Abstract
The phenolic, aromatic and nitrogen composition of a wine determines its organoleptic profile and quality. Elicitors have been used as a tool to stimulate the plant’s defense systems, favoring the synthesis of secondary metabolites. In this pioneering study, the elicitor methyl jasmonate in conventional form (MeJ) and in nanoparticle form (ACP-MeJ), with a concentration ten times lower, was applied in a Tempranillo vineyard over two seasons. The phenolic, nitrogen and volatile composition and the sensory properties of the MeJ-based wines were determined. The results showed that the effects of foliar applications of MeJ modify the wine composition. Thus, although the total concentration of most of the groups of phenolic compounds was not altered, several compounds, such as petunidin-3-glucoside, quercetin-3-glucoside, epigallocatechin and most of the stilbenes, increased, in both years, in the treated wines. Amino acids were influenced differently in each of the years studied, and volatile compounds generally did not improve in the treated wines. However, the ACP-MeJ wines were the best rated by the tasters, highlighting their equilibrium on the taste and their genuineness and odor quality. Therefore, foliar applications of ACP-MeJ can be considered a useful tool to improve wine quality.
1. Introduction
Foliar applications of phytohormones, compounds that regulate plant development, have been effective in reducing the adverse effects of different abiotic stresses on several plant species [1]. Jasmonic acid and its methyl ester, methyl jasmonate (MeJ), play an essential role in the regulation of reactions associated with biotic and abiotic stresses in plants [2] and acts as a signal molecule and inductor of plant secondary metabolites [3,4,5]. These compounds are endogenous messenger molecules that are ubiquitous in a wide range of higher plant species, where their levels are high in the reproductive tissues and flowers, but very low in the mature leaves and roots [6]. MeJ activates the defense mechanisms of plants in response to pathogens, insect wounds and various environmental stresses [7] as well as modulates many crucial processes in plant growth and development, such as vegetative growth, cell cycle regulation, anthocyanin biosynthesis, fruit ripening, nitrogen and phosphorus uptake and glucose transport, among other processes [6,8]. Different studies have shown that MeJ applications in the vineyard induce an improvement or modification of the nitrogen, volatile and phenolic composition of grapes of different varieties and under different climatic conditions. Thus, Garde-Cerdán et al. [9] reported an increase in some amino acids in Tempranillo musts after applying MeJ in the vineyard. Other authors such as Flores et al. [10] showed that the MeJ application as a postharvest treatment enhances anthocyanin accumulation in grapes, and Larrondo et al. [11] reported that the MeJ application is able to stimulate accumulation of stilbene in leaves and berries of grapevines. Portu et al. [3] observed increases of secondary metabolites in Tempranillo grapes after MeJ application in grapevines. Meanwhile, Garde-Cerdán et al. [12] described the influence of MeJ applications to vineyards on grape volatile composition. In their study on Grenache grapes, Marín-San Román et al. [13] observed that MeJ applications in the vineyard increased the content of volatile compounds, mainly favoring terpenoids and C13 norisoprenoids. In addition, the mixed phenylalanine + MeJ treatment favored the increase in terpenoids and benzenoids content in the grapes. However, few authors have studied the effect of foliar applications of MeJ on the nitrogen, phenolic and volatile composition of the wines. Therefore, to the best of our knowledge, only authors such as Ruiz-García et al. [14] and Ruiz-García and Gómez-Plaza [15] in Monastrell, Portu et al. [4] in Tempranillo, and Gil-Muñoz et al. [16] in Monastrell, Merlot and Syrah, reported increases of secondary metabolites in grapes and wines after using a foliar application of MeJ in the vineyard.
Nowadays, nanotechnology is becoming as a promising tool with great potential to release agrochemicals to the crops in a more efficient and safer way [17]. Compared to bulk materials, nanomaterials (size < 100 nm) are generally highly reactive, due to their high surface to volume ratio and their small dimensions [18]. The use of nanoparticles in agriculture could reduce the quantities of chemical products applied in the field, since it has been demonstrated that they minimize product losses, increase product absorption by the plant and inhibit rapid changes in the chemical properties of nutrients [19]. Thus, Parra-Torrejón et al. [20] developed amorphous calcium phosphate nanoparticles (ACP) (mimicking the precursor phase of bone mineral) doped with MeJ, which allows the particles to be retained on the leaf surface for a longer period of time, increasing the efficiency of MeJ action after foliar application, being delivered slowly and gradually over time [21]. This also makes it possible to reduce some of the disadvantages of foliar applications of MeJ, such as its high volatility, low water solubility and its high economic cost [22]. Additionally, Xiong et al. [23] and Epple [24] have shown that ACP used in agriculture as fertilizers is safe as long as bioavailability, movement in soils and human toxicity issues are taken into account. Although, in viticulture, the use of nanoparticles is increasing, especially as an environmentally sustainable fertilizer (for instance, urea-doped nanoparticles such as reported Gaiotti et al. [18] and Pérez-Álvarez et al. [19]) or a winegrowing practice that improves nitrogen plant uptake, increasing the nitrogen quality of the grapes [21], their implications in the composition of wines as a final product in the wine sector are not receiving as much attention. Therefore, the aim of this work was to study, for the first time, the effects of foliar treatments of MeJ, in conventional and nano-size form (with a dose of MeJ ten times lower than the conventional form), on phenolic, aromatic and nitrogen composition of Tempranillo wines over two vintages.
2. Materials and Methods
2.1. Vineyard Site, Grapevine Treatments, Vinification and Samples
The trial was conducted during 2019 and 2020 seasons on an experimental vineyard of Tempranillo (Vitis vinifera L.) cultivar grafted onto R-110 rootstock, located in Finca La Grajera, Logroño, La Rioja (Spain). The vines were trained to a VSP (vertical shoot positioned) trellis system and were planted in 1997 with 2.80 m intra-row × 1.25 m inter-row space. The annual rainfall and mean temperature in 2019 and 2020, were, respectively, 519 and 498 mm and 13.8 °C for both seasons. In the grape-growing period from 1 April to end-September, the rainfall and the mean temperature were 248 and 218 mm, and 18.3 and 18.6 °C, in the 2019 and 2020 seasons, respectively.
The experiment design included 10 vines per replicate of each treatment, and they were arranged in a complete randomized block design, in three randomized blocks, assigned to the following treatments: (i) control, (ii) foliar application of methyl jasmonate (MeJ), and (iii) foliar application of nanoparticles doped with this elicitor (ACP-MeJ). Control plants were sprayed only with a water solution of Tween 80, used as wetting agent (1 mL/L). To carry out the MeJ-based treatments, aqueous solutions were prepared with a MeJ concentration of 10 mM (according to previous works, Garde-Cerdán et al. [9,12]) and 1 mM of ACP-MeJ, according to Pérez-Álvarez et al. [21], using Tween 80. The foliar applications of each of the three treatments were performed twice, at veraison and one week later, applying 200 mL/plant over leaves for each application.
Grapes from all grapevines and treatments were manually harvested at their optimum technological maturity, i.e., when the weight of 100 berries remained constant and the probable alcohol reached 13 (% v/v). At the winery, the grape clusters were destemmed and crushed separately for each treatment and repetition. The resulted pomace was introduced in one 30 L tank for each one to carry out the maceration-fermentation. Therefore, 9 elaborations were carried out (3 treatments × 3 repetitions/treatment). They were protected by the addition of 50 mg SO2/kg of grapes and inoculated (at a dosage of 20 g/hL) with a commercial Saccharomyces cerevisiae strain (Safoeno SC22, Fermentis, Marcq-en-Barœul, France) responsible for carrying out alcoholic fermentation (at 20 +/− 2 °C). Once the alcoholic fermentation was finished (i.e., when sugar concentration was lower than 2.5 g/L), the wines were racked and placed in 12 L tanks. Then, a commercial Oenococcus oeni strain (Viniflora CiNe, CHR Hansen, Hørsholm, Denmark) at 1 g/hL was inoculated into the wines, in order to perform the malolactic fermentation (at 17 +/− 1 °C). For each wine and for each group of compounds studied (amino acids, phenolic compounds and volatile compounds), aliquots samples were frozen and stored at −20 °C until their analysis.
2.2. Determination of Enological Parameters of Wines
The basic enological parameters, alcoholic degree, pH, total acidity and volatile acidity were analyzed using the official methods established by OIV [25]. Malic acid, lactic acid, amino and ammonium nitrogen content, which sum represent the yeast assimilable nitrogen (YAN), and total phenols were determined using a Miura One enzymatic equipment (Tecnología Difusión Ibérica, TDI, Barcelona, Spain). Total anthocyanins content was measured by bleaching using sulfur dioxide [26]. Color intensity (CI) was determined by spectrophotometric absorbance and expressed as the sum of the absorbance at 420, 520 and 620 nm. Total polyphenols index (TPI) was determined by spectrophotometric absorbance at 280 nm after previous dilution of samples.
As the field treatments and the vinifications were performed in triplicate, the results of these parameters are shown as the average of three analyses (n = 3).
2.3. Analysis of Wine Phenolic Compounds by HPLC-DAD
2.3.1. Sample Preparation for the Analysis of Non-Anthocyanin Phenolic Compounds
An amount of 3 mL of each wine sample was diluted with 3 mL of 0.1 N HCl and later was passed through the PCX SPE cartridges (500 mg, 6 mL; Bond Elut Plexa, Agilent, Palo Alto, CA, USA), previously conditioned (5 mL of methanol and 5 mL of water). Then, the cartridges were washed with 5 mL of 0.1 N HCl and 5 mL of water [3]. In order to analyze the non-anthocyanin phenolic compounds (flavonols, flavanols, hydroxybenzoic and hydroxycinnamic acids and stilbenes), the anthocyanin-free fraction was used. The non-anthocyanin phenolic compounds fraction was eluted with 3 × 5 mL of ethanol and dried at 35 °C in a centrifugal evaporator (miVac, Genevac Ltd., Lpswich, Suffolk, UK) and re-solved in 1.5 mL of 20% (v/v) methanol aqueous solution.
2.3.2. Analysis of Phenolic Compounds by HPLC-DAD
Phenolic compounds were analyzed utilizing an Agilent 1260 Infinity II chromatograph (Palo Alto, Santa Clara, CA, USA) equipped with a diode array detector (DAD). According to Portu et al. [3], wine samples were filtered and injected with a flow rate of 0.630 mL/min on a Licrospher® 100 RP-18 reversed-phase column (250 × 4.0 mm; 5 μm packing: Agilent, Santa Clara, CA, USA) with a pre-column Licrospher® 100 RP-18 (4 × 4 mm; 5 μm packing; Agilent, Santa Clara, CA, USA), both thermostated at 40 °C. In order to analyze the anthocyanins, 10 μL of wine sample was injected, using two different eluents: (A) acetonitrile/water/formic acid (3:88:5:8.5, v/v/v) and (B) acetonitrile/water/formic acid (50:41.5:8.5, v/v/v). The gradient used for the anthocyanin separation was: 0 min, 6% B; 15 min, 30% B; 30 min, 50% B; 35 min, 60% B, 38 min, 60% B, 46 min, 6% B. In order to analyze the non-anthocyanin phenolic compounds fraction, 20 μL of sample was injected and three eluents were used: (A) and (B) as for anthocyanins and a third eluent, (C) methanol/water/formic acid (90:1.5:8.5, v/v/v). The gradient used for the non-anthocyanin separation was: 0 min, 4% B and 0% C; 7 min, 4% B and 0% C; 38 min, 17% B and 13% C; 52 min, 30% B and 20% C; 52.5 min, 40% B and 30% C; 57 min, 50% B and 50% C; 58 min, 50% B and 50% C; 65 min, 4% B and 0% C.
The retention times of available pure compounds and the UV-Vis data obtained from authentic standards and/or published in previous studies [27] were used for identifying the phenolic compounds. In order to quantify the compounds, DAD chromatograms were extracted at 520 nm (anthocyanins), 360 nm (flavonols), 320 nm (hydroxycinnamic acids and stilbenes) and 280 nm (gallic acid and flavanols) and the calibration graphs of the respective standards (R2 > 0.99) were used. If no standard was available, quantification was performed according to the calibration of the most similar compound. Therefore, for the quantification of the anthocyanins in the samples, malvidin-3-O-glucoside was used, for flavonols, quercetin-3-O-glucoside was used, for free hydroxycinnamic acids and the corresponding tartaric esters, trans-caftaric acid was used, for procyanidins B1 and B2 the catechin calibration was used, for epigallocatechin the epicatechin was used, and for trans-piceid and trans-resveratrol calibration their respective cis isomers were used. Phenolic compounds’ concentrations in wines were expressed as milligrams per liter of wine (mg/L).
Since field treatments and vinifications were performed in triplicate, the results for phenolic compounds are the average of the analyses of three samples (n = 3).
2.4. Determination of Wine Aromatic Compounds by GC-MS
The determination of the wine volatile compounds was carried out based on Garde-Cerdán et al.’s [28] method. Briefly, 8 mL of each wine sample was centrifuged (3220× g, at 4 °C for 15 min) and placed in a 10 mL tube containing a magnetic stir bar and 10 μL of the internal standard 2-octanol (Sigma-Aldrich, Madrid, Spain). The wine volatile compounds extraction was performed by stirring the sample with 400 μL of dichloromethane (Merck, Darmstadt, Germany) for 15 min. After cooling for 10 min at 0 °C, the organic phase was separated by centrifugation (5031× g, 10 min, 4 °C) and the extract was recovered into a vial.
The analytes determination was carried out using a Gas Chromatograph (GC) with a Mass Detector (MS) (Agilent, Santa Clara, CA, USA) and a VF-Wax 52 CB (60 m × 0.25 mm i.d. × 0.25 μm) capillary column (Agilent, Santa Clara, CA, USA) was used. The volume of injection of each sample was 2 μL and the injector temperature was programmed from 40 °C to 250 °C, at 180 °C/min. The oven temperature was held for 2 min at 50 °C. After that, the oven was programmed to increase at 3 °C/min from 50 °C to 250 °C. The detector was operated at electronic impact mode (70 eV), with an acquisition range (m/z) from 29 to 260. The NIST library and the comparison of results with the mass spectrum of available standards (Sigma-Aldrich) was used to identify the volatile compounds. A semi-quantification was carried out, relating the areas of each volatile compound with the area and the known concentration of 2-octanol, the internal standard. The concentrations of wine aromatic compounds were expressed as milligrams per liter of wine (mg/L).
As the field treatments and vinifications were performed in triplicate, the results of wine volatile compounds are shown as the average of three analyses (n = 3).
2.5. Analysis of Wine Nitrogen Compounds by HPLC-DAD
The analysis of amino acids in wines was performed according to the methodology reported by Garde-Cerdán et al. [29]. Briefly, amino acids were derivatizated in a basic methanolic medium reaction performed in a screw-cap test tube over 30 min in an ultrasound bath (Sonorel Digital 10 P, Bandelin, Berlin, Germany): 1.75 mL of borate buffer 1 M (pH 9), 750 μL of methanol (Merck, Darmstadt, Germany), 1 mL of sample (previously filtered), 20 μL of internal standard (L-2-aminoadipic acid, 1 g/L) (Sigma-Aldrich, Madrid, Spain) and 30 μL of derivatization reagent diethyl ethoxymethylenemalonate (DEEMM) (Sigma-Aldrich, Spain) were mixed. In order to complete degradation of excess DEEMM and reagent by-products, the wine sample was heated at 70–80 °C in a constant temperature heater (Dri-Block DB 3D, Techne, Newcastle upon Tyne, England) for 2 h.
The analyses were performed using a Shimadzu Nexera X2 Ultra High-Performance Liquid Chromatograph (UHPLC) (Shimadzu, Kyoto, Japan) equipped with an automatic liquid sampler and a diode array detector (DAD). An ACE HPLC column (C18-HL) (Aberdeen, Scotland) with particle size 5 μm (250 mm × 4.6 mm) was used in order to perform the chromatographic separation. According to Garde-Cerdán et al. [29], two eluents, previously filtered through a 0.45 μm Durapore ® membrane pore filter (Merck), were used as mobile phases (gradient elution): Phase (A), 25 mM acetate buffer, pH 5.8, with 0.4 g of sodium azide; phase (B), 80:20 (% v/v) mixture of acetonitrile and methanol (Merck). DAD monitoring at 280, 269 and 300 nm was used for detection. The injected volume of derivatized samples was 50 μL. The target compounds aspartic acid, glutamic acid, asparagine, serine, glutamine, histidine, glycine, threonine + citrulline, arginine, α-alanine, γ-aminobutyric acid (Gaba), proline, tyrosine, valine, methionine, cysteine, isoleucine + tryptophan, leucine, phenylalanine, ornithine and lysine were separated, identified and quantified. The identification was performed according to the retention times and the UV–Vis spectral characteristics of their corresponding standards (Sigma-Aldrich) when derivatizated. Quantification was carried out by using the calibration graphs (R2 > 0.98) of the respective standards in 0.1 N HCl, which underwent the same process of derivatization as the samples. The concentrations of amino acids in wine samples were expressed as milligrams per liter (mg/L).
Since the field treatments and vinifications were performed in triplicate, the results of free amino acids correspond to the average of 3 analyses (n = 3).
2.6. Sensory Analysis of the Wines
Approximately 12 months after the completion of malolactic fermentation, the 9 wines of each year were sensorially evaluated by a 12-member panel who were experienced with Appellation D’Origine Contrôlée (A.O.C., Rioja) Rioja wine tasting methodology. For this, the wines were evaluated in a comparative way, using a totally randomized-order blind tasting system. An amount of 50 mL of wine, approximately, was served to each taster in standard tasting glasses, each one with a random three-digit combination code. The wines were kept at a cool temperature until just before they were served to each of the tasters. Each panel member was provided with a specific tasting file comprising the general odor and taste attribute, following the 100-point method approved by the OIV [30]. It has a scale for each evaluated attribute ranging between 40 (insufficient) to 100 (excellent). The tasting file also included a descriptive evaluation of olfactory attributes (raisined, reds, blacks and white fruit, floral, spicy, alcoholic, herbaceous-vegetal, balsamic, underbrush-forest floor, lactic, oxidation and reduction) as well as the gustatory characteristics (sweetness, acidity, bitterness, alcohol, astringency and equilibrium), on an intensity scale of 1 to 6 (1 the lowest and 6 the highest). These descriptors were selected according to the standard attributes from A.O.C. Rioja Tempranillo wines.
Since the treatments and the wines were performed in triplicate, the results of the sensory analysis of the wines correspond to the average of 3 analyses (n = 3).
2.7. Statistical Analysis
The statistical elaboration of the data was performed using SPSS Version 21.0 statistical package for Windows (SPSS, Chicago, IL, USA). General parameters and phenolic, aromatic and nitrogen compounds data were processed using a two-way variance analysis (ANOVA) (p ≤ 0.05). The differences between means were compared using the Duncan test (p ≤ 0.05).
3. Results and Discussion
3.1. Effect of MeJ and ACP-MeJ Foliar Applications on Wine Enological Parameters
General parameters of wines elaborated with grape samples after the applied control, methyl jasmonate (MeJ) and nanoparticles doped with methyl jasmonate (ACP-MeJ) treatments in the vineyard in 2019 and 2020 are shown in Table 1. In 2019, wines from the MeJ and ACP-MeJ groups had lower alcohol content than those from the control treatment. This result could be an advantage of the foliar application of these elicitors as a strategy to reduce the alcohol content of wines, which is strongly demanded by the consumer, and which is increasing due to the climate change. Furthermore, ACP-MeJ reduced the total acidity of the wines with respect to the control wines and MeJ increased the volatile acidity (Table 1). However, all the volatile acidity values were well below 0.6 g/L, which is usually perceived as a spoilage character for wine [31]. The MeJ and ACP-MeJ treatments increased the yeast assimilable nitrogen (YAN) content in wines regarding the control wines. The YAN content is relevant since nitrogen has a key role in the formation of aromatic compounds in wine as well as biogenic amines. Thus, higher amounts of residual nitrogen in wines, together with other factors, increase the risks of microbiological instability and the production of ethyl carbamate and biogenic amines in wines [32]. Regarding the total anthocyanins, wines from the MeJ group had increased content in comparison to the ACP-MeJ wines. The color index (CI) values in wines from ACP-MeJ treatment were reduced with respect to the control wines, with intermediate values for the wines from the MeJ treatment. However, the pH, lactic acid and TPI values did not change in the treated wines with respect to the control wines (Table 1).

Table 1.
Basic enological parameters in wines from control, methyl jasmonate (MeJ) and nanoparticles doped with this elicitor (ACP-MeJ) treatments, in 2019 and 2020 seasons.
In 2020, the effects of the treatments applied in the vineyard on the enological parameters were less than those described for the 2019 samples. Thus, volatile acidity was reduced in the MeJ wines compared to the wines from the other two treatments (control and ACP-MeJ), but lactic acid was higher than in the control wines (Table 1). These seasonal differences are probably because of the differences between the precipitations in both seasons. Thus, the accumulated rainfall in 2019 (519.7 mm) was higher than in 2020 (497.60 mm), as well as the rainfall through the grapevine cycle (April–September), which was higher in 2019 (247.8 mm) vs. 2020 (217.8 mm), whereas the average temperature in both seasons was the same (13.8 °C).
The slight differences observed in the enological parameters of the wines are in agreement with the results obtained by Pérez-Álvarez et al. [21] in cv. Monastrell musts after ACP-MeJ applications.
3.2. Influence of the Foliar MeJ and ACP-MeJ Treatments on Wine Phenolic Compounds
Table 2 and Table 3 show the phenolic composition of wines (mg/L) elaborated from grapes of Tempranillo vines foliarly treated with control, methyl jasmonate (MeJ) and nanoparticles doped with MeJ (ACP-MeJ) treatments, in the 2019 and 2020 seasons.

Table 2.
Anthocyanins content (mg/L) in wines from control, methyl jasmonate (MeJ) and nanoparticles doped with this elicitor (ACP-MeJ) treatments, in 2019 and 2020 seasons.

Table 3.
Flavonols, flavanols, phenolic acids and stilbenes content (mg/L) in wines from control, methyl jasmonate (MeJ) and nanoparticles doped with this elicitor (ACP-MeJ) treatments, in 2019 and 2020 seasons.
In 2019, regarding the non-acylated anthocyanins, only wines from the MeJ treatment increased the peonidin-3-O-glc content in comparison to the control and ACP-MeJ wines. The content of some of the acylated anthocyanins in the wines was affected by the treatments. Thus, the concentration of cyanidin-3-O-aglc, peonidin-3-O-aglc, cyanidin-3-O-cmglc, peonidin-3-O-cmglc and malvidin-3-O-cfglc increased in MeJ wines with respect to the control ones. Wines from the ACP-MeJ treatment increased the malvidin-3-O-cis-cmlg content with respect to the control wines (Table 2). In this first year of the study, neither the total non-acylated anthocyanins nor the acylated anthocyanins and total anthocyanins content of the wines were affected by the application of the elicitors in the vineyard. However, the vitisin A content decreased in wines from the two MeJ treatments in comparison to the content in the control wines (Table 2).
In 2020, the content of non-acylated anthocyanins in the wines was more affected by the treatments than in 2019, although the total non-acylated anthocyanins content did not show a difference in the treated wines compared to the control wines. Delphinidin-3-O-glc, petunidin-3-O-glc and peonidin-3-O-glc content in wines from MeJ treatment were higher than the content of control and ACP-MeJ wines (Table 2). Regarding the acylated anthocyanins, only cyaniding-3-O-acglc content was affected, decreasing in the ACP-MeJ wines with respect to the wines from both the MeJ and control treatments. In 2020, the foliar treatments did not affect the total acylated anthocyanins or total anthocyanins or either of the two vitisins determined in the wines (Table 2). Anthocyanins are the compounds responsible for the color of grapes and red wines. Their synthesis takes place in the skins and the profile or proportion in which each one is found in the grape is specific to each variety [33], which makes it possible to distinguish varieties [34] or even characterize certain wines [35]. The results of the total anthocyanin contents obtained in our study did not match with the increase in the phenolic composition of both grapes and wines, especially the content of total and non-acylated anthocyanins found by other authors after the foliar application of MeJ on plants of Barbera [36], Monastrell [14], Syrah [37], Tempranillo [3,4] and Graciano [5]. Thus, it has been shown that malolactic fermentation of wines, which leads to an increase in pH and changes in chemical composition, influences the ability of anthocyanins to react with other compounds such as pyruvic acid, acetaldehyde, or various copigments such as phenolic acids [38]. In this sense, after applications with MeJ in grapes of Syrah, Fernández-Marín et al. [37] reported significant decreases in the concentration of anthocyanins in the wines once malolactic fermentation was completed with respect to freshly pressed wines.
In 2019, many of the individual flavonols in the wines were affected by foliar treatments as shown in Table 3. Thus, quercetin-3-glcU, quercetin-3-glc and kaempferol-3-galcU + 3-glc content decreased in wines from the MeJ treatment with respect to the control wines, meanwhile, free-myricetin content was the highest in MeJ wines. The ACP-MeJ treatment reduced the content of free-myricetin, free-quercetin, free-kaempferol and free-isoharmenetin + syringetin with respect to the wines of both control and MeJ treatments (Table 3). However, in the wines from 2020, it was observed that the ACP-MeJ treatment increased the content of all flavonols except free-kaempferol, free-laricitrin and free-isoharmenetin + syringetin, in comparison to the control wines. MeJ treatment increased the myricetin-3-glc, quercetin-3-glc and isorhamnetin-3-glc content in the wines compared to these from the control treatment (Table 3). Although the treatments favored the synthesis of some of the flavonols studied in the wines, compared to the control, the total flavonol compounds were not affected in either of the two years of study (Table 3). Similar to the anthocyanins, flavonols are located in the skin of grapes, and are of great importance in the color stability of red wines due to their copigmentation reactions with the anthocyanins [39]. Furthermore, flavonols contribute to the taste sensations of wine, since quercetin derivatives are related to the wine bitterness, while other compounds such as syringetin-3-glc contribute to the wine astringency [40]. Although anthocyanins and flavonols largely share their synthesis pathway, the response to MeJ treatments observed in the wines was diverse.
Thus, after the application of MeJ on a Graciano variety vineyard, Portu et al. [5] also observed in the second year of the study significant increases in flavonols content, both in grapes and wines, while in the first year, they did not observe differences between control and treated samples. Portu et al. [3] reported a significant increase of 40% of flavonols content in Tempranillo wines from MeJ grapes treated in comparison to the control ones, mainly due to the increase in the concentration of quercetin-3-glc, kaempferol-3-glc, isorhamnetin-3-glc and free myricetin content. However, in another study with Tempranillo wines, Portu et al. [4] did not observe differences in flavonols concentration between those from the MeJ treated grapes and the control.
Regarding the flavanols content in wines, in 2019, MeJ treatment increased the epigallocatechin and procyanidin B1 concentration respect to the control wines, but both treatments reduced the procyanidin B2 content compared to the control wines. In the 2020 wines, neither epicatechin-3-gallate nor procyanidin B2 were detected. On the other hand, the MeJ treatment increased the epicatechin content, and both treatments increased the procyanidin B1 content in comparison to the content in the control wines.
Thus, only in the 2020 wines, total flavonol content increased in the MeJ treatment wines compared to the control (Table 3). These results did not match with those reported by other authors such as Portu et al. [3,4,5] after studying wines from grapes that have been treated with MeJ, which did not show differences in flavanols content compared to untreated wines. Flavanols are located in the skin of grapes and also, mainly, in the pips [41]. They are of special relevance in the taste properties of wines [42], being closely related to the astringency of wines as well as their color stability [40].
The only hydroxybenzoic acid determined in the wine samples, gallic acid, decreased its content in the MeJ wines in 2019 but increased in those of 2020, in comparison to the control wines (Table 3). This acid is found in high concentration in grapes and also in wines, especially those aged in oak barrels, since it is released by the hydrolysis of hydrolysable tannins in the wood [43]. In general, the applications with the MeJ-elicitor have not modified the content of this hydroxybenzoic acid in the treated wines, compared to the control as also reported Portu et al. [3,5].
Among the hydroxycinnamic acids analyzed in the wines, in 2019, neither of the two treatments favored their increase in comparison to the control wines. In the 2020 wines, MeJ treatment increased the concentration of trans-caftaric acids, trans + cis-coutaric acids and trans-fertaric acid, whereas ACP-MeJ also increased the trans-fertaric acid content compared to the control wines (Table 3). Total hydroxycinnamic acids in the wines were only increased with respect to the control treatment in 2020 MeJ wines. Hydroxycinnamic compounds, released by hydrolysis during fermentation, can have a great organoleptic impact on the wines, since they react with anthocyanins to form copigments, and thus contribute to the color stability of young wines [39]. Moreover, in aged wines and because of the activity of contaminating yeasts such as Brettanomyces/Dekkera, these compounds are precursors of the ethylphenols, volatile compounds with unpleasant notes in wines [44]. As previously mentioned in the case of the hydroxybenzoic acid content, foliar applications with MeJ carried out by other authors had no effect on the content of hydroxycinnamic acids in wines.
Finally, regarding the stilbenes, in the 2019 wines, both treatments increased the cis-piceid content and the MeJ treatment also increased the trans-resveratrol content in comparison to the control wines (Table 3). For its part, the 2020 wines showed an increase in all stilbenes (except cis-piceid in the case of the MeJ treatment), compared to the control wines. However, this increase was not reflected in the total stilbene content of the wines, which statistically did not differ from the control in either the 2019 or 2020 wines (Table 3). Stilbenes are phytoalexins synthesized by the plant in response to fungal attacks and other situations of biotic and abiotic stress [45]. Their concentration in grapes and wines depends on multiple factors such as the intrinsic properties of grape variety, climate, cultivar management, season and enological procedures [46]. In 2020, trans-resveratrol was the major stilbene in wines, accounting for up to 49% of the total stilbenes content (Table 3). This agrees with the results of other authors, who reported that trans-resveratrol was the major stilbene in wines [45]. However, in wines from 2019, trans-piceid was the majority stilbene comprising around 66% of the total stilbenes, followed by trans-resveratrol (48%) content (Table 3).
Portu et al. [3] observed that the application of the MeJ elicitor produced increases in trans-piceid content in their Tempranillo wines, doubling the content of total stilbenes compared to the control wines. Portu et al. [5] observed significant increases in Graciano wines after applications in the vineyard with MeJ and important trends of increase (between 30 and 13% higher than the control) in the case of Tempranillo wines, compared to the control wines. Authors such as Vezzulli et al. [36], Ruiz García et al. [14] and Fernández-Martín et al. [37] also reported increases of the total stilbenes content in wines after applying MeJ to the Barbera, Monastrell and Syrah grapevines, respectively. However, Portu et al. [4] observed that the differences in stilbenes concentration found in the Tempranillo grapes treated with MeJ were not reflected in the wines. After applying MeJ (at dose of 5 mM and 10 mM) and nanoparticles of MeJ (1 mM) to Monastrell grapevines, Parra-Torrejón et al. [20] observed that all of the treatments increased the trans-resveratrol concentration in wines, while the cis-resveratrol content only increased compared to the control wines when MeJ was applied at 5 mM and as nano-MeJ. Additionally, all the MeJ-based treatments, including the nano-MeJ (with five and ten times lower MeJ concentration than conventional MeJ treatments), increased the cis- and trans-piceid concentration in their Monastrell wines compared to the control ones.
3.3. Effect of the Foliar MeJ and ACP-MeJ Applications on Wine Aromatic Compounds
Figure 1 shows the concentration (mg/L) of esters in the wines made with grapes after the application in the vineyard of control, methyl jasmonate (MeJ) and nanoparticles doped with MeJ (ACP-MeJ) treatments in the 2019 and 2020 seasons. In 2019, acetate esters (isoamyl acetate and 2-phenyletyl acetate) as well as the total acetate esters content were reduced in the treatments compared to the control, especially in ACP-MeJ wines (Figure 1a–c). However, even in the ACP-MeJ treatment wines, which had the lowest concentration of both acetate esters, its content was above the perception thresholds of 0.03 mg/L in the case of isoamyl acetate, with banana aroma, and 0.25 mg/L in the case of 2-phenylethyl acetate, with rose aroma [47]. In 2020, wines from the MeJ treatment had lower content of both acetate esters and the total acetate esters than the control wines, but the wines from ACP-MeJ treatment had intermediate values of 2-phenylethyl acetate (Figure 1a–c).
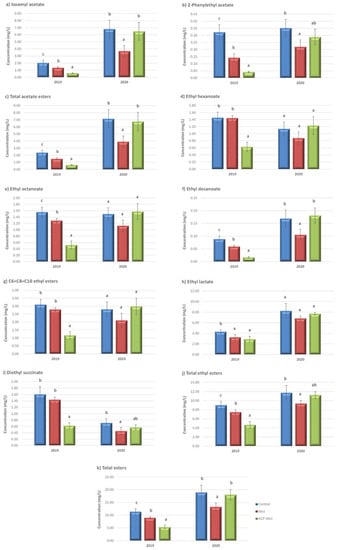
Figure 1.
Esters concentration (mg/L) in control wines and from methyl jasmonate (MeJ) and apatite doped with methyl jasmonate (ACP-MeJ) treatments, in the two seasons (2019 and 2020). All parameters are listed with their standard deviation (n = 3). For each season and compound, different letters indicate significant differences between the samples (p ≤ 0.05).
Regarding the ethyl esters, in 2019 wines, the concentration of ethyl hexanoate, ethyl octanoate, ethyl decanoate, C6 + C8 + C10 ethyl ester, diethyl succinate as well as the total ethyl esters in ACP-MeJ wines was the lowest. Furthermore, the ethyl lactate content was similar in both MeJ and ACP-MeJ wines, but lower than in the control wines (Figure 1d–j). In 2020, there was a tendency to show a lower content of most of the ethyl esters in MeJ wines. However, this reduction was significant, compared to control wines, only for the content of ethyl decanoate, diethyl succinate and total ethyl esters (Figure 1d–j). Among all the ethyl esters found in the wines of all treatments and in both seasons, only ethyl hexanoate (perception threshold = 0.014 mg/L) and ethyl octanoate (0.05 mg/L) contribute with pleasant fruity and floral notes [48]. However, other esters such as ethyl decanoate, ethyl lactate and diethyl succinate would be below its perception threshold (0.2 mg/L, 154 mg/L and 6 mg/L, respectively) [49] in all samples, not contributing directly to the aroma of the wines. The content of total esters in 2019 and 2020 wines reflected what was commented for acetate esters and ethyl esters individually, since, in 2019, the elicitor-treated wines reduced the total esters content compared to the control wines and, in 2020, the MeJ wine was the one with the lowest total esters content compared to the wines from the other two treatments (control and ACP-MeJ) (Figure 1k).
In 2019, the concentration of higher alcohols in the elicitor-treated wines decreased compared to the control wines. In the case of the isoamyl alcohol, 2-phenylethanol, (E)-3-hexenol and total alcohols content, the MeJ wines had intermediate values and ACP-MeJ wines had the lowest concentration (Figure 2a–g). In 2020, the treatments affected the content of higher alcohols in the wines to a lesser extent. Thus, the MeJ treatment reduced the isoamyl alcohol and (E)-3-hexenol content regarding the control wines and n-hexanol and (E)-3-hexenol in comparison to the ACP-MeJ wines (Figure 2a–g). Only in the case of the ACP-MeJ wines for the 2019 season was the concentration of isoamyl alcohol lower than the perception threshold (30 mg/L). This compound contributes with aromatic notes of cheese and alcohol [31]. The 2-phenylethanol concentration was higher in the 2019 wines compared to those from 2020, where there was no difference between treatments although all of the wines were above the threshold. The 2-phenylethanol perception is related with floral notes, rose and honey when its concentration is above the threshold established in wines for this compound (14 mg/L) [49]. The higher alcohols group was the most abundant of the three studied, followed by esters and acids, as found by Garde-Cerdán et al. [28] in their Tempranillo and Tempranillo Blanco wines. The low concentration of compounds of this group observed in all of our wines (less than 300 mg/L), suggests that they contribute to the desirable complexity of the wines [50]. However, if the concentration of these higher alcohols exceeded 400 mg/L, their contribution would negatively influence the aroma of the wines [28].
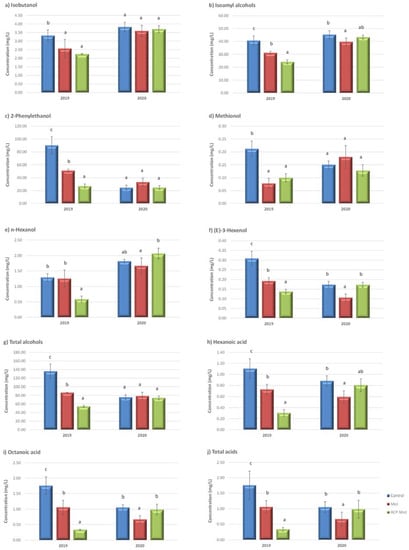
Figure 2.
Alcohols and acids concentration (mg/L) in control wines and from methyl jasmonate (MeJ) and apatite doped with methyl jasmonate (ACP-MeJ) treatments, in the two seasons (2019 and 2020). All parameters are listed with their standard deviation (n = 3). For each season and compound, different letters indicate significant differences between the samples (p ≤ 0.05).
In samples from the 2019 season, elicitor treatments reduced the content of both hexanoic and octanoic acids, as well as total acids content regarding the control wines, with intermediate values for the MeJ treatment samples (Figure 2h–j). In the case of the 2020 samples, MeJ treatment decreased the hexanoic acid content in wines compared to control ones, and also reduced the octanoic acid and total acids content in comparison to the wines of both control and ACP-MeJ treatments (Figure 2h–j). For both acids (hexanoic and octanoic), the concentrations of the 2019 ACP-MeJ wines were found to be below their perception thresholds (0.42 and 0.50 mg/L, respectively) [48]. This may be a positive aspect since these compounds can contribute a fresh flavor to the wines or, conversely, an unpleasant rancid flavor if they are in excess [51].
3.4. Influence of the Foliar MeJ and ACP-MeJ Treatments on Wine Nitrogen Compounds
Table 4 shows the amino acids content of wines from the control, methyl jasmonate (MeJ) and MeJ-doped nanoparticle (ACP-MeJ) treatments from the 2019 and 2020 seasons. In 2019, MeJ treatment increased the content of aspartic acid, asparagine, threonine + citrulline, γ-aminobutyric acid and ornithine in wines compared to control wines. In addition, the wines from this MeJ treatment had the highest concentrations of leucine, phenylalanine and lysine, followed by those from the ACP-MeJ treatment, compared to the control, which had the lowest concentrations. The ACP-MeJ treatment increased the glycine content in comparison to the control wines (Table 4).

Table 4.
Amino acids content (mg/L) in wines from control, methyl jasmonate (MeJ) and nanoparticles doped with this elicitor (ACP-MeJ) treatments, in 2019 and 2020 seasons.
The total amino acids content did not differ between the wines, but the total amino acids without proline content increased in the MeJ treatment wines compared to those from the other two treatments (control and ACP-MeJ). Since proline is not among the amino acids preferred by yeasts as a nitrogen source during the wine fermentation, and it requires the presence of molecular oxygen for its metabolism, it is one of the most released amino acids by the yeast at the end of the alcoholic fermentation. Therefore, proline is the amino acid found in the highest concentration in all wines (Table 4), as also reported by other authors [29].
In 2020, treatments affected the amino acids content of wines to a different extent than in 2019. In general, wines from the control treatment had higher amino acids content than the treated wines, although this was not reflected in the total amino acids content. In addition, the total amino acids content without proline was higher in the control wines than in wines from the two elicitors (Table 4). Thus, MeJ treatment decreased the content of arginine, tyrosine, leucine, phenylalanine, ornithine and lysine in wines compared to the control one. ACP-MeJ reduced the content of aspartic and glutamic acids, alanine γ-aminobutyric acid, tyrosine, valine, methionine, isoleucine + tryptophan and lysine regarding the control wines, whereas increased the proline and ornithine content in comparison to the MeJ treatment (Table 4). In a study with Monastrell grapevines in rainfed and RDI regime, Pérez-Álvarez et al. [21] also did not observe the influence of ACP-MeJ in the total amino acids content of the musts. They suggested that, although the ACP nanoparticle has nitrates in its structure [19], which are a source of nitrogen for the plant, the coverage of the surface by the MeJ (ACP-MeJ) would not allow such an easy release of nitrogen. This would explain the little effect observed on the amino acids content of wines treated with ACP-MeJ compared to those from the MeJ treatment. Our work is pioneering in the study of the effects of these foliar applications of ACP-MeJ on wines composition. Therefore, although it is very likely that the wines are the reflection of the quality of the grapes, more studies are needed to increase knowledge of the influence on the wines’ composition of this kind of plant elicitor, since wine is the product that reaches the consumer.
3.5. Wine Sensory Analysis
The sensorial evaluation (visual, odor and taste properties, as well as the total score given by tasters) of the control, MeJ and ACP-MeJ wines are shown in Table 5. Figure 3 shows a “spider web” diagram for the average scores of a) odor and b) taste attribute intensities of Tempranillo wines obtained from control and treated grapevines with MeJ and ACP-MeJ in the 2019 and 2020 seasons. Wines from the ACP-MeJ treatment were better evaluated by the tasters in their odor characteristics (genuineness and quality) in comparison to the wines from the other treatments (Table 5). In addition, these ACP-MeJ wines obtained the highest total score and harmony from the panelists. On the other hand, the tasters rated the 2019 wines higher than the 2020 wines, highlighting the attributes of odor genuineness and quality, as well as taste intensity (Table 5).

Table 5.
Factor analysis of the sensory evaluation of the wines with the two factors studied: treatment (Control, MeJ, ACP-MeJ) and season (2019 and 2020).
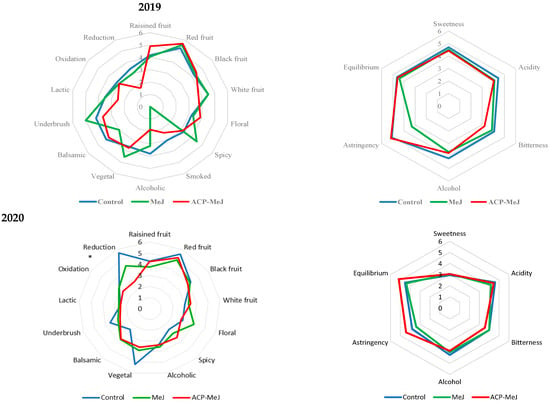
Figure 3.
Polar coordinate (spider web) plot of mean intensity ratings of sensory descriptors (odor and taste attributes) for control wines and from methyl jasmonate (MeJ) and apatite doped with methyl jasmonate (ACP-MeJ) treatments, in the two seasons (2019 and 2020). At the origin, intensity = 0; at the perimeter, intensity = 6. * indicates significant differences between treatments (p ≤ 0.05).
Regarding odor attributes, in 2019 wines (Figure 3), no significant differences were observed between treatments. In 2020 wines, the only significant difference was the greater perception of reduction by the tasters in the control wines. Concerning taste characteristics, in 2019, the control and ACP-MeJ wines were appreciated as more astringent than the MeJ wines. In 2020, the wines that were described as more astringent but at the same time more equilibrate were those from the ACP-MeJ treatment (Figure 3).
4. Conclusions
This is the first time that the effects of foliar applications of the elicitor methyl jasmonate (MeJ) doped in nanoparticles of calcium phosphate apatite (ACP-MeJ) on phenolic, nitrogen and volatile composition and sensory properties of Tempranillo wines have been studied. Thus, foliar applications of control, MeJ and ACP-MeJ were carried out in a Tempranillo vineyard during two seasons, and wines from those grapes were produced and analyzed. Although the vinifications generally homogenize the wines and it would seem that the effect of the elicitor could not be observed in the wines from the treated grapes, certain differences in the wine profiles can be noted in comparison to the control wines, having a positive impact on the taste and color properties, in which phenolic and volatile compounds are mainly involved. Thus, anthocyanins such as peonidin-3-O-glc, flavanols such as free-myricetin and free-quercetin, flavanols such as procyanidin B1, the hydroxybenzoic acid gallic acid, some hydroxycinnamics acids and stilbenes such as cis-piceid and cis-resveratrol increased their content in wines treated in comparison to the control ones, although the differences were greater with the MeJ wines than with the ACP-MeJ wines. The impact of the treatments also influenced the amino acids concentration, many of which were higher in the treated wines in 2019 but higher in 2020 in the control wine. In the case of volatile compounds, few were those that increased in the elicitor-treated wines compared to the control wine; however, the tasters rated all the wines as good, without detracting from the treated wines, even highlighting the ACP-MeJ wines in their overall rating.
In conclusion, applications of elicitors based on methyl jasmonate have an impact on the phenolic, nitrogen and aromatic composition of Tempranillo wines, affecting their quality and sensory perception by consumers.
Author Contributions
Conceptualization: T.G.-C.; methodology, T.G.-C. and E.P.P.-Á.; formal analysis and investigation: P.R.-B., S.M.-S.R., I.S.d.U. and R.M.-P.; funding acquisition and supervision: T.G.-C.; writing—original draft: T.G.-C. and E.P.P.-Á.; writing—review and editing: E.P.P.-Á., I.S.d.U., P.R.-B., S.M.-S.R., R.M.-P., B.P.-T., G.B.R.-R., J.M.D.-L. and T.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been carried out thanks to funding from the Ministerio de Ciencia, Innovación y Universidades through the Projects RTI2018-096549-B-I00 and RTI-2018-095794-A-C22.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
E.P.P.-Á. thanks the Ministerio de Ciencia, Innovación y Universidades for her Juan de la Cierva-Incorporación contract. S.M.-S.R. thanks Gobierno de La Rioja and R.M.-P. thanks INIA for their predoctoral contracts.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jamalian, S.; Truemper, C.; Pawelzik, E. Jasmonic and abscisic acid contribute to metabolism re-adjustment in strawberry leaves under NaCl stress. Int. J. Fruit Sci. 2020, 20 (Suppl. 2), S123–S144. [Google Scholar] [CrossRef]
- Yue, X.; Shi, P.; Tang, Y.; Zhang, H.; Ma, X.; Ju, Y.; Zhang, Z. Effects of methyl jasmonate on the monoterpenes of Muscat Hamburg grapes and wine. J. Sci. Food Agric. 2021, 101, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; Santamaría, P.; López-Alfaro, I.; López, R.; Garde-Cerdán, T. Methyl jasmonate foliar application to Tempranillo vineyard improved grape and wine phenolic content. J. Agric. Food Chem. 2015, 63, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Methyl jasmonate treatment to increase grape and wine phenolic content in Tempranillo and Graciano varieties during two growing seasons. Sci. Hortic. 2018, 240, 378–386. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Meng, Y.; Hu, J.; Ding, M.; Bian, J.; Yan, M.; Han, J.; Zhou, M. Jasmonic acid/ethylene signaling coordinates hydroxycinnamic acid amides biosynthesis through ORA59 transcription factor. Plant J. 2018, 95, 444–457. [Google Scholar] [CrossRef]
- Camposa, M.L.; Kanga, J.-H.; Howea, G.A. Jasmonate-triggered plant immunity. J. Chem. Ecol. 2014, 40, 657–675. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Portu, J.; López, R.; Santamaría, P. Effect of methyl jasmonate application to grapevine leaves on grape amino acid content. Food Chem. 2016, 203, 536–539. [Google Scholar] [CrossRef]
- Flores, G.; Blanch, G.P.; del Castillo, M.L.R. Postharvest treatment with (-) and (+)-methyl jasmonate stimulates anthocyanin accumulation in grapes. LWT-Food Sci. Technol. 2015, 62, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Larrondo, F.; Gaudillère, J.P.; Krisa, S.; Decendi, A.; Deffieux, G.; Mérillon, J.M. Airborne methyl jasmonate induces stilbene accumulation in leaves and berries of grapevine plants. Am. J. Enol. Vitic. 2003, 54, 63–66. [Google Scholar]
- Garde-Cerdán, T.; Gutiérrez-Gamboa, G.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Influence of methyl jasmonate foliar application to vineyard on grape volatile composition over three consecutive vintages. Food Res. Int. 2018, 112, 274–283. [Google Scholar] [CrossRef]
- Marín-San Román, S.; Garde-Cerdán, T.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Foliar application of phenylalanine plus methyl jasmonate as a tool to improve Grenache grape aromatic composition. Sci. Hortic. 2020, 272, 109515. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving grape phenolic content and wine chromatic characteristics through the use of two different elicitors: Methyl jasmonate versus benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A tool for improving fruit phenolic content. Agriculture 2013, 3, 33–52. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Improving phenolic and chromatic characteristics of Monastrell, Merlot and Syrah wines by using methyl jasmonate and benzothiadiazole. J. Int. Sci. Vigne Vin 2017, 51, 17–27. [Google Scholar]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Gaiotti, F.; Lucchetta, M.; Rodegher, G.; Lorenzoni, D.; Longo, E.; Boselli, E.; Cesco, S.; Belfiore, N.; Lovat, L.; Delgado-López, J.M.; et al. Urea-doped calcium phosphate nanoparticles as sustainable nitrogen nanofertilizers for viticulture: Implications on yield and quality of Pinot Gris grapevines. Agronomy 2021, 11, 1026. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Ramírez-Rodríguez, G.B.; Carmona, F.J.; Martínez-Vidaurre, J.M.; Masciocchi, N.; Guagliardi, A.; Garde-Cerdán, T.; Delgado-López, J.M. Towards a more sustainable viticulture: Foliar application of N-doped calcium phosphate nanoparticles on Tempranillo grapes. J. Sci. Food Agric. 2021, 101, 1307–1313. [Google Scholar] [CrossRef]
- Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Gil-Muñoz, R.; Delgado-López, J.M. Nanoelicitors with prolonged retention and sustained release to produce beneficial compounds in wines. Environ. Sci. Nano 2021, 8, 3524–3535. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Rubio-Bretón, P.; Intrigliolo, D.S.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Garde-Cerdán, T. Year, watering regime and foliar methyl jasmonate doped nanoparticles treatments: Effects on must nitrogen compounds in Monastrell grapes. Sci. Hortic. 2022, 297, 110944. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. Effect of methyl jasmonate doped nanoparticles on nitrogen composition of Monastrell grapes and wines. Biomolecules 2021, 11, 1631. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, P.; Hunter, M.N.; Kopittke, P.M. Bioavailability and movement of hydroxyapatite nanoparticles (HA-NPs) applied as a phosphorus fertiliser in soils. Environ. Sci. Nano 2018, 5, 2888–2898. [Google Scholar] [CrossRef]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of Internationals Methods of Wine and Must Analysis; OIV: Paris, France, 2016. [Google Scholar]
- Ribéreau-Gayon, P.; Stonestreet, E. Determination of anthocyanins in red wine. [Le dosage des anthocyanes dans le vin rouge]. Bull. Soc. Chim. Fr. 1965, 9, 2649–2652. [Google Scholar]
- Castillo-Muñoz, N.; Fernández-González, M.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Red-color related phenolic composition of Garnacha Tintorera (Vitis vinifera L.) grapes and red wines. J. Agric. Food Chem. 2009, 57, 7883–7891. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Rubio-Bretón, P.; Marín-San Román, S.; Baroja, E.; Sáenz de Urturi, I.; Pérez-Álvarez, E.P. Study of wine volatile composition of Tempranillo versus Tempranillo blanco, a new white grape variety. Beverages 2021, 7, 72. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Martínez-Gil, A.M.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Salinas, M.R. Implications of nitrogen compounds during alcoholic fermentation from some grape varieties at different maturation stages and cultivation systems. Food Chem. 2011, 124, 106–116. [Google Scholar] [CrossRef]
- OIV. Resolution OIV/Concours 332A/2009. OIV Standard for International Wine and Spirituous Beverages of Vitivinicultural Origin Competitions. Annex 3.1. Score Sheet. 2009. Available online: https://www.oiv.int/public/medias/4661/oiv-concours-332a-2009-en.pdf (accessed on 20 July 2022).
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Seaweed foliar applications at two dosages to Tempranillo blanco (Vitis vinifera L.) grapevines in two seasons: Effects on grape and wine volatile composition. Food Res. Int. 2020, 130, 108918. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Garde-Cerdán, T.; Cabrita, M.J.; García-Escudero, E.; Peregrina, F. Influence on wine biogenic amine composition of modifications to soil N availability and grapevine N by cover crops. J. Sci. Food Agric. 2017, 97, 4800–4806. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Vila-López, R.; Martinez-Cutillas, A. Anthocyanin profile in Monastrell grapes in six different areas from Denomination of Origen Jumilla during ripening stage. Int. J. Food Sci. Technol. 2010, 45, 1870–1877. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; López-Roca, J.M.; Ros-García, J.M.; Gómez-Plaza, E. Anthocyanin fingerprint of grapes: Environmental and genetic variations. J. Sci. Food Agric. 2006, 86, 1460–1467. [Google Scholar] [CrossRef]
- Kelebek, H.; Canbas, A.; Selli, S. HPLC-DAD-MS analysis of anthocyanins in rose wine made from cv. Okuzgiozu grapes, and effect of maceration time on anthocyanin content. Chromatographia 2007, 66, 207–212. [Google Scholar] [CrossRef]
- Vezzulli, S.; Civardi, S.; Ferrari, F.; Bavaresco, L. Methyl jasmonate treatment as a trigger of resveratrol synthesis in cultivated grapevine. Am. J. Enol. Vitic. 2007, 58, 530–533. [Google Scholar]
- Fernández-Marín, M.I.; Puertas, B.; Guerrero, R.F.; García-Parrilla, M.C.; Cantos-Villar, E. Preharvest methyl jasmonate and postharvest UVC treatments: Increasing stilbenes in wine. J. Food Sci. 2014, 79, C310–C317. [Google Scholar] [CrossRef]
- Burns, T.R.; Osborne, J.P. Impact of malolactic fermentation on the color and color stability of Pinot noir and Merlot Wine. Am. J. Enol. Vitic. 2013, 64, 370–377. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Cassasa, L.F.; Harbertson, J.F. Extraction, evolution and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Souquet, J.M.; Cheynier, V.; Brossaud, F.; Moutounet, M. Polymeric proanthocyanidins from grape skins. Phytochemistry 1996, 43, 509–512. [Google Scholar] [CrossRef]
- Gonzalo-Diago, A.; Dizy, M.; Fernández-Zurbano, P. Contribution of low molecular weight phenols to bitter taste and mouthfell properties in red wines. Food Chem. 2014, 154, 187–198. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols- A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Suárez, R.; Suárez-Lepe, J.A.; Morata, A.; Calderón, F. The production of ethylphenols in wine by yeast of the genera Brettanomyces and Dekkera: A review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Bavaresco, L.; Fregoni, C.; Van Zeller De Macedo Basto Goçalves, M.I.; Vezzulli, S. Physiology and molecular biology of grapevine stilbenes: An update. In Grapevine Molecular Physiology and Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer Science+Business Media, B.V.: Dordrecht, The Netherlands, 2009; pp. 341–364. [Google Scholar]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Crespo-Villegas, O.; Garde-Cerdán, T. Elicitors used as a tool to increase stilbenes in grapes and wines. Food Res. Int. 2017, 98, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Jarauta, I.; Salinas, M.R.; Ancín-Azpilicueta, C. Comparative study of the volatile composition in wines obtained from traditional vinification and from the Ganimede method. J. Sci. Food Agric. 2008, 88, 1777–1785. [Google Scholar] [CrossRef]
- Rapp, A.; Mandery, H. Wine aroma. Experientia 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; G-Alegría, E.; Polo, M.C.; Tenorio, C.; Martín-Álvarez, P.J.; Calvo De La Banda, M.T.; Ruiz-Larrea, F.; Moreno-Arribas, M.V. Wine volatile and amino acid composition after malolactic fermentation: Effect of Oenococcus oeni and Lactobacillus plantarum starter cultures. J. Agric. Food Chem. 2005, 53, 8729–8735. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).