Modeling the Thermal Inactivation of Ascospores from Heat-Resistant Molds in Pineapple Juice and Evaluating Disinfection Efficiency of Sodium Hypochlorite and Chlorine Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Production of Ascospores
2.3. Preparation of Pineapple Juice
2.4. Thermal Inactivation Study
2.5. Mathematical Modeling for Thermal Inactivation
2.6. Sanitizer Treatment
2.7. Statistical Analysis
3. Results and Discussion
3.1. Inactivation Kinetics of Heat-Resistant Mold Ascospores in Pineapple Juice
3.1.1. Log-Linear Model
3.1.2. Weibull Model
3.1.3. Comparison of Thermal Inactivation Models
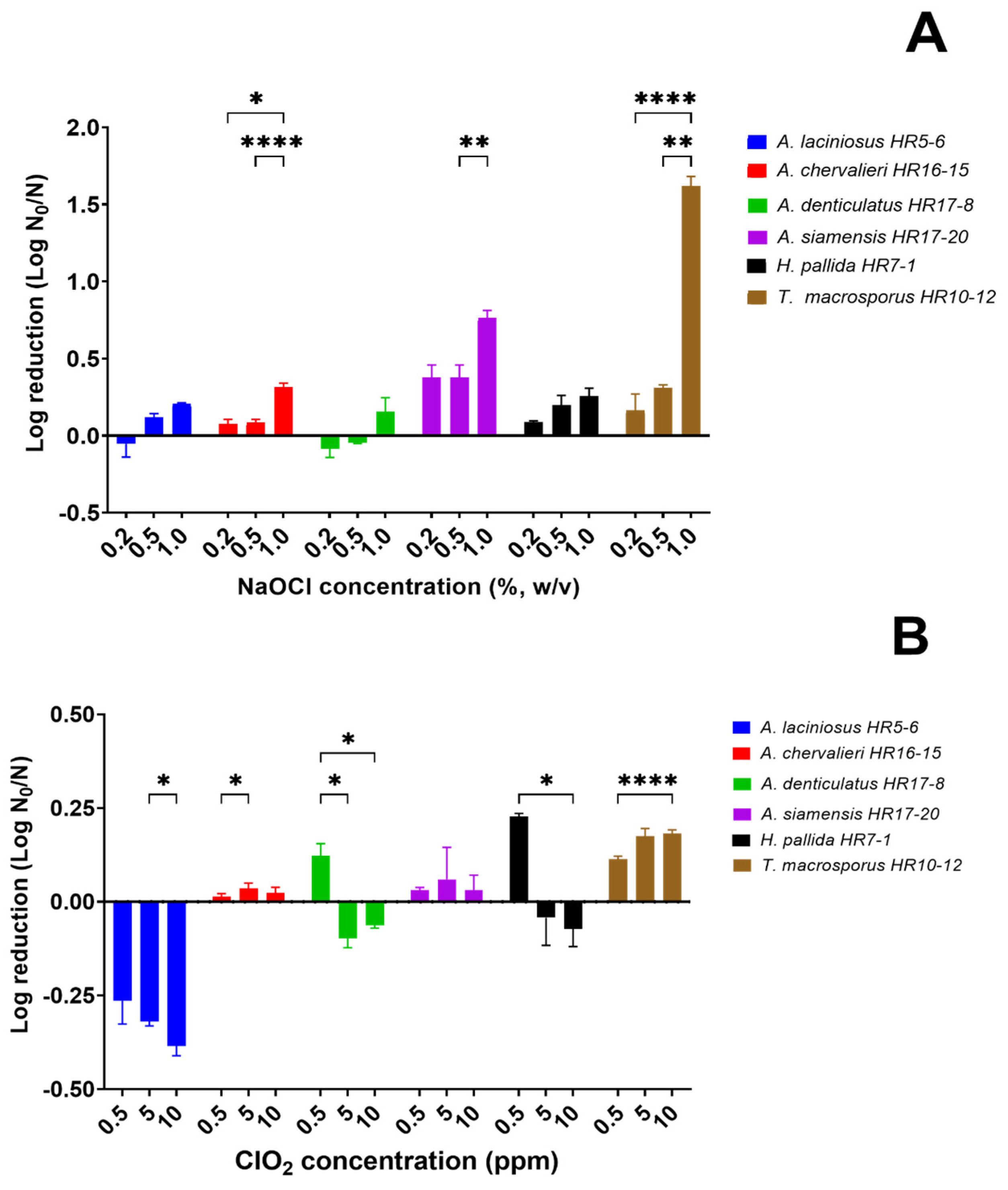
3.2. Inactivation of Ascospores by Chlorine-Based Sanitizers
3.2.1. Efficacy of Sodium Hypochlorite
3.2.2. Efficacy of Chlorine Dioxide
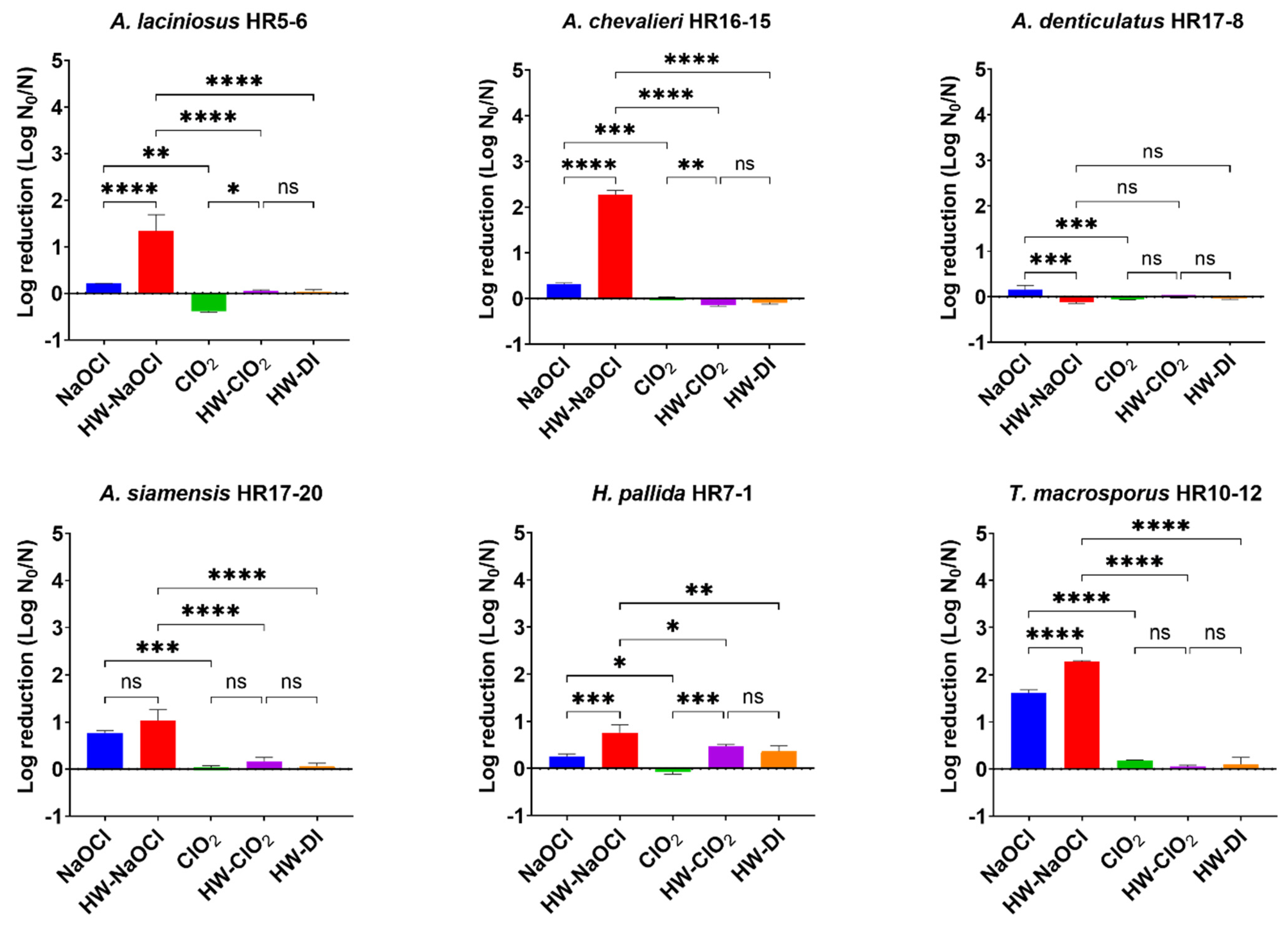
3.2.3. Effect of Hot Water Pretreatment on the Efficacy of Chlorine-Based Sanitizers
3.2.4. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houbraken, J.; Samson, R.A. Phylogeny of Penicillium and the Segregation of Trichocomaceae into Three Families. Stud. Mycol. 2011, 70, 1–51. [Google Scholar] [CrossRef]
- Houbraken, J.; Samson, R.A.; Frisvad, J.C. Byssochlamys: Significance of Heat Resistance and Mycotoxin Production. Adv. Exp. Med. Biol. 2006, 571, 211–224. [Google Scholar]
- Aydin, A.; Ulusoy, B.H.; Ergün, Ö. A Survey on Heat-Resistant Moulds in Heat Treated Milk, Milk Products and Fruit Juices. Arch. Leb. 2005, 56, 58–60. [Google Scholar]
- de Cássia Martins Salomão, B.; Muller, C.; do Amparo, H.C.; de Aragão, G.M.F. Survey of Molds, Yeast and Alicyclobacillus spp. From a Concentrated Apple Juice Productive Process. Braz. J. Microbiol. 2014, 45, 49–58. [Google Scholar] [CrossRef]
- Kocakaya Yildiz, A.; Çoksöyler, N. Heat-Resistance Characteristics of Ascospores of Eurotium chevalieri Isolated from Apricot Juice. Nahrung 2002, 46, 28–30. [Google Scholar] [CrossRef]
- Scaramuzza, N.; Berni, E. Heat-Resistance of Hamigera avellanea and Thermoascus crustaceus Isolated from Pasteurized Acid Products. Int. J. Food Microbiol. 2014, 168–169, 63–68. [Google Scholar] [CrossRef]
- Samson, R.; Houbraken, J.; Varga, J.; Frisvad, J. Polyphasic Taxonomy of the Heat Resistant Ascomycete Genus Byssochlamys and Its Paecilomyces Anamorphs. Persoonia 2009, 22, 14–27. [Google Scholar] [CrossRef]
- Rico-Munoz, E. Heat Resistant Molds in Foods and Beverages: Recent Advances on Assessment and Prevention. Curr. Opin. Food Sci. 2017, 17, 75–83. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. Inactivation of Byssochlamys nivea Ascospores in Strawberry Puree by High Pressure, Power Ultrasound and Thermal Processing. Int. J. Food Microbiol. 2015, 214, 129–136. [Google Scholar] [CrossRef]
- Souza, P.B.; Poltronieri, K.F.; Alvarenga, V.O.; Granato, D.; Rodriguez, A.D.; Sant’ana, A.S.; Peña, W.E. Modeling of Byssochamys nivea and Neosartorya fischeri Inactivation in Papaya and Pineapple Juices as a Function of Temperature and Soluble Solids Content. LWT-Food Sci. Technol. 2017, 82, 90–95. [Google Scholar] [CrossRef]
- Panagou, E.; Tassou, C.; Manitsa, C.; Mallidis, C. Modelling the Effect of High Pressure on the Inactivation Kinetics of a Pressure-Resistant Strain of Pediococcus damnosus in Phosphate Buffer and Gilt-Head Seabream (Sparus aurata). J. Appl. Microbiol. 2007, 102, 1499–1507. [Google Scholar] [CrossRef]
- Copetti, M.V. Sanitizers for Controlling Fungal Spoilage in Some Food Industries. Curr. Opin. Food Sci. 2023, 52, 101072. [Google Scholar] [CrossRef]
- Frąc, M.; Jezierska-Tys, S.; Yaguchi, T. Occurrence, detection, and molecular and metabolic characterization of heat-resistant fungi in soils and plants and their risk to human health. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 161–204. [Google Scholar]
- Wyatt, T.T.; Van Leeuwen, M.R.; Golovina, E.A.; Hoekstra, F.A.; Kuenstner, E.J.; Palumbo, E.A.; Snyder, N.L.; Visagie, C.; Verkennis, A.; Hallsworth, J.E.; et al. Functionality and Prevalence of Trehalose-Based Oligosaccharides as Novel Compatible Solutes in Ascospores of Neosartorya fischeri (Aspergillus fischeri) and Other Fungi. Environ. Microbiol. 2015, 17, 395–411. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; Meijer, M.; van Doorn, T.; Samson, R.; Rico-Munoz, E. Inactivation of Stress-Resistant Ascospores of Eurotiales by Industrial Sanitizers. Int. J. Food Microbiol. 2018, 285, 27–33. [Google Scholar] [CrossRef]
- Stefanello, A.; Magrini, L.N.; Lemos, J.G.; Garcia, M.V.; Bernardi, A.O.; Cichoski, A.J.; Copetti, M.V. Comparison of Electrolized Water And multiple Chemical Sanitizer Action against Heat-Resistant Molds (HRM). Int. J. Food Microbiol. 2020, 335, 108856. [Google Scholar] [CrossRef]
- Maneeboon, T.; Sangchote, S.; Hongprayoon, R.; Chuaysrinule, C.; Mahakarnchanakul, W. Occurrence of Heat-Resistant Mold Ascospores in Pineapple and Sugarcane Field Soils in Thailand. Int. J. Microbiol. 2023, 2023, 8347560. [Google Scholar] [CrossRef]
- Rico-Munoz, E.; Houbraken, J.; Samson, R.A. Detection and enumeration of heat-resistant molds. In Compendium of Methods for the Microbiological Examination of Foods; Salfinger, Y., Tortorello, M.L., Eds.; American Public Health Association: Washington, DC, USA, 2015; pp. 387–397. [Google Scholar]
- Peterson, S.W.; Jurjevic, Z.; Bills, G.F.; Stchigel, A.M.; Guarro, J.; Vega, F.E. Genus Hamigera, Six New Species and Multilocus DNA Sequence Based Phylogeny. Mycologia 2010, 102, 847–864. [Google Scholar] [CrossRef]
- Santos, J.L.; Samapundo, S.; Gülay, S.M.; Van Impe, J.; Sant’Ana, A.S.; Devlieghere, F. Inter- and Intra-Species Variability in Heat Resistance and the Effect of Heat Treatment Intensity on Subsequent Growth of Byssochlamys Fulva and Byssochlamys Nivea. Int. J. Food Microbiol. 2018, 279, 80–87. [Google Scholar] [CrossRef]
- Sant’Ana, A.; Rosenthal, A.; Massaguer, P.R. Heat Resistance and the Effects of Continuous Pasteurization on the Inactivation of Byssochlamys fulva Ascospores in Clarified Apple Juice. J. Appl. Microbiol. 2009, 107, 197–209. [Google Scholar] [CrossRef]
- Geeraerd, A.; Valdramidis, V.; Van Impe, J. Ginafit, a Freeware Tool to Assess Non-Log-Linear Microbial Survivor Curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef]
- Bigelow, W.D.; Esty, J.R. The Thermal Death Point in Relation to Time of Typical Thermophilic Organisms. J. Infect. Dis. 1920, 27, 602–617. [Google Scholar] [CrossRef]
- Colás-Medà, P.; Nicolau-Lapeña, I.; Viñas, I.; Neggazi, I.; Alegre, I. Bacterial Spore Inactivation in Orange Juice and Orange Peel by Ultraviolet-C Light. Foods 2021, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguérinel, I. On Calculating Sterility in Thermal Preservation Methods: Application of the Weibull Frequency Distribution Model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Pinto, G.; Taylor-Roseman, R.; Cogan, K.; D’Alesandre, G.; Kovac, J. Evaluation of the Thermal Inactivation of a Salmonella Serotype Oranienburg Strain During Cocoa Roasting at Conditions Relevant to the Fine Chocolate Industry. Front. Microbiol. 2021, 12, 576337. [Google Scholar] [CrossRef]
- European Union. Bs-En 13697: 2001: Quantitative Non-Porous Surface Test for the Evaluation of Bactericidal and/or Fungicidal Activity of Chemical Disinfectants Used in Food, Industrial, Domestic and Institutional Areas. Test Method and Requirements without Mechanical Action; European Committee for Standardization: Brussels, Belgium, 2001. [Google Scholar]
- Bernardi, A.O.; Garcia, M.V.; Copetti, M.V. Food Industry Spoilage Fungi Control through Facility Sanitization. Curr. Opin. Food Sci. 2019, 29, 28–34. [Google Scholar] [CrossRef]
- Ruiz, V.; Alonso, R.; Salvador, M.; Condón, S.; Condón-Abanto, S. Impact of Shoulders on the Calculus of Heat Sterilization Treatments with Different Bacterial Spores. Food Microbiol. 2021, 94, 103663. [Google Scholar] [CrossRef]
- Pohůnek, V.; Adamcova, M.; Kulišanová, I.; Šístková, I.; Rohlik, B.A.; Ševčík, R. Thermal Inactivation of Aspergillus lacinosus Ascospores as a Function of Temperature and Soluble Solids Content in Fruit Jam. J. Food Nutr. Res. 2019, 58, 275–282. [Google Scholar]
- Dijksterhuis, J.; Wyatt, T.; Hanssen, M.; Golovina, E.; Hoekstra, F.; Lugones, L. Abundant Small Protein Icarus inside the Cell Wall of Stress-Resistant Ascospores of Talaromyces Macrosporus Suggests a Novel Mechanism of Constitutive Dormancy. J. Fungi 2021, 7, 216. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; van Driel, K.G.; Sanders, M.G.; Molenaar, D.; Houbraken, J.A.; Samson, R.A.; Kets, E.P. Trehalose Degradation and Glucose Efflux Precede Cell Ejection During Germination of Heat-Resistant Ascospores of Talaromyces macrosporus. Arch. Microbiol. 2002, 178, 1–7. [Google Scholar] [CrossRef]
- Kikoku, Y. Heat Activation Characteristics of Talaromyces Ascospores. J. Food Sci. 2003, 68, 2331–2335. [Google Scholar] [CrossRef]
- King, A.D.; Michener, H.D.; A Ito, K. Control of Byssochlamys and Related Heat-Resistant Fungi in Grape Products. Appl. Microbiol. 1969, 18, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Evelyn, E.; Muria, S.R.; Chairul, C.; Fozla, D.; Khoirunnisa, F.K. Thermal Inactivation of Talaromyces flavus Ascospores in Pineapple Juice as Influenced by Temperature, Soluble Solids, and Spore Age. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 69, 111–119. [Google Scholar] [CrossRef]
- Kikoku, Y.; Tagashira, N.; Nakano, H. Heat Resistance of Fungi Isolated from Frozen Blueberries. J. Food Prot. 2008, 71, 2030–2035. [Google Scholar] [CrossRef] [PubMed]
- van Boekel, M.A.J.S. On the Use of the Weibull Model to Describe Thermal Inactivation of Microbial Vegetative Cells. Int. J. Food Microbiol. 2002, 74, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Couvert, O.; Gaillard, S.; Savy, N.; Mafart, P.; Leguérinel, I. Survival Curves of Heated Bacterial Spores: Effect of Environmental Factors on Weibull Parameters. Int. J. Food Microbiol. 2005, 101, 73–81. [Google Scholar] [CrossRef]
- Chuaysrinule, C.; Mahakarnchanakul, W.; Maneeboon, T. Comparative Study on the Effect of Temperature and Water Activity on Aspergillus flavus and Aspergillus carbonarius Isolates Growth and Mycotoxin Production on a Chili Powder Medium. Cogent Food Agric. 2020, 6, 1782097. [Google Scholar] [CrossRef]
- Racchi, I.; Scaramuzza, N.; Hidalgo, A.; Cigarini, M.; Berni, E. Sterilization of Food Packaging by Uv-C Irradiation: Is Aspergillus brasiliensis Atcc 16404 the Best Target Microorganism for Industrial Bio-Validations? Int. J. Food Microbiol. 2021, 357, 109383. [Google Scholar] [CrossRef]
- Visconti, V.; Coton, E.; Rigalma, K.; Dantigny, P. Effects of Disinfectants on Inactivation of Mold Spores Relevant to the Food Industry: A Review. Fungal Biol. Rev. 2021, 38, 44–66. [Google Scholar] [CrossRef]
- Cheshchevik, V.T.; Krylova, N.G.; Cheshchevik, N.G.; Lapshina, E.A.; Semenkova, G.N.; Zavodnik, I.B. Role of Mitochondrial Calcium in Hypochlorite Induced Oxidative Damage of Cells. Biochimie 2021, 184, 104–115. [Google Scholar] [CrossRef]
- Wan, Q.; Xia, Y.; Li, Y.; Wu, G.; Wang, J.; Huang, T.; Wen, G. Enhanced Solar Inactivation of Fungal Spores by Addition of Low-Dose Chlorine: Efficiency and Mechanism. Water Res. 2022, 222, 118964. [Google Scholar] [CrossRef]
- Bernardi, A.O.; Stefanello, A.; Lemos, J.G.; Garcia, M.V.; Copetti, M.V. Antifungal Activity of Commercial Sanitizers against Strains of Penicillium roqueforti, Penicillium paneum, Hyphopichia burtonii and Aspergillus pseudoglaucus: Bakery Spoilage Fungi. Food Microbiol. 2019, 83, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.H.; Karabucak, B.; Lee, S.M. Effect of Sodium Hypochlorite on Conventional and Heat-Treated Nickel-Titanium Endodontic Rotary Instruments—An In vitro Study. J. Dent. Sci. 2021, 16, 738–743. [Google Scholar]
- López-Gálvez, F.; Allende, A.; Truchado, P.; Martínez-Sánchez, A.; Tudela, J.A.; Selma, M.V.; Gil, M.I. Suitability of Aqueous Chlorine Dioxide Versus Sodium Hypochlorite as an Effective Sanitizer for Preserving Quality of Fresh-Cut Lettuce While Avoiding by-Product Formation. Postharvest Biol. Technol. 2010, 55, 53–60. [Google Scholar] [CrossRef]
- Ran, Y.; Qingmin, C.; Maorun, F. Chlorine Dioxide Generation Method and Its Action Mechanism for Removing Harmful Substances and Maintaining Quality Attributes of Agricultural Products. Food Bioprocess Technol. 2019, 12, 1110–1122. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Z.; Yu, G. Fungicidal Mechanism of Chlorine Dioxide on Saccharomyces cerevisiae. Ann. Microbiol. 2013, 63, 495–502. [Google Scholar] [CrossRef]
- Insignares-Carrione, E.; Gómez, B.B.; Kalcker, A.L. Chlorine Dioxide in COVID-19: Hypothesis About the Possible Mechanism of Molecular Action in SARS-CoV-2. Mol. Genet. Med. 2020, 14, 468. [Google Scholar]
- Wen, G.; Xu, X.; Huang, T.; Zhu, H.; Ma, J. Inactivation of Three Genera of Dominant Fungal Spores in Groundwater Using Chlorine Dioxide: Effectiveness, Influencing Factors, and Mechanisms. Water Res. 2017, 125, 132–140. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, W.; Du, Y.; Chen, Q.; Su, Z.; Fu, M. Chlorine Dioxide Controls Green Mold Caused by Penicillium digitatum in Citrus Fruits and the Mechanism Involved. J. Agric. Food Chem. 2020, 68, 13897–13905. [Google Scholar] [CrossRef]
- Bundgaard-Nielsen, K.; Nielsen, P.V. Fungicidal Effect of 15 Disinfectants against 25 Fungal Contaminants Commonly Found in Bread and Cheese Manufacturing. J. Food Prot. 1996, 59, 268–275. [Google Scholar] [CrossRef]
- Pandit, A.; Maheshwari, R. Life-History of Neurospora intermedia in a Sugar Cane Field. J. Biosci. 1996, 21, 57–79. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; Teunissen, P. Dormant Ascospores of Talaromyces macrosporus Are Activated to Germinate after Treatment with Ultra High Pressure. J. Appl. Microbiol. 2004, 96, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Reyns, K.M.; Veraverbeke, E.A.; Michiels, C.W. Activation and Inactivation of Talaromyces macrosporus Ascospores by High Hydrostatic Pressure. J. Food Prot. 2003, 66, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Kikoku, Y.; Tagashira, N.; Gabriel, A.A.; Nakano, H. Heat Activation of Neosartorya and Talaromyces Ascospores and Enhancement by Organic Acids. Biocontrol Sci. 2009, 14, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Løvdal, I.S.; Hovda, M.B.; Granum, P.E.; Rosnes, J.T. Promoting Bacillus cereus Spore Germination for Subsequent Inactivation by Mild Heat Treatment. J. Food Prot. 2011, 74, 2079–2089. [Google Scholar] [CrossRef]
- Rodriguez-Palacios, A.; LeJeune, J.T. Moist-Heat Resistance, Spore Aging, and Superdormancy in Clostridium difficile. Appl. Environ. Microbiol. 2011, 77, 3085–3091. [Google Scholar] [CrossRef]
- Holah, J.; Lavaud, A.; Peters, W.; Dye, K.A. Future Techniques for Disinfectant Efficacy Testing. Int. Biodeterior. Biodegrad. 1998, 41, 273–279. [Google Scholar] [CrossRef]
- Masuku, S.M.; Babu, D.; Martin, E.M.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C. Lethality of Moist Heat and Silver Dihydrogen Citrate Sanitizer Combinations on Listeria spp. Adhered to Components of a Deli Meat Slicer. Food Control 2014, 44, 227–232. [Google Scholar] [CrossRef]
- Taormina, P.J.; Dorsa, W.J. Evaluation of Hot-Water and Sanitizer Dip Treatments of Knives Contaminated with Bacteria and Meat Residue. J. Food Prot. 2007, 70, 648–654. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Gibbs, P.A.; Nunez, H.; Almonacid, S.; Simpson, R. Thermal Process|Pasteurization. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 577–595. [Google Scholar]
- Mertz, A.W.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C.; Morawicki, R. The Elimination of Listeria monocytogenes Attached to Stainless Steel or Aluminum Using Multiple Hurdles. J. Food Sci. 2015, 80, M1557–M1562. [Google Scholar] [CrossRef]
- Cai, S.; Snyder, A.B. Thermoresistance in Black Yeasts Is Associated with Halosensitivity and High Pressure Processing Tolerance but Not with Uv Tolerance or Sanitizer Tolerance. J. Food Prot. 2022, 85, 203–212. [Google Scholar] [CrossRef]


| Source | Species | Isolate | GenBank Accession Number |
|---|---|---|---|
| Pineapple field soils, Thailand | A. laciniosus | HR5-6 | OP480885 |
| H. pallida | HR7-1 | OP480891 | |
| T. macrosporus | HR10-12 | OP480922 | |
| Sugarcane field soils, Thailand | A. chevalieri | HR16-15 | OP480965 |
| A. denticulatus | HR17-8 | OP480975 | |
| A. siamensis | HR17-20 | OP480981 |
| Species | D-Value (min) | Z-Value (°C) | |||||
|---|---|---|---|---|---|---|---|
| 75 °C | 80 °C | 85 °C | 90 °C | 95 °C | 97 °C | ||
| A. laciniosus | NR | NR | 74.93 ± 3.86 Aa | 7.78 ± 0.35 Ba | 1.09 ± 0.09 Ca | 0.89 ± 0.06 Ca | 6.19 |
| A. chevalieri | 69.85 ± 3.17 Aa | 9.53 ± 0.42 Bbc | 1.52 ± 0.13 Cd | 0.44 ± 0.01 Cd | - * | - | 6.75 |
| A. denticulatus | 31.01 ± 3.94 Ab | 13.56 ± 2.44 Bb | 4.69 ± 0.69 Cc | 2.22 ± 0.16 Cc | - | - | 12.87 |
| A. siamensis | 25.32 ± 1.04 Ab | 4.73 ± 0.25 Bc | 1.64 ± 0.14 Cd | - | - | - | 8.40 |
| H. pallida | 31.29 ± 2.43 Ab | 13.77 ± 0.67 Bb | 2.15 ± 0.11 Ccd | 0.46 ± 0.04 Cd | - | - | 7.91 |
| T. macrosporus | NR | 137.33 ± 9.21 Aa | 23.85 ± 2.81 Bb | 3.98 ± 0.35 Cb | 1.02 ± 0.08 Ca | 0.5 6 ± 0.03 Cb | 7.14 |
| Species | 75 °C | 80 °C | 85 °C | 90 °C | 95 °C | 97 °C | Z*-Value (°C) |
|---|---|---|---|---|---|---|---|
| Scale parameter (δ-value, min) | |||||||
| A. laciniosus | NR | NR | 104.59 ± 1.68 Aa | 7.39 ± 0.56 Ba | 1.38 ± 0.08 Ca | 0.81 ± 0.13 Ca | 5.71 |
| A. chevalieri | 68.23 ± 1.89 Aa | 8.65 ± 1.37 Bbc | 2.57 ± 0.33 Cc | 0.51 ± 0.01 Ccd | - | - | 7.02 |
| A. denticulatus | 24.29 ± 5.57 Ab | 6.04 ± 3.69 Bc | 0.79 ± 0.17 Bc | 0.74 ± 0.16 Bc | - | - | 9.36 |
| A. siamensis | 26.02 ± 2.41 Ab | 3.39 ± 0.35 Bc | 1.11 ± 0.19 Bc | - | - | - | 7.30 |
| H. pallida | 25.71 ± 4.07 Ab | 10.57 ± 1.42 Bbc | 1.70 ± 0.21 Cc | 0.48 ± 0.10 Ccd | - | - | 8.38 |
| T. macrosporus | NR | 205.13 ± 10.26 Aa | 28.70 ± 6.55 Bb | 5.49 ± 0.17 Cb | 0.94 ± 0.16 Cb | 0.76 ± 0.04 Ca | 6.86 |
| Shape parameter (p-value) | |||||||
| A. laciniosus | NR | NR | 1.95 ± 0.12 Aa | 0.79 ± 0.13 Cc | 1.65 ± 0.20 Ba | 0.91 ± 0.13 Cb | - |
| A. chevalieri | 1.32 ± 0.11 Aa | 0.93 ± 0.11 BCbc | 1.52 ± 0.25 Ab | 1.02 ± 0.33 Bb | - | - | - |
| A. denticulatus | 0.37 ± 0.05 Bc | 0.33 ± 0.07 Bd | 0.38 ± 0.03 Bd | 0.52 ± 0.04 Acd | - | - | - |
| A. siamensis | 1.03 ± 0.09 Aab | 0.73 ± 0.06 Bc | 0.63 ± 0.10 Bcd | - | - | - | - |
| H. pallida | 0.47 ± 0.06 Cc | 0.77 ± 0.08 Bc | 0.80 ± 0.08 Bc | 1.09 ± 0.28 Ab | - | - | - |
| T. macrosporus | NR | 1.88 ± 0.32 Aa | 1.26 ± 0.34 Bb | 1.92 ± 0.18 Aa | 0.92 ± 0.12 Cb | 1.44 ± 0.09 Ba | - |
| Species | RMSE | R2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 75 °C | 80 °C | 85 °C | 90 °C | 95 °C | 97 °C | 75 °C | 80 °C | 85 °C | 90 °C | 95 °C | 97 °C | |
| Log-linear model | ||||||||||||
| A. laciniosus | NR | NR | 0.089 | 0.057 | 0.213 | 0.197 | NR | NR | 0.972 | 0.960 | 0.909 | 0.946 |
| A. chevalieri | 0.056 | 0.223 | 0.282 | 0.090 | - * | - | 0.960 | 0.962 | 0.929 | 0.992 | - | - |
| A. denticulatus | 0.197 | 0.393 | 0.438 | 0.328 | - | - | 0.756 | 0.645 | 0.770 | 0.908 | - | - |
| A. siamensis | 0.139 | 0.155 | 0.197 | - | - | - | 0.967 | 0.963 | 0.929 | - | - | - |
| H. pallida | 0.138 | 0.170 | 0.162 | 0.264 | - | - | 0.907 | 0.955 | 0.963 | 0.929 | - | - |
| T. macrosporus | NR | 0.122 | 0.383 | 0.185 | 0.259 | 0.199 | NR | 0.941 | 0.868 | 0.884 | 0.919 | 0.964 |
| Weibull model | ||||||||||||
| A. laciniosus | NR | NR | 0.040 | 0.055 | 0.132 | 0.201 | NR | NR | 0.994 | 0.963 | 0.965 | 0.944 |
| A. chevalieri | 0.045 | 0.226 | 0.270 | 0.032 | - | - | 0.975 | 0.961 | 0.933 | 0.999 | - | - |
| A. denticulatus | 0.128 | 0.308 | 0.122 | 0.199 | - | - | 0.918 | 0.815 | 0.982 | 0.975 | - | - |
| A. siamensis | 0.142 | 0.102 | 0.145 | - | - | - | 0.966 | 0.984 | 0.962 | - | - | - |
| H. pallida | 0.114 | 0.148 | 0.142 | 0.282 | - | - | 0.940 | 0.966 | 0.972 | 0.918 | - | - |
| T. macrosporus | NR | 0.118 | 0.382 | 0.106 | 0.239 | 0.092 | NR | 0.941 | 0.869 | 0.961 | 0.942 | 0.992 |
| Measures | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. D-value | 1 | |||||
| 2. δ-value | 0.988 ** | 1 | ||||
| 3. Z-value | −0.422 | −0.376 | 1 | |||
| 4. Z*-value | −0.572 | −0.473 | 0.828 * | 1 | ||
| 5. Log reduction for NaOCl | −0.118 | −0.238 | −0.392 | −0.567 | 1 | |
| 6. Log reduction for ClO2 | −0.820 * | −0.897 ** | 0.104 | 0.100 | 0.588 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maneeboon, T.; Sangchote, S.; Hongprayoon, R.; Chuaysrinule, C.; Mahakarnchanakul, W. Modeling the Thermal Inactivation of Ascospores from Heat-Resistant Molds in Pineapple Juice and Evaluating Disinfection Efficiency of Sodium Hypochlorite and Chlorine Dioxide. Beverages 2023, 9, 96. https://doi.org/10.3390/beverages9040096
Maneeboon T, Sangchote S, Hongprayoon R, Chuaysrinule C, Mahakarnchanakul W. Modeling the Thermal Inactivation of Ascospores from Heat-Resistant Molds in Pineapple Juice and Evaluating Disinfection Efficiency of Sodium Hypochlorite and Chlorine Dioxide. Beverages. 2023; 9(4):96. https://doi.org/10.3390/beverages9040096
Chicago/Turabian StyleManeeboon, Thanapoom, Somsiri Sangchote, Ratchanee Hongprayoon, Chananya Chuaysrinule, and Warapa Mahakarnchanakul. 2023. "Modeling the Thermal Inactivation of Ascospores from Heat-Resistant Molds in Pineapple Juice and Evaluating Disinfection Efficiency of Sodium Hypochlorite and Chlorine Dioxide" Beverages 9, no. 4: 96. https://doi.org/10.3390/beverages9040096





