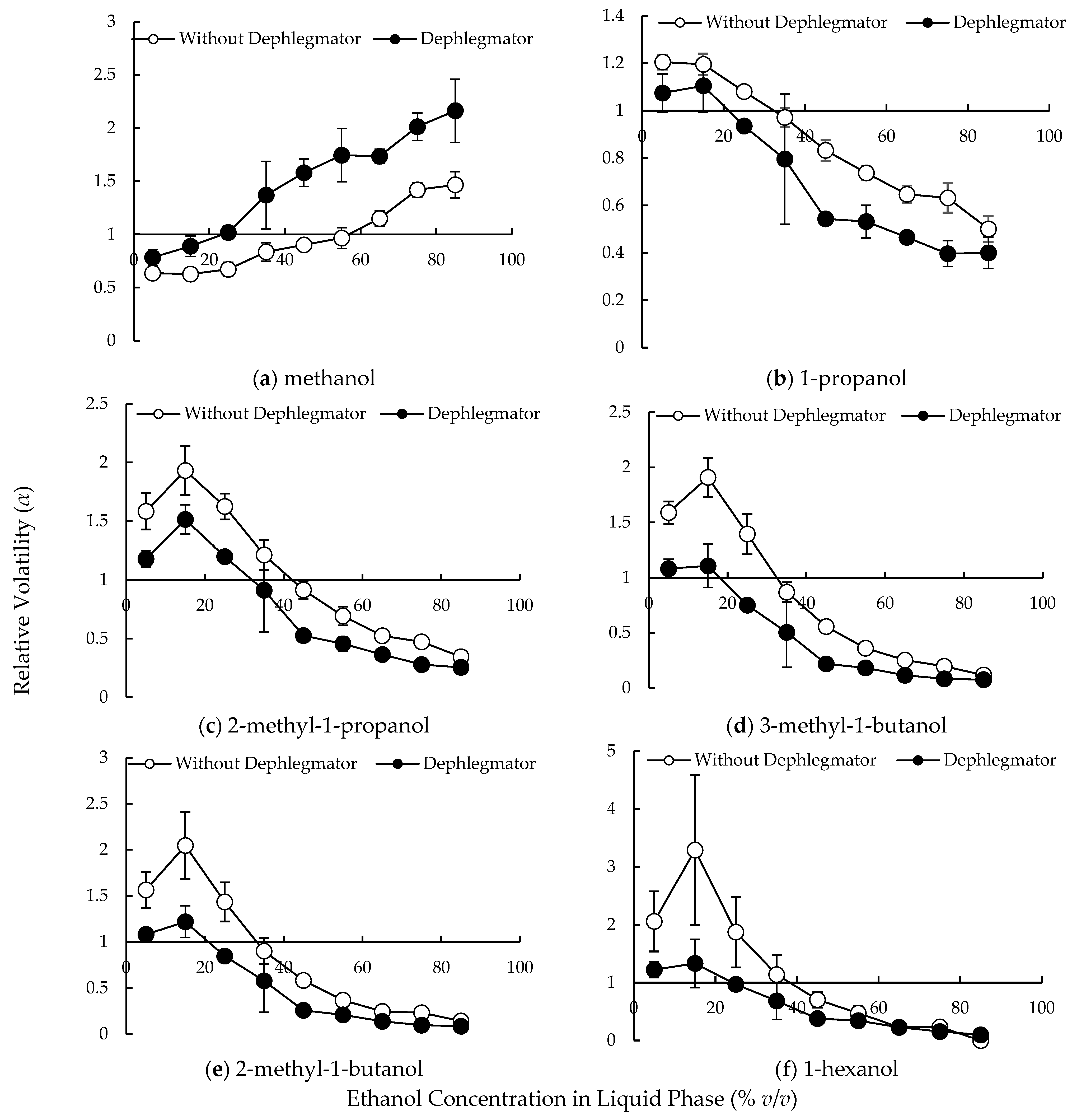
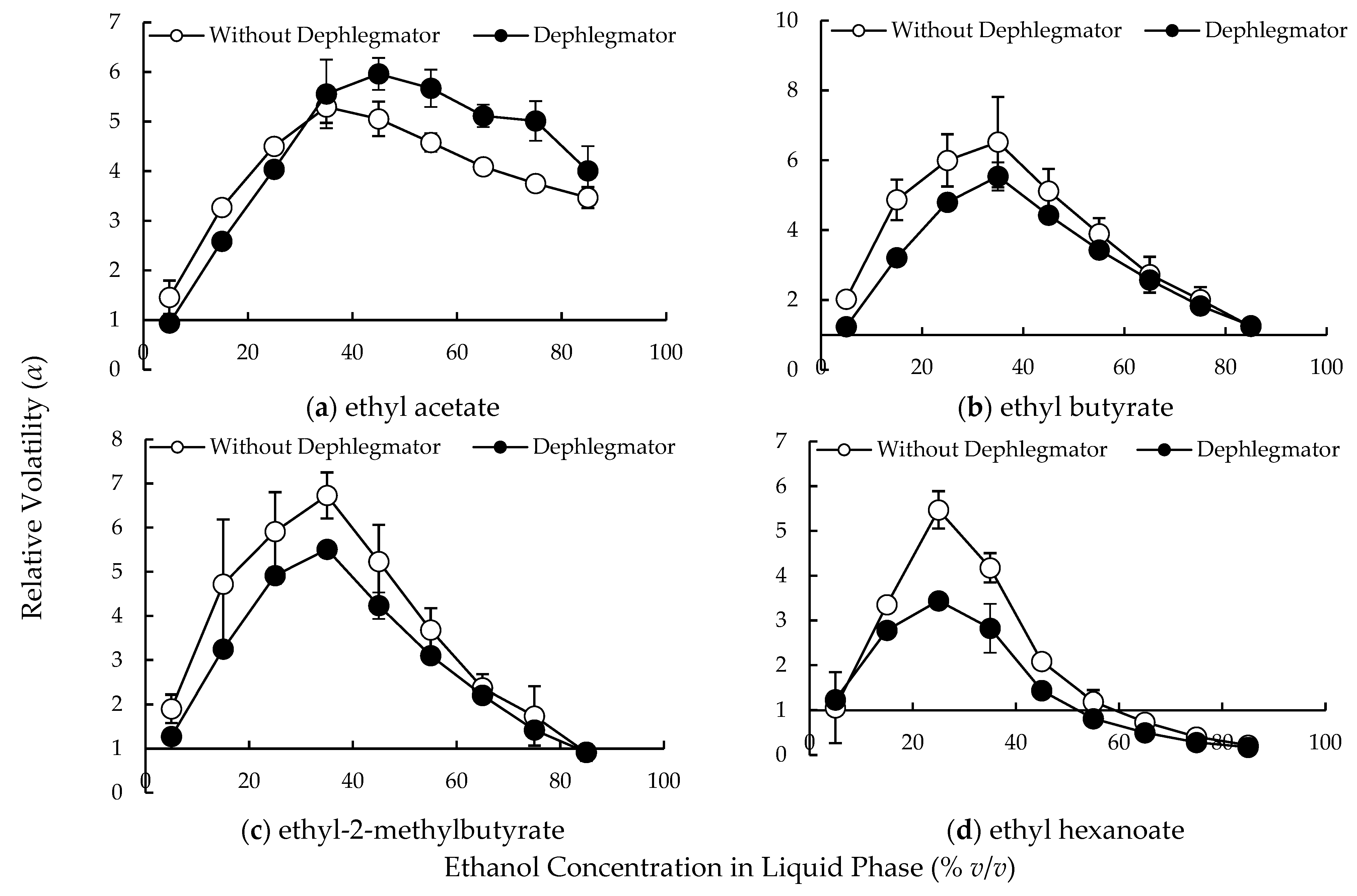
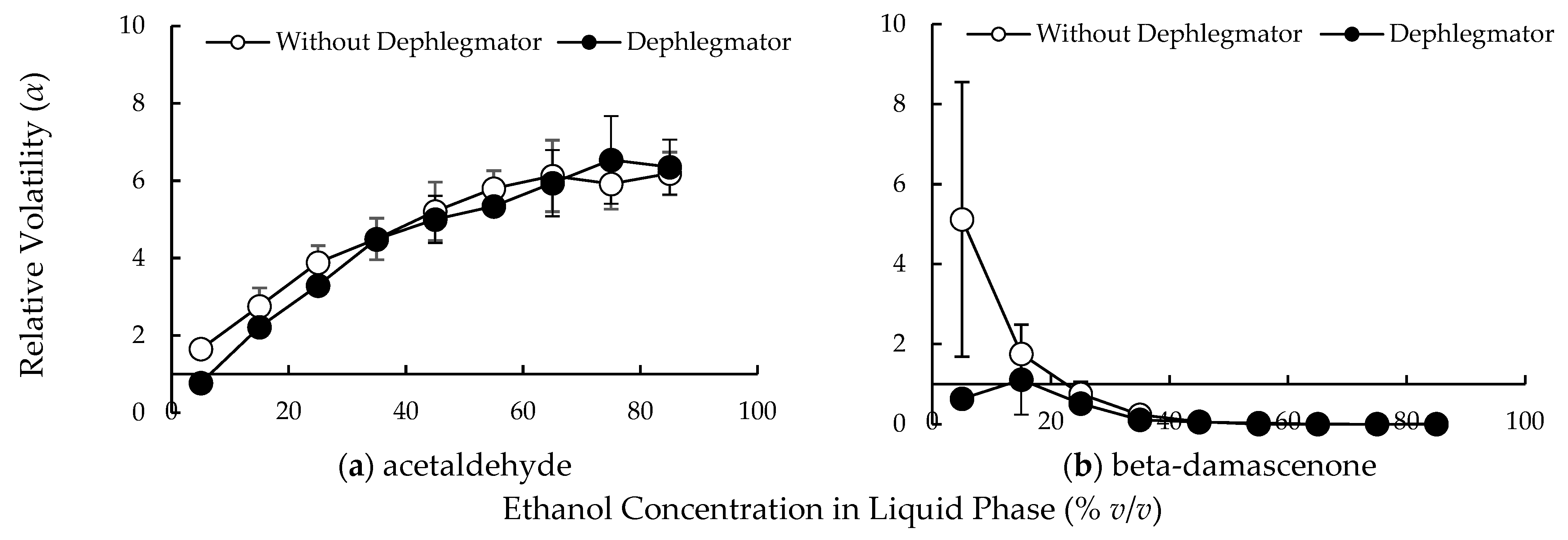
3.1. Relative Volatilities of Compounds in the Lab-Scale Dephlegmator
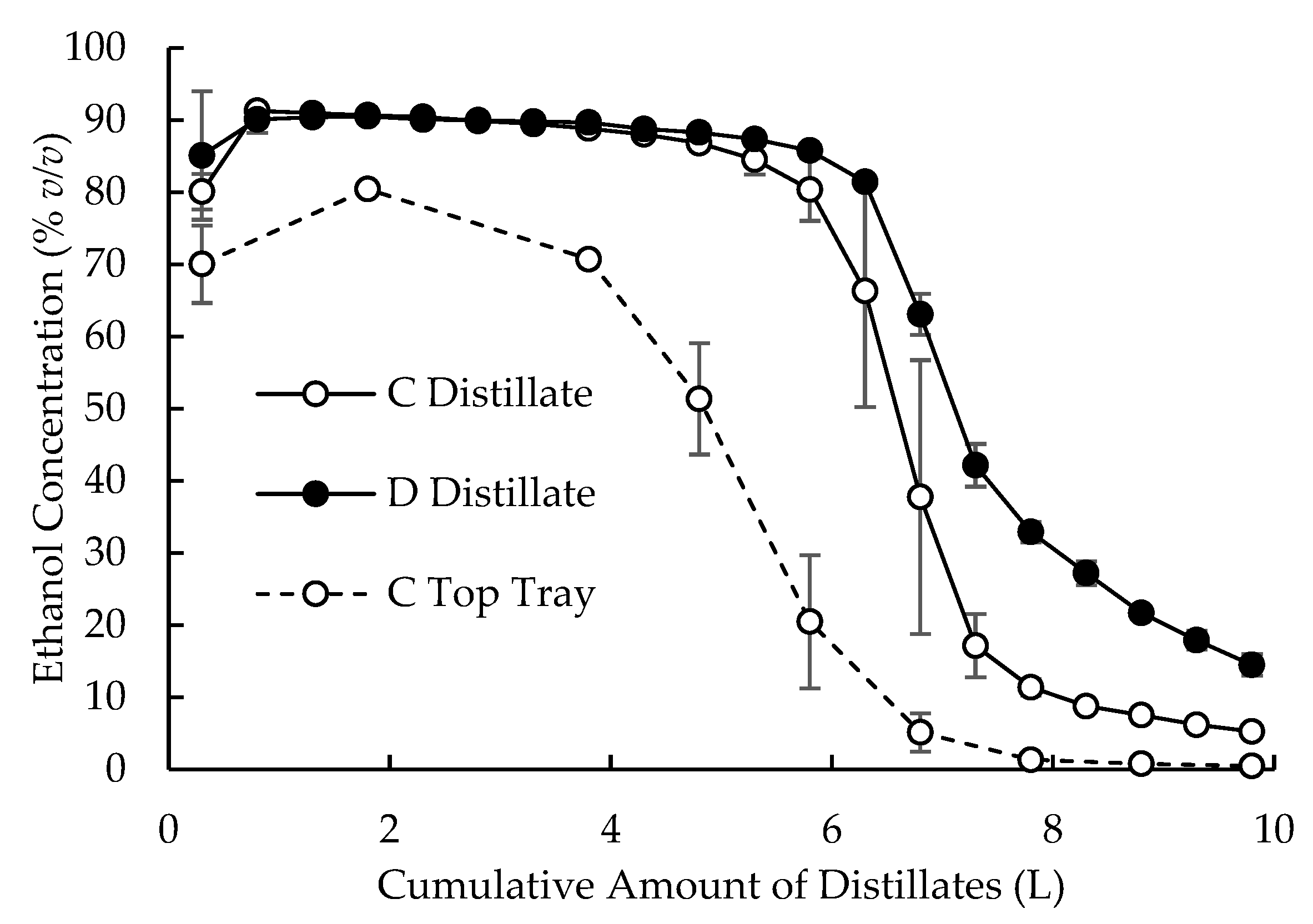
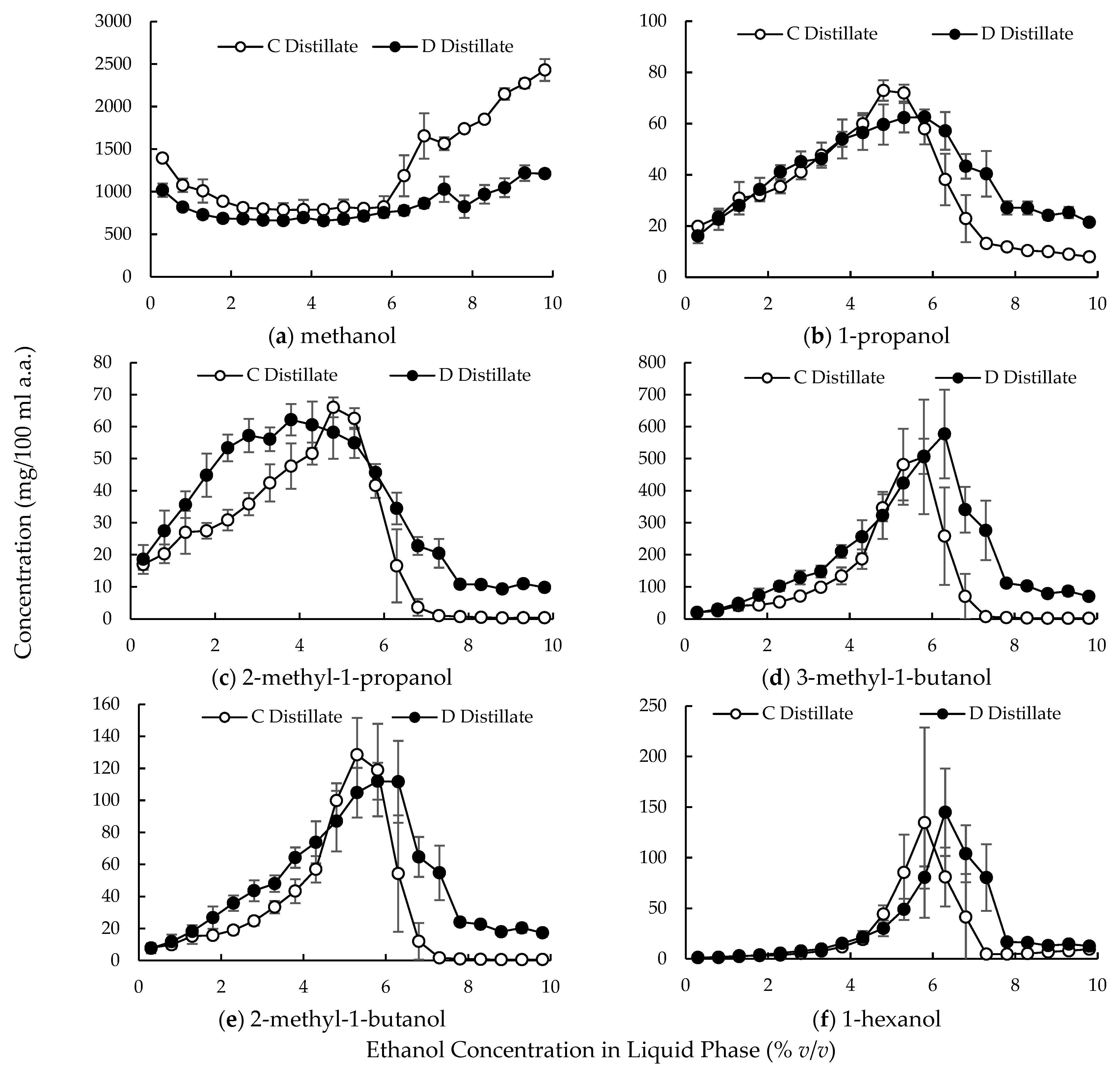
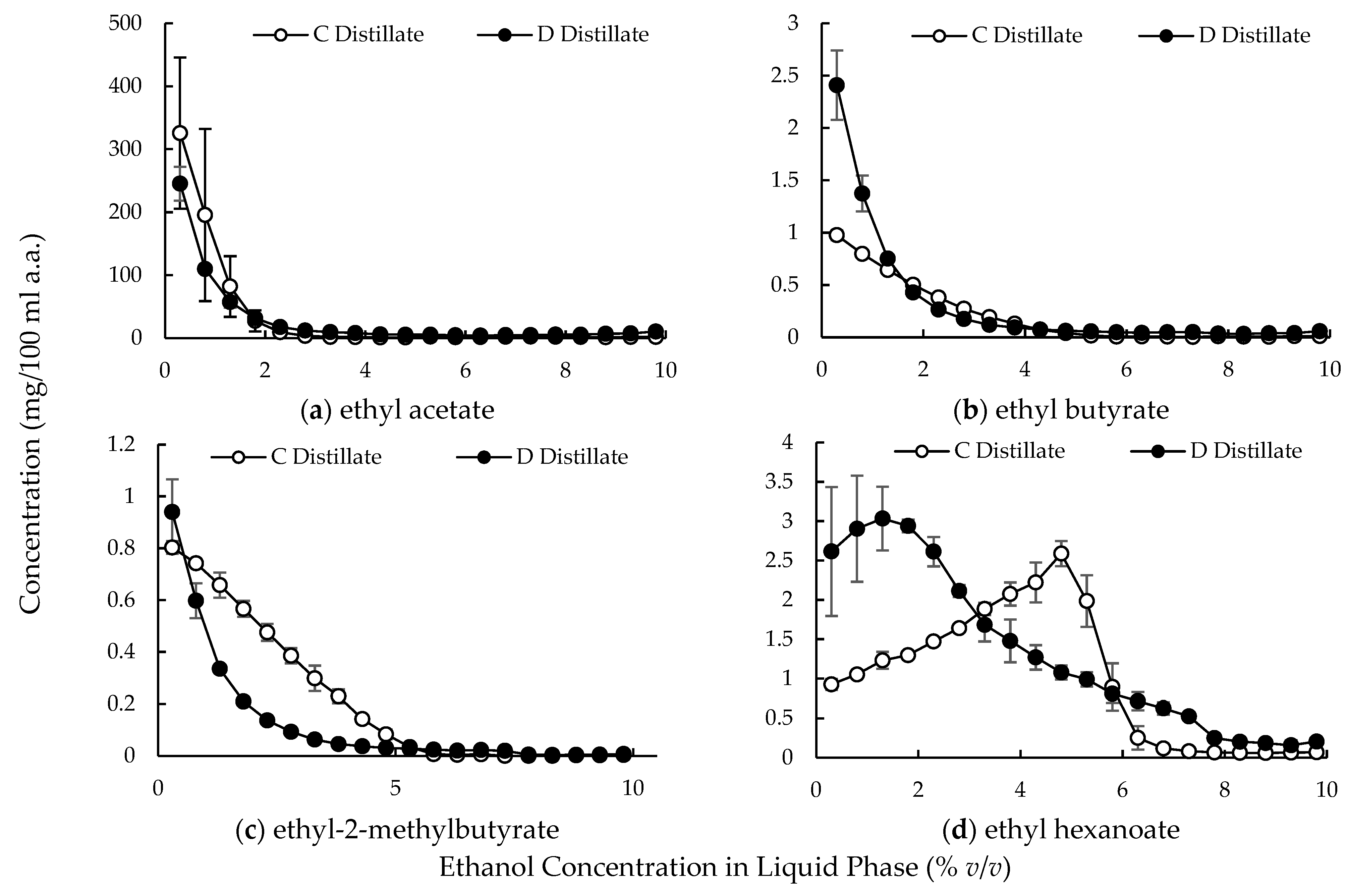
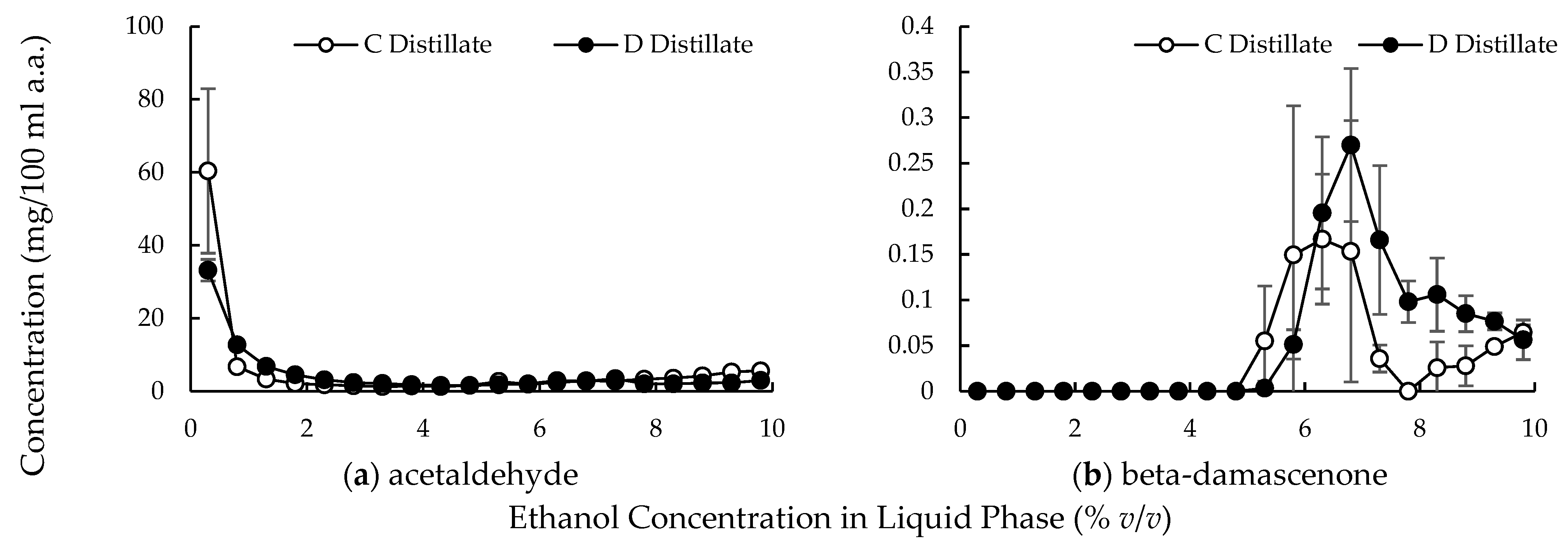
3.2. Large-Scale Experiments
3.3. Yields of Hearts and Tails Fractions from C and D Distillations
3.4. Sensory Evaluation
4. Discussion
4.1. Complex Condensation Behavior in the Lab-Scale Dephlegmator
4.2. Compound Behavior in the Distillation Column with Multiple Dephlegmators
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Ethanol Concentration (% v/v) | Sampling Duration (s) |
|---|---|
| 5 | 735 ± 94 |
| 15 | 422 ± 45 |
| 25 | 364 ± 35 |
| 35 | 324 ± 25 |
| 45 | 318 ± 46 |
| 55 | 244 ± 18 |
| 65 | 186 ± 14 |
| 75 | 204 ± 27 |
| 85 | 156 ± 28 |
References
- Kockmann, N. 200 years in innovation of continuous distillation. Chem. Bio Eng. Rev. 2014, 1, 40–49. [Google Scholar] [CrossRef]
- Webber, A.H.; Bridger, L.G. Dephlegmator efficiency typical data and graphical calculation. Ind. Eng. Chem. 1938, 30, 315–318. Available online: https://pubs.acs.org/sharingguidelines (accessed on 14 May 2022). [CrossRef]
- Kent, R.E.; Pigford, L.R. Fractionation during condensation of vapor mixtures. AIChE J. 1956, 2, 363–371. [Google Scholar] [CrossRef]
- Röhm, J.H. Simulation of the unsteady state behaviour of the dephlegmation of binary vapour mixtures. Lett. Heat Mass Transf. 1978, 5, 307–314. [Google Scholar] [CrossRef]
- Röhm, J.H. The simulation of steady state behavior of the dephlegmation of multi-component mixed vapours. Int. J. Mass Transf. 1980, 23, 141–146. [Google Scholar] [CrossRef]
- Wang, J.; Smith, R. Dephlegmator design in low temperature gas separation. Chem. Eng. Res. Des. 2005, 83, 1133–1144. [Google Scholar] [CrossRef]
- Matias-Guiu, P.; Rodríguez-Bencomo, J.J.; Orriols, I.; Pérez-Correa, R.J.; López, F. Floral aroma improvement of Muscat spirits by packed column distillation with variable internal reflux. Food Chem. 2016, 213, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bencomo, J.J.; Pérez-Correa, R.J.; Orriols, I.; López, F. Spirit distillation strategies for aroma improvement using variable internal column reflux. Food Bioprocess Technol. 2016, 9, 1885–1892. [Google Scholar] [CrossRef]
- Yagishita, M.; Kölling, R.; Einfalt, D. Introducing a simple method to investigate relative volatilities of flavour compounds in fruit brandies. Beverages 2023, 9, 32. [Google Scholar] [CrossRef]
- Heller, D.; Einfalt, D. Reproducibility of fruit spirit distillation processes. Beverages 2022, 8, 20. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, H.; Zhou, R.; Duan, Z. Influence of salt added to solvent on extractive distillation. Chem. Eng. J. 2002, 87, 149–156. [Google Scholar] [CrossRef]
- Tsakiris, A.; Kallithraka, S.; Kourkoutas, Y. Grape brandy production, composition and sensory evaluation. J. Sci. Food Agric. 2014, 94, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.; Samy Ei-Shall, M. Condensation of supersaturated vapors of hydrogen bonding molecules: Ethylene glycol, propylene glycol, trimethylene glycol, and glycerol. J. Chem. Phys. 1996, 105, 7617–7631. [Google Scholar] [CrossRef]
- Zhao, J.; Khalizov, A.; Zhang, R.; McGraw, R. Hydrogen-bonding interaction in molecular complexes and clusters of aerosol nucleation precursors. J. Phys. Chem. A 2009, 113, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Studies on the conformation, thermodynamics, and evaporation rate characteristics of sulfuric acid and amines molecular clusters. Results Chem. 2022, 4, 100527. [Google Scholar] [CrossRef]

| Functional Group | Compound | Concentration in Solutions (mg/100 mL a.a. *) | MW (g/mol) | Boiling Point (°C) | Supplier | Purity (≥%) |
|---|---|---|---|---|---|---|
| alcohol | methanol | 1300 | 32.04 | 64.7 | CARL ROTH, Karlsruhe, Germany | 99.9 |
| 1-propanol | 350 | 60.1 | 97 | Merck, Darmstadt, Germany | 99.5 | |
| 2-methyl-1-propanol | 70 | 74.12 | 108 | Fluka Chemie, Buchs, Schweiz | 99.5 | |
| 3-methyl-1-butanol | 150 | 88.15 | 131 | Sigma-Aldrich Chemie, Taufkirchen Germany | 98.5 | |
| 2-methyl-1-butanol | 50 | 88.15 | 129 | ROTH, Karls-ruhe, Germanyh | 97.5 | |
| 1-hexanol | 15 | 102.16 | 157 | Fluka Chemie, Buchs, Schweiz | 99 | |
| aldehyde | acetaldehyde | 80 | 44.05 | 20.2 | ROTH, Karls-ruhe, Germanyh | 99.5 |
| ester | ethyl butyrate | 5 | 116.16 | 121 | Sigma-Aldrich ChemieSig-ma-Aldrich, Taufkirchen Germany | 99.5 |
| ethyl hexanoate | 5 | 144.21 | 168 | Sigma-Aldrich ChemieSig-ma-Aldrich, Taufkirchen Germany | 99.0 | |
| ethyl-2-methylbutyrate | 5 | 130.18 | 133 | Sigma-Aldrich ChemieSig-ma-Aldrich, Taufkirchen Germany | 98 | |
| ethyl acetate | 500 | 88.11 | 77.1 | Sigma-Aldrich ChemieSig-ma-Aldrich, Taufkirchen Germany | 99.9 | |
| ketone | β-damascenone | 5 | 190.28 | 116 | Sigma-Aldrich ChemieSig-ma-Aldrich, Taufkirchen Germany | 98 |
| Fractions | Starting Point | U | |
|---|---|---|---|
| C distillation | D distillation | ||
| Hearts | 6.5 ± 0.5 | 7.0 ± 0 | 0.5 |
| Tails | 53 ± 0 | 45.5 ± 0.5 | 0 * |
| Participants | Correct Answer | Wrong Answer | χ2 Value | |
|---|---|---|---|---|
| Group 1 a | 28 | 15 | 13 | 5.16 * |
| Group 2 a | 29 | 16 | 13 | 6.22 * |
| Triangle test total | 57 | 31 | 26 | 11.38 ** |
| Participants a | C Favored | D Favored | χ2 Value | |
|---|---|---|---|---|
| Group 1 | 15 | 10 | 5 | 1.67 |
| Group 2 | 16 | 5 | 11 | 2.25 |
| Preference test total | 31 | 15 | 16 | 3.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagishita, M.; Reber, O.; Alter, D.; Kölling, R.; Einfalt, D. Effect of Partial Condensation (Dephlegmation) in Fruit Brandy Distillation Equipment on the Composition of Apple Brandies. Beverages 2025, 11, 52. https://doi.org/10.3390/beverages11020052
Yagishita M, Reber O, Alter D, Kölling R, Einfalt D. Effect of Partial Condensation (Dephlegmation) in Fruit Brandy Distillation Equipment on the Composition of Apple Brandies. Beverages. 2025; 11(2):52. https://doi.org/10.3390/beverages11020052
Chicago/Turabian StyleYagishita, Manami, Oliver Reber, Daniela Alter, Ralf Kölling, and Daniel Einfalt. 2025. "Effect of Partial Condensation (Dephlegmation) in Fruit Brandy Distillation Equipment on the Composition of Apple Brandies" Beverages 11, no. 2: 52. https://doi.org/10.3390/beverages11020052
APA StyleYagishita, M., Reber, O., Alter, D., Kölling, R., & Einfalt, D. (2025). Effect of Partial Condensation (Dephlegmation) in Fruit Brandy Distillation Equipment on the Composition of Apple Brandies. Beverages, 11(2), 52. https://doi.org/10.3390/beverages11020052






