In Vitro Screening of Potential Role of Green and Roasted Coffee Extracts in Type 2 Diabetes Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extracts Production
2.3. Determination of Total Phenolic Content
2.4. Determination of Total Flavonoid Content
2.5. Determination of Total Tannin Content
2.6. Evaluation of the Antioxidant Activity
2.7. Resazurin Cell Viability Assay
2.8. Determination of Glucose Uptake Capacity by Yeast Cells
2.9. In Vitro α-Glucosidase Inhibition Assay
2.10. In Vitro α-Amylase Inhibition Assay
2.11. Statistical Analysis
3. Results
3.1. Phytochemical Profile
3.2. Antioxidant Activity
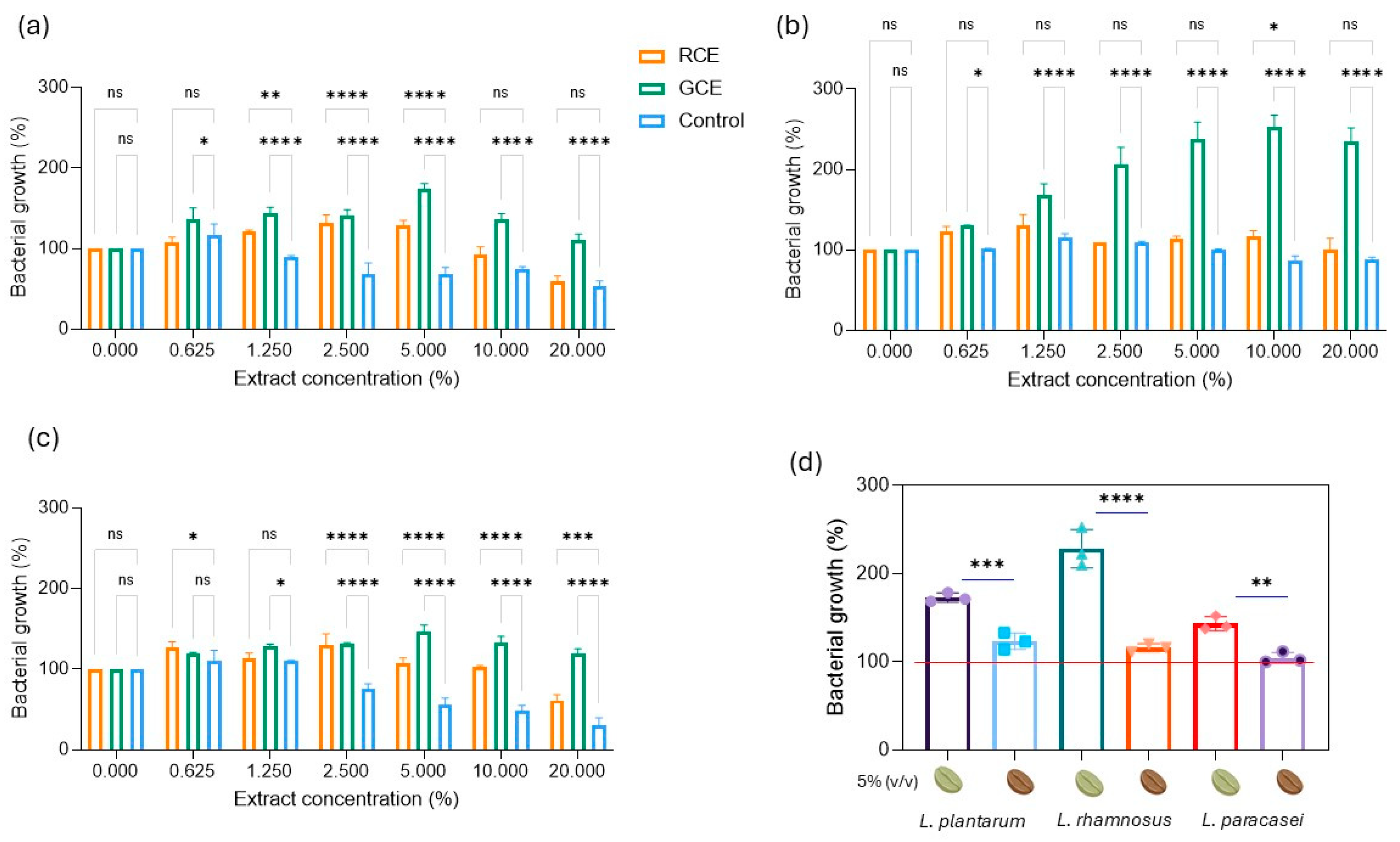
3.3. Effect of GCE and RCE on Probiotics Growth
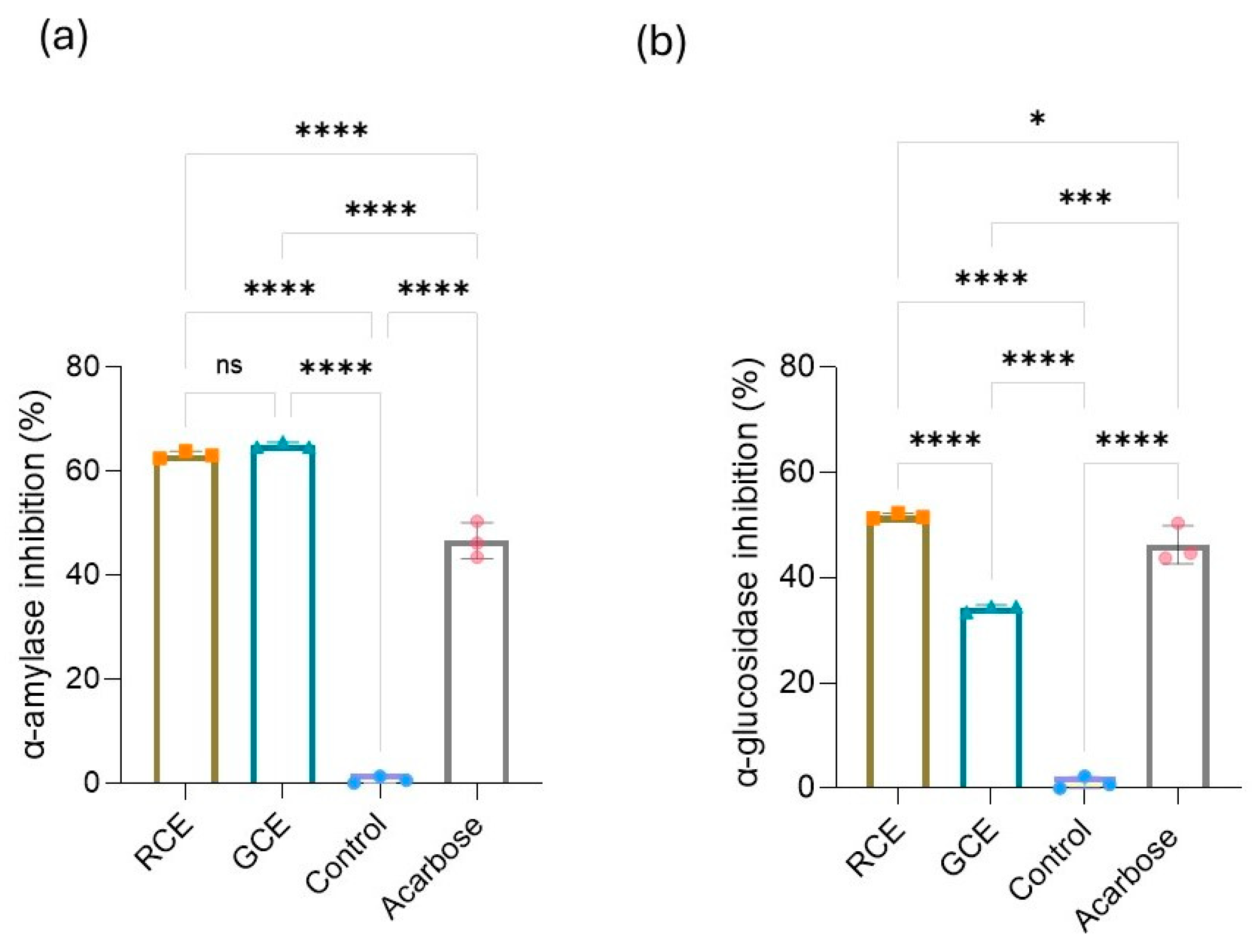
3.4. Effect of Coffee Extracts on Carbohydrate-Cleavage Enzymes
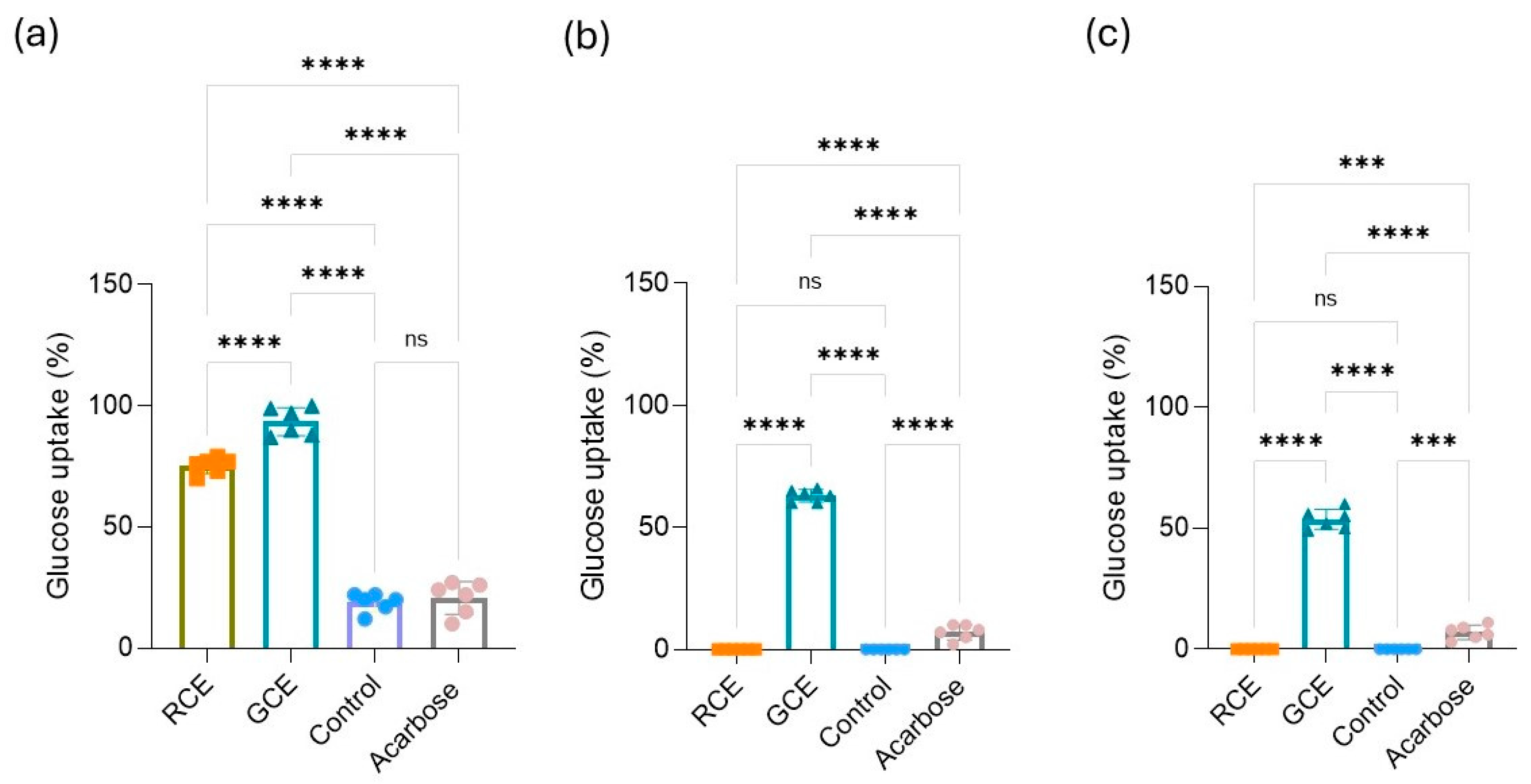
3.5. Modulation of Glucose Cell Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 10. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, K.M.; Kemps, E.; White, M.J.; Bartlett, S.E. The Impact of Free Sugar on Human Health-A Narrative Review. Nutrients 2023, 15, 4. [Google Scholar] [CrossRef]
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 Diabetes; StatPearl: Petersburg, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513253/ (accessed on 23 June 2023).
- Institute for Quality and Efficiency in Health Care (IQWiG). Overview: Type 2 Diabetes. In Informed Health; Institute for Quality and Efficiency in Health Care (IQWiG): Cologne, Germany, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279509/ (accessed on 18 December 2023).
- Smith, J.; Doe, A.; Johnson, L. The impact of diet on type 2 diabetes management. Lancet Diabetes Endocrinol. 2024, 12, 5. [Google Scholar]
- Rehman, G.; Hamayun, M.; Iqbal, A.; Ul Islam, S.; Arshad, S.; Zaman, K.; Ahmad, A.; Shehzad, A.; Hussain, A.; Lee, I. In Vitro Antidiabetic Effects and Antioxidant Potential of Cassia nemophila Pods. BioMed Res. Int. 2018, 2018, 1824790. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Martinez-González, M.Á.; Bulló, M.; Ros, E. The role of diet in the prevention of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2011, 21, B32–B48. [Google Scholar] [CrossRef] [PubMed]
- Ardisson Korat, A.V.; Willett, W.C.; Hu, F.B. Diet, lifestyle, and genetic risk factors for type 2 diabetes: A review from the Nurses’ Health Study, Nurses’ Health Study 2, and Health Professionals’ Follow-up Study. Curr. Nutr. Rep. 2014, 3, 4. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. A review. Nutrients 2020, 12, 8. [Google Scholar] [CrossRef]
- Jagadeesan, G.; Muniyandi, K.; Manoharan, A.L.; Nataraj, G.; Thangaraj, P. Understanding the bioaccessibility, α-amylase and α-glucosidase enzyme inhibition kinetics of Allmania nodiflora (L.) R.Br. ex Wight polyphenols during in vitro simulated digestion. Food Chem. 2022, 372, 131294. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut microbiota and complications of type-2 diabetes. Nutrients 2021, 14, 1. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Yen, Y.-Y.; Hung, K.-C.; Hsu, S.-W.; Lan, S.-J.; Lin, H.-C. Inhibitory effects of pu-erh tea on alpha glucosidase and alpha amylase: A systemic review. Nutr. Diabetes 2019, 9, 23. [Google Scholar] [CrossRef]
- Poovitha, S.; Parani, M. In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.). BMC Complement. Altern. Med. 2016, 16, 185. [Google Scholar] [CrossRef]
- Holman, R.R.; Cull, C.A.; Turner, R.C. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (UK Prospective Diabetes Study 44). Diabetes Care 1999, 22, 960–964. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.-J.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoon, S.; Park, Y.H.; Lee, J.-S.; Rhyu, D.Y.; Kim, K.-T. Microbiota dysbiosis associated with type 2 diabetes-like effects caused by chronic exposure to a mixture of chlorinated persistent organic pollutants in zebrafish. Environ. Pollut. 2023, 334, 122108. [Google Scholar] [CrossRef]
- Wang, G.; Si, Q.; Yang, S.; Jiao, T.; Zhu, H.; Tian, P.; Wang, L.; Li, X.; Gong, L.; Zhao, J.; et al. Lactic acid bacteria reduce diabetes symptoms in mice by alleviating gut microbiota dysbiosis and inflammation in different manners. Food Funct. 2020, 11, 5898–5914. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y. Mechanism and application of Lactobacillus in type 2 diabetes-associated periodontitis. Front Public Health 2023, 11, 1248518. [Google Scholar] [CrossRef]
- Sarriá, B.; Martínez-López, S.; Mateos, R.; Bravo-Clemente, L. Long-term consumption of a green/roasted coffee blend positively affects glucose metabolism and insulin resistance in humans. Food Res. Int. 2016, 89, 1023–1028. [Google Scholar] [CrossRef]
- Gamboa-Gómez, C.I.; Morales-Castro, J.; Barragan-Zuñiga, J.; Herrera, M.D.; Zamilpa-Álvarez, A.; González, J.L.; Martínez-Aguilar, G.; Morales-Castro, E.P.; Anese, M.; Alongi, M. Influence of coffee roasting degree on antioxidant and metabolic parameters: Comprehensive in vitro and in vivo analysis. Curr. Res. Food Sci. 2024, 9, 100861. [Google Scholar] [CrossRef]
- Van Dam, R.M. Coffee and type 2 diabetes: From beans to beta-cells. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Chen, S. Effects of coffee on type 2 diabetes mellitus. Nutrition 2014, 30, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Parker, E.D.; Folsom, A.R. Coffee consumption and risk of type 2 diabetes mellitus: An 11-year prospective study of 28,812 postmenopausal women. Arch. Intern. Med. 2006, 166, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Liczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops. Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Palmieri, M.G.S.; Cruz, L.T.; Bertges, F.S.; Húngaro, H.M.; Batista, L.R.; Silva, S.S.; Fonseca, M.J.V.; Rodarte, M.P.; Vilela, F.M.P.; Amaral, M.P.H. Enhancement of antioxidant properties from green coffee as promising ingredient for food and cosmetic industries. Biocatal. Agric. Biotechnol. 2018, 16, 43–48. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef]
- Awwad, S.; Issa, R.; Alnsour, L.; Albals, D.; Al-Momani, I. Quantification of caffeine and chlorogenic acid in green and roasted coffee samples using HPLC-DAD and evaluation of the effect of degree of roasting on their levels. Molecules 2021, 26, 7502. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Sun, L.; Liu, Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure–activity relationship of its eight flavonoids by a refined assign-score method. Chem. Cent. J. 2018, 12, 82. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed electric field assisted extraction of bioactive compounds from cocoa bean shell and coffee silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Calvo, L.G.; Villarino, R.A.; Rama, J.L.R.; Abril, A.G.; de Miguel, T. A modification of the resazurin cell viability assay, suitable for the quantification of lactic acid producing bacteria. LWT 2025, 215, 117259. [Google Scholar] [CrossRef]
- Cirillo, V.P. Mechanism of glucose transport across the yeast cell membrane. J. Bacteriol. 1962, 84, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Duangjai, A.; Trisat, K.; Saokaew, S. Effect of roasting degree, extraction time, and temperature of coffee beans on anti-hyperglycaemic and anti-hyperlipidaemic activities using ultrasound-assisted extraction. Prev. Nutr. Food Sci. 2021, 26, 338–345. [Google Scholar] [CrossRef]
- Ali, A.; Zahid, H.F.; Cottrell, J.J.; Dunshea, F.R. A comparative study for nutritional and phytochemical profiling of Coffea arabica (C. arabica) from different origins and their antioxidant potential and molecular docking. Molecules 2022, 27, 16. [Google Scholar] [CrossRef]
- Lawag, I.L.; Nolden, E.S.; Schaper, A.A.M.; Lim, L.Y.; Locher, C. A modified Folin-Ciocalteu assay for the determination of total phenolics content in honey. Appl. Sci. 2023, 13, 4. [Google Scholar] [CrossRef]
- Bonitati, J.; Elliott, W.B.; Miles, P.G. Interference by carbohydrate and other substances in the estimation of protein with the Folin-Ciocalteu reagent. Anal. Biochem. 1969, 31, 399–404. [Google Scholar] [CrossRef]
- Shafras, M.; Sabaragamuwa, R.; Suwair, M. Role of dietary antioxidants in diabetes: An overview. Food Chem. Adv. 2024, 4, 100666. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials, and mechanism(s) of action. Front Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 1–9. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Szajewska, H.; Scott, K.P.; de Meij, T.; Forslund-Startceva, S.K.; Knight, R.; Koren, O.; Little, P.; Johnston, B.C.; Łukasik, J.; Suez, J.; et al. Antibiotic-perturbed microbiota and the role of probiotics. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Telagari, M.; Hullatti, K. In-vitro α-amylase and α-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar] [PubMed]
- Lankatillake, C.; Luo, S.; Flavel, M.; Lenon, G.B.; Gill, H.; Huynh, T.; Dias, D.A. Screening natural product extracts for potential enzyme inhibitors: Protocols, and the standardisation of the usage of blanks in α-amylase, α-glucosidase and lipase assays. Plant Methods 2021, 17, 3. [Google Scholar] [CrossRef]
- Leto, D.; Saltiel, A. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef]
- Brown, G.K. Glucose transporters: Structure, function and consequences of deficiency. J. Inherit. Metab. Dis. 2000, 23, 237–246. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Strocchi, G.; Rubiolo, P.; Cordero, C.; Bicchi, C.; Liberto, E. Acrylamide in coffee: What is known and what still needs to be explored. A review. Food Chem. 2022, 393, 133406. [Google Scholar] [CrossRef]
- Mestanza, M.; Mori-Culqui, P.L.; Chavez, S.G. Changes of Polyphenols and Antioxidants of Arabica Coffee Varieties during Roasting. Front. Nutr. 2023, 10, 1078701. [Google Scholar] [CrossRef] [PubMed]
- Mehaya, F.M.; Mohammad, A.A. Thermostability of Bioactive Compounds During Roasting Process of Coffee Beans. Heliyon 2020, 6, e05508. [Google Scholar] [CrossRef]
- Souza Ldos, S.; Horta, I.P.C.; de Souza Rosa, L.; Lima, L.G.; da Rosa, J.S.; Montenegro, J.; da Silva Santos, L.; de Castro, R.B.; Freitas-Silva, O.; Teodoro, A.J. Effect of the roasting levels of Coffea arabica L. extracts on their potential antioxidant capacity and antiproliferative activity in human prostate cancer cells. RSC Adv. 2020, 10, 30115–30126. [Google Scholar] [CrossRef] [PubMed]
- Haile, M.; Kang, W.H. Antioxidant Activity, Total Polyphenol, Flavonoid and Tannin Contents of Fermented Green Coffee Beans with Selected Yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- Gobbi, L.; Maddaloni, L.; Prencipe, S.A.; Vinci, G. Bioactive Compounds in Different Coffee Beverages for Quality and Sustainability Assessment. Beverages 2023, 9, 3. [Google Scholar] [CrossRef]
- Priftis, A.; Stagos, D.; Konstantinopoulos, K.; Tsitsimpikou, C.; Spandidos, D.A.; Tsatsakis, A.M.; Tzatzarakis, M.N.; Kouretas, D. Comparison of antioxidant activity between green and roasted coffee beans using molecular methods. Mol. Med. Rep. 2015, 12, 7293–7302. [Google Scholar] [CrossRef]
- Machado, M.; Ferreira, H.; Oliveira, M.B.P.P.; Alves, R.C. Coffee by-products: An underexplored source of prebiotic ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 7181–7200. [Google Scholar] [CrossRef]
- Piekarska-Radzik, L.; Klewicka, E. Mutual influence of polyphenols and Lactobacillus spp. bacteria in food: A review. Eur. Food Res. Technol. 2021, 247, 9–24. [Google Scholar] [CrossRef]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar] [CrossRef]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic Responses of Lactobacillus plantarum Strains during Fermentation and Storage of Vegetable and Fruit Juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef]
- McCarty, M.F. A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med. Hypotheses 2005, 64, 848–853. [Google Scholar] [CrossRef]
- Arion, W.J.; Canfield, W.K.; Ramos, F.C.; Schindler, P.W.; Burger, H.J.; Hemmerle, H.; Schubert, G.; Below, P.; Herling, A. W Chlorogenic acid and hydroxynitrobenzaldehyde: New inhibitors of hepatic glucose 6-phosphatase. Arch. Biochem. Biophys. 1997, 339, 315–322. [Google Scholar] [CrossRef]
- Anandh Babu, P.V.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef]

| Extract | TPC (GAE µg/mL) | TTC (CE µg/mL) | TFC (RE µg/mL) | AA (TE µg/mL) |
|---|---|---|---|---|
| GCE | 3531 ± 321 | 547 ± 206 | 19,523 ± 1317 | 40,457 ± 298 |
| RCE | 3008 ± 741 | 2391 ± 218 | 29,103 ± 794 | 40,276 ± 333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, L.G.; de Monte Vidal, V.; Díaz-Tomé, V.; Otero Espinar, F.J.; de Miguel, T. In Vitro Screening of Potential Role of Green and Roasted Coffee Extracts in Type 2 Diabetes Management. Beverages 2025, 11, 56. https://doi.org/10.3390/beverages11030056
Calvo LG, de Monte Vidal V, Díaz-Tomé V, Otero Espinar FJ, de Miguel T. In Vitro Screening of Potential Role of Green and Roasted Coffee Extracts in Type 2 Diabetes Management. Beverages. 2025; 11(3):56. https://doi.org/10.3390/beverages11030056
Chicago/Turabian StyleCalvo, Lorena G., Vinicius de Monte Vidal, Victoria Díaz-Tomé, Francisco J. Otero Espinar, and Trinidad de Miguel. 2025. "In Vitro Screening of Potential Role of Green and Roasted Coffee Extracts in Type 2 Diabetes Management" Beverages 11, no. 3: 56. https://doi.org/10.3390/beverages11030056
APA StyleCalvo, L. G., de Monte Vidal, V., Díaz-Tomé, V., Otero Espinar, F. J., & de Miguel, T. (2025). In Vitro Screening of Potential Role of Green and Roasted Coffee Extracts in Type 2 Diabetes Management. Beverages, 11(3), 56. https://doi.org/10.3390/beverages11030056







