Development of Low-Caffeine Kombucha Using Lotus Root Tea and an Evaluation of Its Functional Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
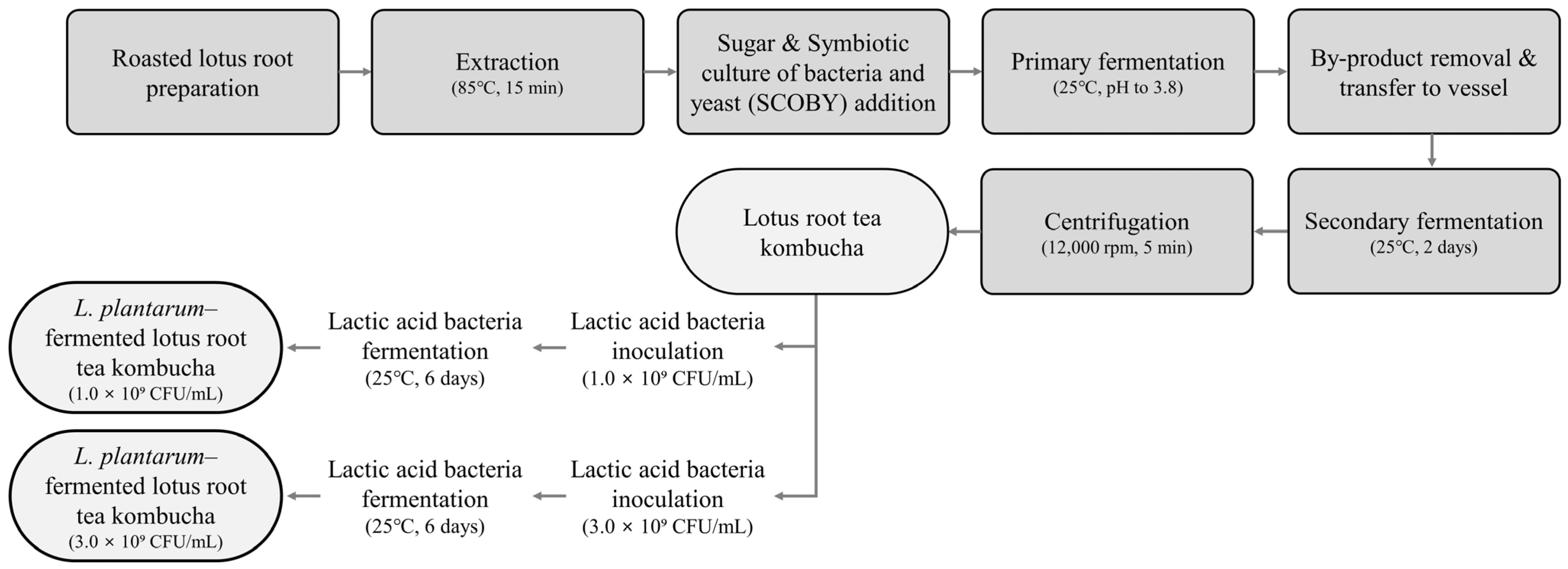
2.2. Preparation and Fermentation of Lotus Root Tea Kombucha
2.2.1. Preparation of Lotus Root Tea Kombucha
2.2.2. Additional Fermentation with Lactobacillus plantarum
2.3. HPLC Analysis of Caffeine and Organic Acids (SCFAs and Lactic Acid)
2.4. The Antioxidant Activity
2.4.1. DPPH Radical Scavenging Activity
2.4.2. ABTS Radical Scavenging Activity
2.5. Antimicrobial Activity Assay
2.6. β-Glucuronidase Inhibition Assay
2.7. C. elegans Assays
2.7.1. Maintenance and Strains
2.7.2. Pharyngeal Pumping Assay
2.7.3. Pathogen Resistance Assays of Salmonella enterica Infection
2.8. Statistical Analysis
3. Results
3.1. Caffeine Concentration of Lotus Root Tea Kombucha
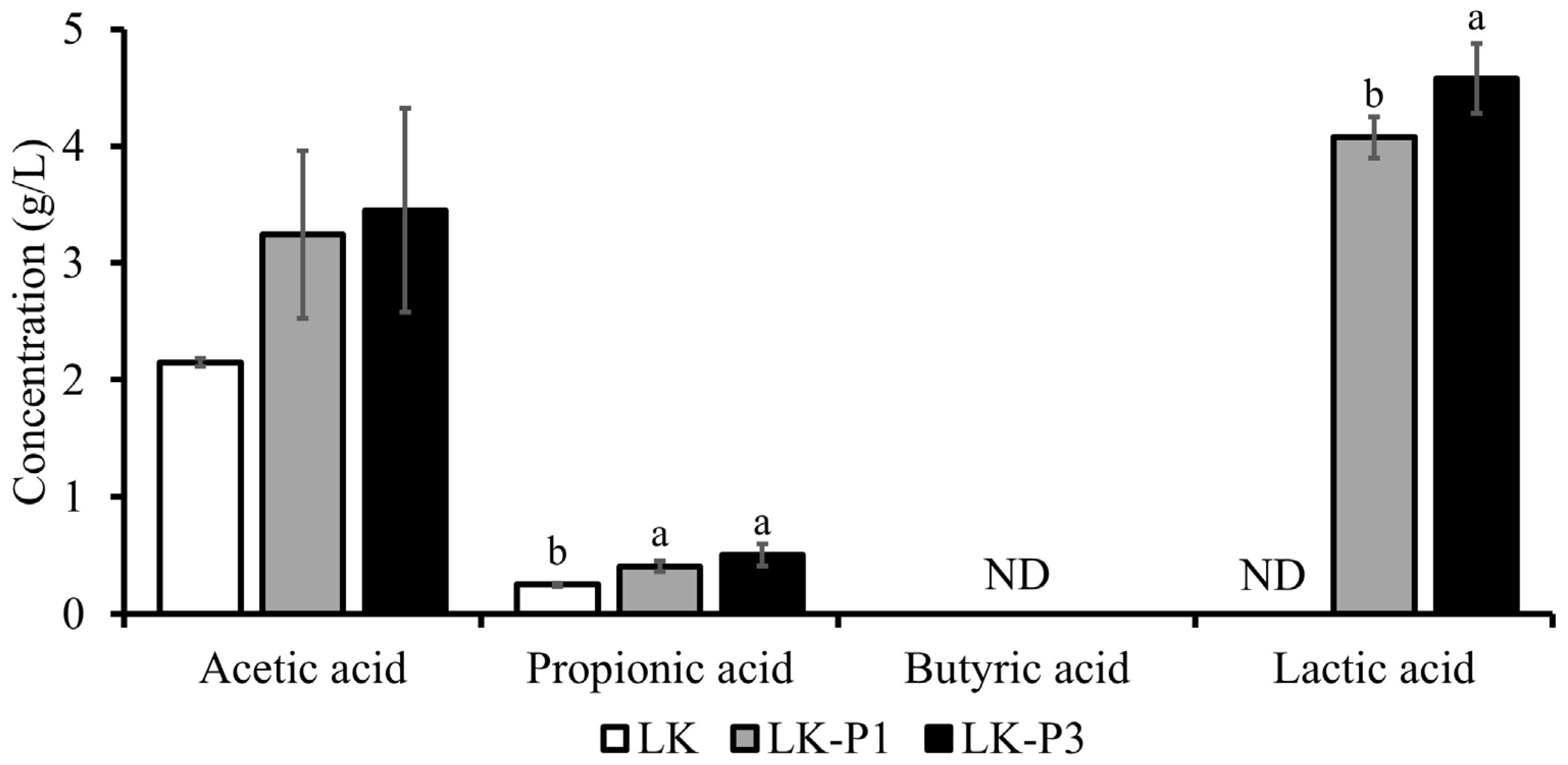
3.2. Effect of L. plantarum Fermentation on Organic Acids in Lotus Root Tea Kombucha
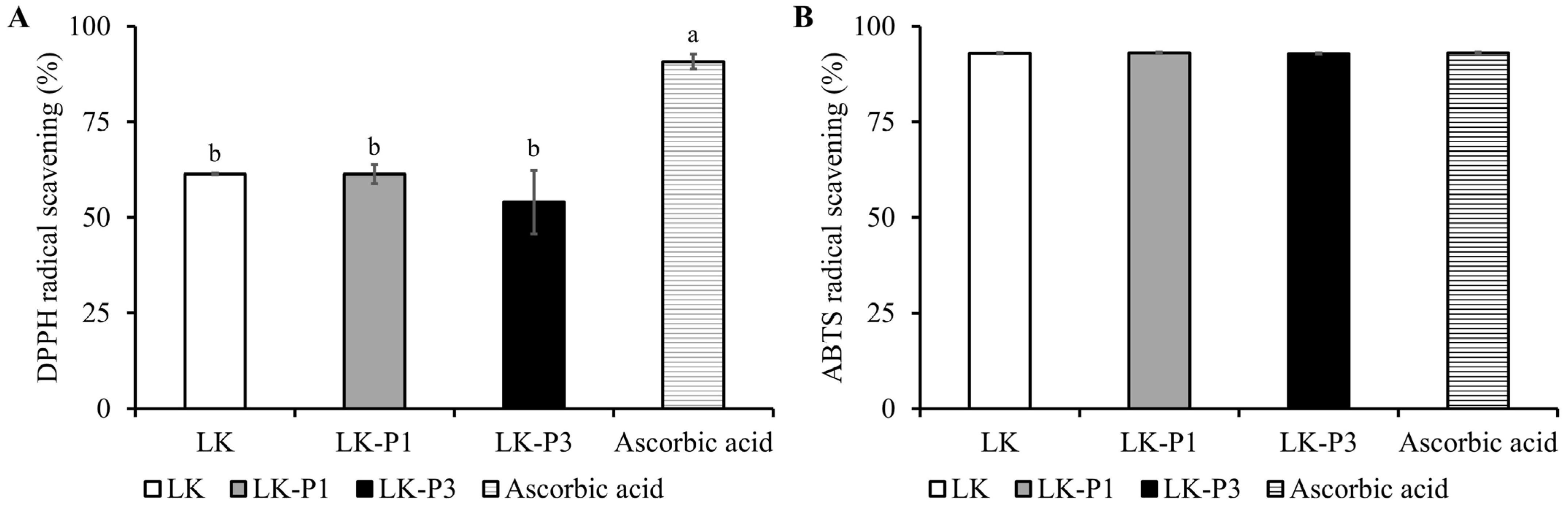
3.3. Antioxidant Activity of Lotus Root Tea Kombucha
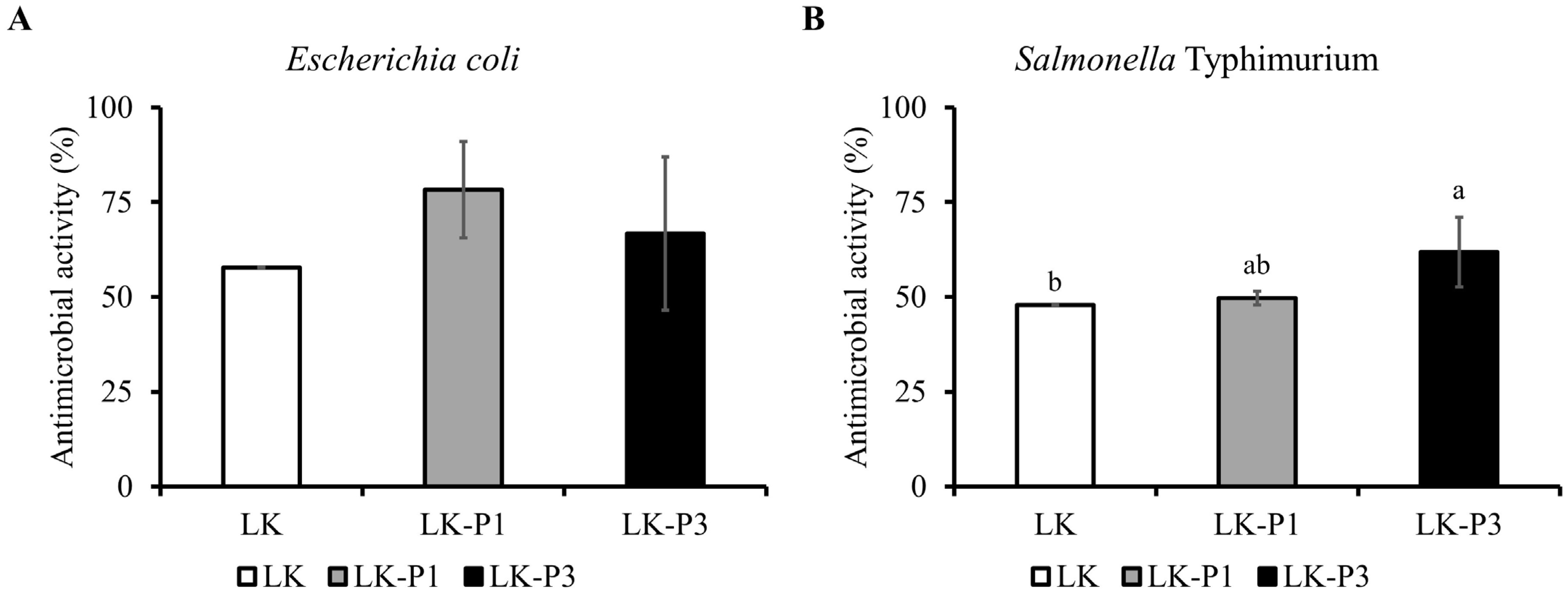
3.4. Antimicrobial Activity of Lotus Root Tea Kombucha
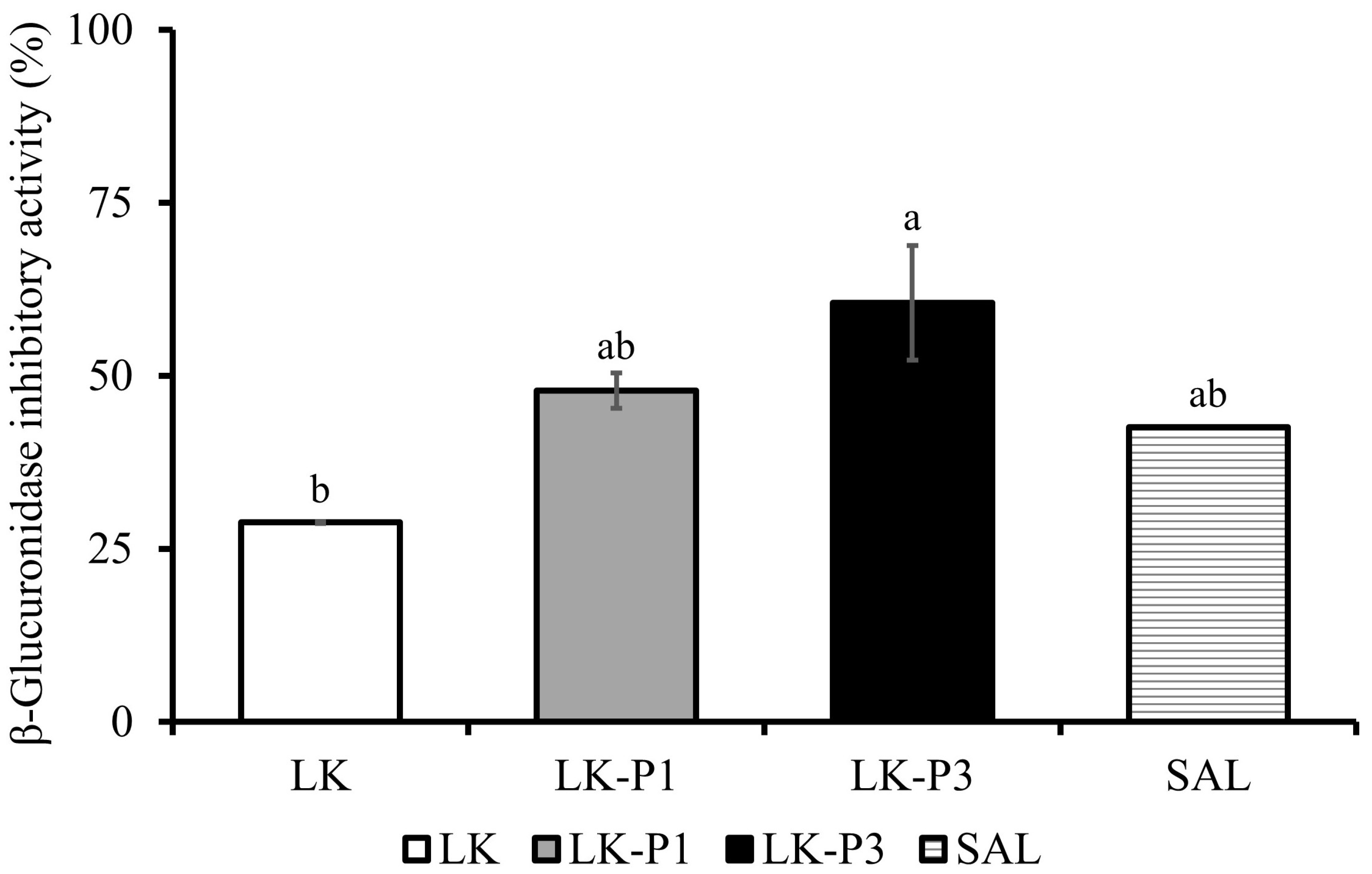
3.5. Inhibitory Effect of Lotus Root Tea Kombucha on β-Glucuronidase Activity
3.6. Protective Effect of Lotus Root Tea Kombucha Against Salmonella Infection Resistance in C. elegans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFU | Colony-forming unit |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| HPLC | High-performance liquid chromatography |
| LAB | Lactic acid bacteria |
| LK | Lotus root tea kombucha |
| LK-P1 | Lotus root tea kombucha supplemented with 1.0 × 109 CFU/mL of L. plantarum |
| LK-P3 | Lotus root tea kombucha supplemented with 3.0 × 109 CFU/mL of L. plantarum |
| NGM | Nematode growth medium |
| OD | Optical density |
| SAL | D-saccharic acid 1,4-lactone |
| SCOBY | Symbiotic culture of bacteria and yeast |
| SCFAs | Short-chain fatty acids |
References
- Troitino, C. Kombucha 101: Demystifying the Past, Present and Future of the Fermented Tea Drink. Forbes Website. Available online: https://www.forbes.com/sites/christinatroitino/2017/02/01/kombucha-101-demystifying-the-past-present-and-future-of-the-fermented-tea-drink/ (accessed on 1 February 2017).
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A.-L. Traditional low-alcoholic and non-alcoholic fermented beverages consumed in European countries: A neglected food group. Nutr. Res. Rev. 2017, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Içen, H.; Corbo, M.R.; Sinigaglia, M.; Korkmaz, B.I.O.; Bevilacqua, A. Microbiology and antimicrobial effects of kombucha, a short overview. Food Biosci. 2023, 56, 103270. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, Ľ.; Kántor, A.; Kačániová, M. The evaluation of chemical, antioxidant, antimicrobial and sensory properties of kombucha tea beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef]
- rDarbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Arpaia, N.; Rudensky, A.Y. Microbial metabolites control gut inflammatory responses. Proc. Natl. Acad. Sci. USA 2014, 111, 2058–2059. [Google Scholar] [CrossRef] [PubMed]
- Anantachoke, N.; Duangrat, R.; Sutthiphatkul, T.; Ochaikul, D.; Mangmool, S. Kombucha beverages produced from fruits, vegetables, and plants: A review on their pharmacological activities and health benefits. Foods 2023, 12, 1818. [Google Scholar] [CrossRef]
- Martínez Leal, J.; Valenzuela Suárez, L.; Jayabalan, R.; Huerta Oros, J.; Escalante-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CyTA-J. Food 2018, 16, 390–399. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Saimaiti, A.; Luo, M.; Huang, S.-Y.; Xiong, R.-G.; Shang, A.; Gan, R.-Y.; Li, H.-B. Fermentation with tea residues enhances antioxidant activities and polyphenol contents in kombucha beverages. Antioxidants 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Crum, H.; LaGory, A. The Big Book of Kombucha: Brewing, Flavoring, and Enjoying the Health Benefits of Fermented Tea; Storey Publishing: North Adams, MA, USA, 2016. [Google Scholar]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Whitsett, T.L.; al’Absi, M.; Sung, B.H.; Vincent, A.S.; Wilson, M.F. Caffeine stimulation of cortisol secretion across the waking hours in relation to caffeine intake levels. Biopsychosoc. Sci. Med. 2005, 67, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Parvin, R.; Bhattacharya, S.; Chaudhury, S.S.; Roy, U.; Mukherjee, J.; Gachhui, R. Production, purification and characterization of a novel thermostable caffeine dehydrogenase from Pichia manshurica strain CD1 isolated from kombucha tea. Microbiology 2023, 92, 230–241. [Google Scholar] [CrossRef]
- Saimaiti, A.; Zhou, D.-D.; Li, J.; Xiong, R.-G.; Gan, R.-Y.; Huang, S.-Y.; Shang, A.; Zhao, C.-N.; Li, H.-Y.; Li, H.-B. Dietary sources, health benefits, and risks of caffeine. Crit. Rev. Food Sci. Nutr. 2023, 63, 9648–9666. [Google Scholar] [CrossRef] [PubMed]
- Showkat, Q.A.; Rather, J.A.; Jabeen, A.; Dar, B.; Makroo, H.A.; Majid, D. Bioactive components, physicochemical and starch characteristics of different parts of lotus (Nelumbo nucifera Gaertn.) plant: A review. Int. J. Food Sci. Technol. 2021, 56, 2205–2214. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, X.-Y.; Zhong, Z.-T.; Huang, F.; Li, S.-Y.; Wang, L.-M.; Min, T.; Wang, H.-X. Structural and biological properties of polysaccharides from lotus root. Int. J. Biol. Macromol. 2019, 130, 454–461. [Google Scholar] [CrossRef]
- Guan, X.; Feng, Y.; Jiang, Y.; Hu, Y.; Zhang, J.; Li, Z.; Song, C.; Li, F.; Hou, J.; Shen, T. Simulated digestion and in vitro fermentation of a polysaccharide from lotus (Nelumbo nucifera Gaertn.) root residue by the human gut microbiota. Food Res. Int. 2022, 155, 111074. [Google Scholar] [CrossRef]
- Zheng, Z.; Gao, W.; Zhu, Z.; Li, S.; Chen, X.; Cravotto, G.; Sui, Y.; Zhou, L. Complexes of Soluble Dietary Fiber and Polyphenols from Lotus Root Regulate High-Fat Diet-Induced Hyperlipidemia in Mice. Antioxidants 2024, 13, 466. [Google Scholar] [CrossRef]
- Bangar, S.P.; Dunno, K.; Kumar, M.; Mostafa, H.; Maqsood, S. A comprehensive review on lotus seeds (Nelumbo nucifera Gaertn.): Nutritional composition, health-related bioactive properties, and industrial applications. J. Funct. Foods 2022, 89, 104937. [Google Scholar] [CrossRef]
- You, J.S.; Lee, Y.J.; Kim, K.S.; Kim, S.H.; Chang, K.J. Ethanol extract of lotus (Nelumbo nucifera) root exhibits an anti-adipogenic effect in human pre-adipocytes and anti-obesity and anti-oxidant effects in rats fed a high-fat diet. Nutr. Res. 2014, 34, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Khatua, T.N.; Venkatesh, P.; Saha, B.; Mukherjee, P.K. Immunomodulatory potential of rhizome and seed extracts of Nelumbo nucifera Gaertn. J. Ethnopharmacol. 2010, 128, 490–494. [Google Scholar] [CrossRef]
- Tsuruta, Y.; Nagao, K.; Shirouchi, B.; Nomura, S.; Tsuge, K.; Koganemaru, K.; Yanagita, T. Effects of lotus root (the edible rhizome of Nelumbo nucifera) on the deveolopment of non-alcoholic fatty liver disease in obese diabetic db/db mice. Biosci. Biotechnol. Biochem. 2012, 76, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Saha, K.; Pal, M.; Saha, B. Effect of Nelumbo nucifera rhizome extract on blood sugar level in rats. J. Ethnopharmacol. 1997, 58, 207–213. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Saha, K.; Balasubramanian, R.; Pal, M.; Saha, B. Studies on psychopharmacological effects of Nelumbo nucifera Gaertn. rhizome extract. J. Ethnopharmacol. 1996, 54, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.M.; Shim, K.J.; Choi, M.J.; Park, S.Y.; Choi, B.-J.; Chang, M.S.; Park, S.K. Novel effects of Nelumbo nucifera rhizome extract on memory and neurogenesis in the dentate gyrus of the rat hippocampus. Neurosci. Lett. 2008, 443, 104–107. [Google Scholar] [CrossRef]
- Yi, Y.; Sun, J.; Xie, J.; Min, T.; Wang, L.-M.; Wang, H.-X. Phenolic profiles and antioxidant activity of lotus root varieties. Molecules 2016, 21, 863. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Zhu, Z.; Cheng, S.; He, J.; Lamikanra, O. Soluble dietary fiber and polyphenol complex in lotus root: Preparation, interaction and identification. Food Chem. 2020, 314, 126219. [Google Scholar] [CrossRef]
- Tan, S.-J.; Lee, C.-K.; Gan, C.-Y.; Olalere, O.A. Statistical Optimization of Flavonoid and Antioxidant Recovery from Macerated Chinese and Malaysian Lotus Root (Nelumbo nucifera) Using Response Surface Methodology. Molecules 2021, 26, 2014. [Google Scholar] [CrossRef]
- Dong, W.; Liu, X.; Yi, Y.; Wang, L.; Hou, W.; Ai, Y.; Wang, H.; Min, T. Evaluation of Pre-Harvest Nutrient Composition and Functional Active Substances in Various Lotus Roots. Foods 2024, 13, 2297. [Google Scholar] [CrossRef]
- Saeed, S.M.G.; Tayyaba, S.; Ali, S.A.; Tayyab, S.; Sayeed, S.A.; Ali, R.; Mobin, L.; Naz, S. Evaluation of the potential of Lotus root (Nelumbo nucifera) flour as a fat mimetic in biscuits with improved functional and nutritional properties. CyTA-J. Food 2020, 18, 624–634. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Lee, S. Quality characteristics, antioxidant activity, and acrylamide content of lotus root chips prepared using different processing methods. Food Sci. Biotechnol. 2024, 33, 1371–1379. [Google Scholar] [CrossRef]
- Dufresne, C.; Farnworth, E. Tea, Kombucha, and health: A review. Food Res. Int. 2000, 33, 409–421. [Google Scholar] [CrossRef]
- Masood, M.I.; Qadir, M.I.; Shirazi, J.H.; Khan, I.U. Beneficial effects of lactic acid bacteria on human beings. Crit. Rev. Microbiol. 2011, 37, 91–98. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, F.; Ji, B.; Li, B.; Luo, Y.; Yang, L.; Li, T. Symbiosis between microorganisms from kombucha and kefir: Potential significance to the enhancement of kombucha function. Appl. Biochem. Biotechnol. 2010, 160, 446–455. [Google Scholar] [CrossRef]
- Beaud, D.; Tailliez, P.; Anba-Mondoloni, J. Genetic characterization of the β-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology 2005, 151, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Ejike, E.N. Probiotic Potentials of Lactobacillus plantarumFMO2, Lactobacillus plantarumZ2 and Lactobacillus pentosusBSR3 Isolated from Fermented Starchy Foods. Curr. Trends Food Sci. 2024, 1. [Google Scholar]
- Chen, J.; Mou, L.; Wang, L.; Wu, G.; Dai, X.; Chen, Q.; Zhang, J.; Luo, X.; Xu, F.; Zhang, M. Mixed Bacillus subtilis and Lactiplantibacillus plantarum-fermented feed improves gut microbiota and immunity of Bamei piglet. Front. Microbiol. 2024, 15, 1442373. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Chen, C.; Fu, Y.; Wang, H.; Zhao, C.; Zhang, J.; Liu, L. The Effect of an Early-Life Lactiplantibacillus plantarum LPJZ-658 Intervention on Performance and Gut Microbiota in Suckling Piglets. Microbiol. Res. 2025, 16, 41. [Google Scholar] [CrossRef]
- Biernat, K.A.; Pellock, S.J.; Bhatt, A.P.; Bivins, M.M.; Walton, W.G.; Tran, B.N.T.; Wei, L.; Snider, M.C.; Cesmat, A.P.; Tripathy, A. Structure, function, and inhibition of drug reactivating human gut microbial β-glucuronidases. Sci. Rep. 2019, 9, 825. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Dong, N.T.; Le, P.H.; Nguyen, H.T. Evaluation of the glucuronic acid production and other biological activities of fermented sweeten-black tea by kombucha layer and the co-culture with different Lactobacillus sp. strains. Int. J. Mod. Eng. Res. 2014, 4, 12–17. [Google Scholar]
- Plessas, S. Advancements in the use of fermented fruit juices by lactic acid bacteria as functional foods: Prospects and challenges of Lactiplantibacillus (Lpb.) plantarum subsp. plantarum application. Fermentation 2021, 8, 6. [Google Scholar] [CrossRef]
- 이미령; 김희송; 양동훈; 김익중. Method for Manufacturing Fermented Tea Using Lotus Root and Fermented Tea Produced Thereby. Korean Patent KR1020200061670, 17 October 2022. (In Korean). [CrossRef]
- Wanyika, H.; Gatebe, E.; Gitu, L.; Ngumba, E.; Maritim, C. Determination of caffeine content of tea and instant coffee brands found in the Kenyan market. Afr. J. Food Sci. 2010, 4, 353–358. [Google Scholar]
- Xie, J.; Schaich, K. Re-evaluation of the 2, 2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.C.; Chinnathambi, A.; Alahmadi, T.A.; Joshi, D.; Jhanani, G.; Haile, A. An Assessment of Anticancer Activity (MCF-7) and Free Radicals Scavenging Potential of Ferula Asafoetida Synthesized AgNPs through In Vitro Analyses. J. Nanomater. 2022, 2022, 6095005. [Google Scholar] [CrossRef]
- Hyrslova, I.; Kana, A.; Nesporova, V.; Mrvikova, I.; Doulgeraki, A.I.; Lampova, B.; Doskocil, I.; Musilova, S.; Kieliszek, M.; Krausova, G. In vitro digestion and characterization of selenized Saccharomyces cerevisiae, Pichia fermentans and probiotic Saccharomyces boulardii. J. Trace Elem. Med. Biol. 2024, 83, 127402. [Google Scholar] [CrossRef]
- Medina-Pérez, G.; Peralta-Adauto, L.; Afanador-Barajas, L.; Fernández-Luqueño, F.; Pérez-Soto, E.; Campos-Montiel, R.; Peláez-Acero, A. Inhibition of urease, elastase, and β-glucuronidase enzymatic activity by applying aqueous extracts of opuntia oligacantha CF först acid fruits: In vitro essay under simulated digestive conditions. Appl. Sci. 2021, 11, 7705. [Google Scholar] [CrossRef]
- Liew, S.Y.; Sivasothy, Y.; Shaikh, N.N.; Isa, D.M.; Lee, V.S.; Choudhary, M.I.; Awang, K. β-Glucuronidase inhibitors from Malaysian plants. J. Mol. Struct. 2020, 1221, 128743. [Google Scholar] [CrossRef]
- Yun, B.; Ryu, S.; Kang, M.; Lee, J.; Yoo, J.; Kim, Y.; Oh, S. Probiotic Lacticaseibacillus rhamnosus GG increased longevity and resistance against foodborne pathogens in Caenorhabditis elegans by regulating microRNA miR-34. Front. Cell. Infect. Microbiol. 2022, 11, 819328. [Google Scholar] [CrossRef]
- Trojanowski, N.F.; Raizen, D.M.; Fang-Yen, C. Pharyngeal pumping in Caenorhabditis elegans depends on tonic and phasic signaling from the nervous system. Sci. Rep. 2016, 6, 22940. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.-R.; Yang, H.; Park, C.-H.; Yun, C.-S.; Jang, B.-C.; Hong, Y.; Park, D.-S. Potential probiotic Lactiplantibacillus plantarum DS1800 extends lifespan and enhances stress resistance in Caenorhabditis elegans model. Front. Physiol. 2024, 15, 1476096. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Yang, J.; Lagishetty, V.; Kurnia, P.; Henning, S.M.; Ahdoot, A.I.; Jacobs, J.P. Microbial and chemical profiles of commercial kombucha products. Nutrients 2022, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-C.; Chen, C. Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chem. 2006, 98, 502–507. [Google Scholar] [CrossRef]
- Han, S.; Hu, F.; Ji, X.; Liu, Y.; Zhang, S.; Wang, Z.; Qiao, K. Polysaccharides from Ziziphus jujuba prolong lifespan and attenuate oxidative stress in Caenorhabditis elegans via DAF-16 and SKN-1. Int. J. Biol. Macromol. 2024, 282, 137482. [Google Scholar] [CrossRef]
- Hadinia, N.; Dovom, M.R.E.; Yavarmanesh, M. The effect of fermentation conditions (temperature, salt concentration, and pH) with lactobacillus strains for producing short chain fatty acids. LWT 2022, 165, 113709. [Google Scholar] [CrossRef]
- Liu, X.-F.; Shao, J.-H.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.-J.; Liu, Z.-z.; He, D.-D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Diez, T.; Cabezas, J.A. Properties of Two Molecular Forms of β-Glucuronidase from the Mollusc Littorina littorea L. Eur. J. Biochem. 1979, 93, 301–311. [Google Scholar] [CrossRef]
- KIM, D.; JIN, Y.; JUNG, E.; HAN, M.; Kobashi, K. Purification and characterization of β-glucuronidase from Escherichia coli HGU-3, a human intestinal bacterium. Biol. Pharm. Bull. 1995, 18, 1184–1188. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.W.; Ji, B.P.; Zhou, F.; Li, B.; Luo, Y.; Yang, L.; Li, T. Hypocholesterolaemic and antioxidant effects of kombucha tea in high-cholesterol fed mice. J. Sci. Food Agric. 2009, 89, 150–156. [Google Scholar] [CrossRef]
- Sreeramulu, G.; Zhu, Y.; Knol, W. Kombucha fermentation and its antimicrobial activity. J. Agric. Food Chem. 2000, 48, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.W.; Liu, Y.-l.; Leung, P.S.; Chan, R.C.; Inciardi, J.F.; Cheng, A.F. Expression of bacterial β-glucuronidase in human bile: An in vitro study. Gastrointest. Endosc. 2001, 54, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Al-Kalifawi, E. Study the antimicrobial effect of kombucha tea on bacteria isolated from diabetic foot ulcer. J. Biotechnol. Res. Cent. (JOBRC) 2014, 8, 27–33. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, I.; Mannaa, M.; Kim, J.; Wang, S.; Park, I.; Kim, J.; Seo, Y.-S. Effect of Kombucha on gut-microbiota in mouse having non-alcoholic fatty liver disease. Food Sci. Biotechnol. 2019, 28, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.-L.; Mahdy, A.S. Iraqi medicinal plants: Total flavonoid contents, free-radical scavenging and bacterial beta-glucuronidase inhibition activities. IOSR J. Dent. Med. Sci. 2014, 13, 72–77. [Google Scholar] [CrossRef]
- Gade, A.; Kumar, M.S. Gut microbial metabolites of dietary polyphenols and their potential role in human health and diseases. J. Physiol. Biochem. 2023, 79, 695–718. [Google Scholar] [CrossRef]
- Dinev, T.; Beev, G.; Tzanova, M.; Denev, S.; Dermendzhieva, D.; Stoyanova, A. Antimicrobial activity of Lactobacillus plantarum against pathogenic and food spoilage microorganisms: A review. Bulg. J. Vet. Med. 2018, 21, 253–268. [Google Scholar] [CrossRef]
- Zare, D.; Aryaee, H.; Mirdamadi, S.; Shirkhan, F. The Benefits and Applications of Lactobacillus plantarum in Food and Health: A Narrative Review. Iran. J. Public Health 2024, 53, 2201. [Google Scholar] [CrossRef]
- Ma, H.; Dong, A.; Xu, Y.; Wu, Q.; Lambo, M.T.; Zhang, Y.; Dou, X.; Li, Y. Regulatory effects of high concentrate diet synergistically fermented with cellulase and lactic acid bacteria: In vitro ruminal fermentation, methane production, and rumen microbiome. Anim. Feed Sci. Technol. 2025, 319, 116194. [Google Scholar] [CrossRef]
- Arenahalli Ningegowda, M.; Siddalingaiya Gurudutt, P. In vitro fermentation of prebiotics by Lactobacillus plantarum CFR 2194: Selectivity, viability and effect of metabolites on β-glucuronidase activity. World J. Microbiol. Biotechnol. 2012, 28, 901–908. [Google Scholar] [CrossRef]
- An, L.; Fu, X.; Chen, J.; Ma, J. Application of Caenorhabditis elegans in lipid metabolism research. Int. J. Mol. Sci. 2023, 24, 1173. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.; Wang, Y.; Parhar, R.S.; Collison, K.S.; Conca, W.; Al-Mohanna, F.; Gaugler, R. A C. elegans model to study human metabolic regulation. Nutr. Metab. 2013, 10, 31. [Google Scholar] [CrossRef]
- Zhang, F.; Berg, M.; Dierking, K.; Félix, M.-A.; Shapira, M.; Samuel, B.S.; Schulenburg, H. Caenorhabditis elegans as a model for microbiome research. Front. Microbiol. 2017, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Cabreiro, F.; Gems, D. Worms need microbes too: Microbiota, health and aging in Caenorhabditis elegans. EMBO Mol. Med. 2013, 5, 1300–1310. [Google Scholar] [CrossRef]
- Labrousse, A.; Chauvet, S.; Couillault, C.; Kurz, C.L.; Ewbank, J.J. Caenorhabditis elegans is a model host for Salmonella typhimurium. Curr. Biol. 2000, 10, 1543–1545. [Google Scholar] [CrossRef] [PubMed]
- DuMez-Kornegay, R.N.; Baker, L.S.; Morris, A.J.; DeLoach, W.L.; Dowen, R.H. Kombucha Tea-associated microbes remodel host metabolic pathways to suppress lipid accumulation. PLoS Genet. 2024, 20, e1011003. [Google Scholar] [CrossRef]
- Aballay, A.; Yorgey, P.; Ausubel, F.M. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 2000, 10, 1539–1542. [Google Scholar] [CrossRef]
- Yang, T.; Fan, X.; Li, D.; Zhao, T.; Wu, D.; Liu, Z.; Long, D.; Li, B.; Huang, X. High Antioxidant capacity of Lacticaseibacillus paracasei TDM-2 and Pediococcus pentosaceus TCM-3 from Qinghai Tibetan plateau and their function towards gut modulation. Foods 2023, 12, 1814. [Google Scholar] [CrossRef]
- Frece, J.; Kos, B.; Beganović, J.; Vuković, S.; Šušković, J. In vivo testing of functional properties of three selected probiotic strains. World J. Microbiol. Biotechnol. 2005, 21, 1401–1408. [Google Scholar] [CrossRef]
- Faghfuri, E.; Gholizadeh, P. The role of Akkermansia muciniphila in colorectal cancer: A double-edged sword of treatment or disease progression? Biomed. Pharmacother. 2024, 173, 116416. [Google Scholar] [CrossRef] [PubMed]
- Feizi, H.; Plotnikov, A.; Rezaee, M.A.; Ganbarov, K.; Kamounah, F.S.; Nikitin, S.; Kadkhoda, H.; Gholizadeh, P.; Pagliano, P.; Kafil, H.S. Postbiotics versus probiotics in early-onset colorectal cancer. Crit. Rev. Food Sci. Nutr. 2024, 64, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Geronikaki, A.A.; Gavalas, A.M. Antioxidants and inflammatory disease: Synthetic and natural antioxidants with anti-inflammatory activity. Comb. Chem. High Throughput Screen. 2006, 9, 425–442. [Google Scholar] [CrossRef] [PubMed]

| LK | LK-P1 | LK-P3 | |
|---|---|---|---|
| Caffeine (µg/mL) | 2.75 ± 0.02 a | 2.29 ± 0.09 b | 2.32 ± 0.10 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.S.; Nam, Y.; Kim, S.; Kim, H.S.; Lee, E.J.; Lee, M.-R.; Kim, S.R. Development of Low-Caffeine Kombucha Using Lotus Root Tea and an Evaluation of Its Functional Properties. Beverages 2025, 11, 55. https://doi.org/10.3390/beverages11020055
Baek JS, Nam Y, Kim S, Kim HS, Lee EJ, Lee M-R, Kim SR. Development of Low-Caffeine Kombucha Using Lotus Root Tea and an Evaluation of Its Functional Properties. Beverages. 2025; 11(2):55. https://doi.org/10.3390/beverages11020055
Chicago/Turabian StyleBaek, Jin Seon, Younhee Nam, Sunghee Kim, Hee Song Kim, Eun Jin Lee, Mee-Ryung Lee, and Soo Rin Kim. 2025. "Development of Low-Caffeine Kombucha Using Lotus Root Tea and an Evaluation of Its Functional Properties" Beverages 11, no. 2: 55. https://doi.org/10.3390/beverages11020055
APA StyleBaek, J. S., Nam, Y., Kim, S., Kim, H. S., Lee, E. J., Lee, M.-R., & Kim, S. R. (2025). Development of Low-Caffeine Kombucha Using Lotus Root Tea and an Evaluation of Its Functional Properties. Beverages, 11(2), 55. https://doi.org/10.3390/beverages11020055








