Abstract
The escalating production of processed prickly pear products has led to a substantial rise in waste materials, particularly peel, which is rich in bioactive chemicals and holds the potential for value-added product development. However, the high perishability of these peels imposes effective preservation techniques. So, this work aimed to explore the potential of prickly pear peels from O. ficus-indica ‘Rossa’, ‘Gialla’, and ‘Bianca’ cultivars for the production of Opuntia beverages, namely infusions. To achieve this goal, two distinct drying methods, namely microwave drying and a food dehydrator, were employed with the latter method, resulting in the higher recovery of phenolic compounds (0.5 mgGAE/mL vs. 0.16 mgGAE/mL) and the higher antioxidant capacity of the resulting infusions. Additionally, various steeping conditions involving water temperatures of 80, 90, and 100 °C were assessed to maximize the levels of phenolic compounds and antioxidant activity. The results demonstrate that the better overall drying method temperature and steeping conditions for the food dehydrator were at 35 °C and boiling water (100 °C) due to its generally better results and practicality. Sensorial trials revealed that the three infusions were generally accepted (score > 7.20 out of 9) but that O. ficus-indica ‘Rossa’ received the highest ratings. This study offers valuable insights for optimizing drying methods and steeping conditions to preserve and enhance the bioactive compounds and antioxidant potential in prickly pear peel infusions, promoting their sustainable utilization as functional ingredients in food and nutraceutical applications.
Keywords:
O. ficus-indica; herbal infusion; food dehydrator; microwave; steeping; phytochemicals; antioxidants 1. Introduction
Herbal teas and infusions, which have been consumed for centuries worldwide, are gaining popularity for their health benefits. An estimated 6.7 billion kilograms of tea and related herbal infusions are now being consumed worldwide, motivated by both therapeutic and pleasurable motives [1]. Infusions encompass a spectrum of botanical components, including leaves, flowers, seeds, fruits, bark, stems, and roots, which are typically steeped in hot water [2] for their particular health-related properties. In general, they represent a rich reservoir of bioactive phytochemicals, primarily polyphenols, which are prized for their powerful antioxidant and antiradical properties and have the potential to be used in therapeutic applications. The levels of these compounds and the benefits of this drink are influenced by distinct processes, such as the herbal drying methodology and the steeping techniques applied [1,3].
The genus Opuntia is one of the most agro-economically important cactus crop species; in fact, due to its well-known health-promoting properties, the crops of the cactus pear are increasingly gaining momentum both for health professionals and consumers [4,5]. Opuntia ficus-indica, commonly known as the prickly pear, is regarded as one of the most commercially valuable species among the Opuntia genus, primarily due to its renowned health-promoting properties. In fact, this plant possesses a wide array of valuable compounds, including betalains, phenolic compounds, sterols, fibers, and vitamins, particularly vitamin C, which is known to be associated with several of its claimed health benefits, which include, among others, antioxidant, anti-inflammatory, antidiabetic, and antimicrobial properties [6,7]. As a result, the cultivation of the prickly pear has experienced a significant surge in recent years, particularly in regions such as Italy, Brazil, North Africa, and Portugal [5,8]. As such, there is an increasing interest in this plant as a source of food, medicine, and industrial raw materials, which has led to the establishment of extensive research efforts aimed at enhancing its cultivation, processing, and utilization. In this context, companies have been struggling to find new approaches and new value-added products using prickly pear fruits.
In parallel with this, the processing of prickly fruits, such as juices and other pulp beverages, produces high quantities of by-products, such as peels and seeds, which still contain important nutrients and bioactive compounds [9], thus being suitable for the production of new value-added products, instead of being treated as waste or used in low-commercial valued products, as feed livestock. To address this issue, some researchers have directed their efforts toward harnessing the potential of Opuntia ficus-indica peel for the development of diverse beverage formulations. A comprehensive review by Barba et al. [10] highlighted the utilization of Opuntia peel in beverage production, primarily due to their rich reservoir of phenolic compounds. In many instances, these peels have been employed in conjunction with other components of the plant, such as pulp and juices, to yield high-value products [11,12]. Nevertheless, it is imperative to acknowledge that the high moisture content inherent in the peel, which typically ranges from 75% to 85% [9,13], renders these by-products exceptionally perishable, necessitating further processing steps to ensure their safe and sustainable utilization.
The dehydration of food represents one of the most important achievements in the history of the food industry, primarily using drying to reduce water content, preventing microbial growth, and extending shelf life [14]. While sun drying and oven drying are traditional methods known for their low cost and simplicity, they are time-consuming and face challenges like weather conditions and microorganism exposure.
Nowadays, the consumers’ demand for high-quality products drives research and the improvement of new food technologies. Processes such as cabinet drying, spray drying, freeze drying, microwave drying, and osmotic dehydration are the most common industrial procedures used to dry vegetal products [15]. Microwaves accelerate the drying process by promoting the rapid vibrational excitation of water molecules, which leads to volumetric heating, high thermal efficiency, and increased product quality. As a result, numerous firms, such as MicroDried® [16] and ENWΛVE [17], have honed their expertise in the application of distinct microwave techniques for the dehydration of their products. Nevertheless, due to the high frequencies of the waves, an increment in temperature can be drastic and lead to the production of burned products [18]. Previous studies demonstrate that drying, an essential thermal process in producing teas and herbal infusions, may cause significant losses of bioactive ingredients and thereby decrease the health benefits and quality of the products [19,20]. Moreover, steeping conditions such as water volume, temperature, and steeping time also claim to affect the content of the desired components in tea infusions [21,22].
A small number of authors have previously studied the impact of different drying methods, namely oven, freeze-drying, and microwave drying, on fruits and peels from O. ficus-indica [13,23,24]. Specifically, these studies have focused on evaluating the nutritional composition of these peels and utilizing oven-dried peels as flour supplements in the development of pastry products without investigating the impact of drying methods. So, there is a notable gap in the existing literature regarding the drying of O. ficus-indica peel, both from a kinetics point of view and the suitability of the resulting material for use in the production of beverages. Furthermore, the scarcity of publications on prickly pear infusion production, which are mostly centered on its flowers [25,26], coupled with the absence of previous investigations into the influence of steeping temperatures, underscores the imperative for further research that is aimed at optimizing the infusion process to yield a beverage characterized by elevated levels of bioactive compounds.
Therefore, in this study, the effects of microwave and dehydrator drying and steeping settings were evaluated to find the best conditions for producing prickly pear peel infusions with a high content of bioactives, antioxidant activity, and sensory quality. These findings support the idea of a simple drying and steeping method that may be applied at home or on a small scale to create high-quality prickly pear infusions.
2. Materials and Methods
2.1. Opuntia ficus-indica Peels
The fruits of three different O. ficus-indica cultivars were cultivated and collected by Cooperativa Agrícola, Figo d’Idanha (Idanha a Nova, Portugal). After harvest, the fruits underwent distinct processing procedures, as outlined by Ferreira et al. [27]. In brief, the cultivar pulps and seeds were separated, and peels were packaged in 3 kg polyethylene bags without additives. The samples were stored at −20 °C for further analysis of the proximal composition (Section 2.2). In addition, just after arrival, a portion of the frozen peel was thawed and divided for drying in a food dehydrator or in a microwave (Section 2.3).
2.2. Proximal Composition of Prickly Pear Peels
The relative moisture content was assessed by placing 1–2 g of prickly pear peels in a porcelain crucible and drying them at 105 °C overnight before weighing. To measure the ash content, the residual material was pre-incinerated on a heating plate for 20 min and then subjected to a muffle furnace at 550 °C for 6 h, with subsequent gravimetric quantification. The mineral composition was analyzed using microwave-assisted acid digestion following the method described by Marçal et al. [28]. A sample (up to 0.5 g) was digested with 4 mL of 69% (w/w) HNO3 in a microwave digester (Speedwave MWS-3+). Two cycles of microwave radiation were applied as follows: initially, the temperature was raised to 130 °C and then 170 °C with varying power levels and durations, followed by a cooling process. After cooling, 0.5 mL of 30% (w/w) H2O2 was added, and a second digestion cycle was conducted. Acid digests were diluted to 50 mL with Milli-Q water. The mineral content was determined using an inductively coupled plasma mass spectrometry (ICP-MS) on a Thermo ICP-MS XSeries equipped with a Burgener nebulizer. Total nitrogen, hydrogen, and carbon assays were conducted on a FlashEA 1112 Elemental analyzer (Thermo, Waltham, MA, USA) with helium (130 mL/min) as the carrier and reference gas (100 mL/min). The temperatures of the oxidation and reduction ovens were 900 °C and 680 °C, respectively, and the oxygen flow was 250 mL/min. The protein content was calculated using the nitrogen content multiplied by the universal conversion factor of 6.25. The total carbohydrate content was obtained by differences and calculated using the following formula:
2.3. Drying of Prickly Pear Peels
The peels were dried at 35 °C, 60 °C and 85 °C using a food dehydrator (DEHY, LACOR MENAJE PROFESIONAL S.L, Bergara, Spain) and three different potencies at 125 W, 375 W, and 700 W using a microwave (MW, Eletronia, D70H20LE17, Vanderbijlpark, South Africa), until reaching a moisture content below 8%. During the drying processes, the moisture content of the samples was measured to establish the drying curves at each drying temperature and microwave potency. The dried samples were stored in the dark at room temperature throughout the period of analysis.
2.4. Prickly Pear Peels Infusions
The dried peels were cut by hand into smaller pieces of approximately 2 × 2 cm. Approximately 2 g of the peel were then added to 100 °C water (for the drying methods study) and 80 °C, 90 °C and 100 °C water (for the study of steeping conditions) in a ratio of 100:1 (mLwater/gdried peel); they were left to steep for 5 min. The liquid–solid ratio and the time of steeping were defined using the mean of the quantities used in commercial teas. The resulting prickly pear infusion, steeped under different conditions, was evaluated for total phenolic compounds, total flavonoid content, total betalains, and antioxidant capacity.
2.5. Phytochemicals of Prickly Pear Peel Infusions
2.5.1. Total Phenolic Content (TPC)
The TPC was determined with the Folin–Ciocalteu reagent using the adapted procedure described by Singleton and Rossi [29]. Briefly, 15 µL of the diluted sample was reacted with 15 µL of the 0.2 mol/L Folin–Ciocalteu reagent and 60 µL for 5 min, and then 150 µL of 7% the sodium carbonate solution was added into the reaction mixture. The absorbance readings were taken at 760 nm after incubation at room temperature for 1 h in an automated plate reader (Biotek Instrument Inc, Winooski, VT, USA). Gallic acid was used as a reference standard, and the results were expressed in gallic acid equivalents per milliliter of infusion (GAE)/mL.
2.5.2. Total Flavonoid Content (TFC)
The TFC in prickly pear peel infusions was measured using the colorimetric method (trichloride aluminum method) outlined by Yeddes et al. [30]. An aliquot for each infusion (0.3 mL) was added to a 5 mL volumetric flask containing 0.45 mL of distilled deionized water. After 5 min, 0.75 mL of the 2% aluminum chloride (AlCl3·H2O) solution was added. The mixture was shaken and allowed to rest for 10 min of the reaction. The absorbance was measured at 415 nm versus the prepared methanol blank with a UV-VIS spectrophotometer (Biotek Instrument Inc, Winooski, VT, USA). Quercetin was used as a reference standard, and the results were expressed as the equivalent of quercetin per L of the infusion (mgQuercetin·L−1 of infusion).
2.5.3. UHPLC-DAD-ESI-MSn Analysis
UHPLC-DAD-ESI/MS analyses were carried out, as reported by Ferreira et al. [31,32]. The compounds were separated on a Hypersil GOLD C18 column (100 × 2.1 mm, 1.9 μm particle size, Thermo Scientific, Waltham, MA, USA) at 30 °C. Gradient elution with 0.1% formic acid in water (A) and 30% methanol in acetonitrile (B) was performed at 0.2 mL/min. UV–Vis data were collected from 200 to 700 nm while chromatographic profiles were taped at 280 nm. MS operated in the negative mode, and the instrument scanned in the range of m/z 100–2000. The ESI settings used were a needle voltage of 4.80 kV and a capillary temperature of 275 °C. The identification of compounds was performed through the comparison of retention times, absorption spectra, and MS data with standards in the literature [33].
2.5.4. Total Betalains
The assessment of the amount of betalains present in the prickly pear peel infusion followed the procedure outlined by Stintzing et al. [34]. The samples were mixed with a McIlvaine buffer (citrate-phosphate buffer with a pH of 6.5) to achieve absorption values ranging from 0.9 to 1.0 at their respective absorption maxima. The betalain content (BC) was calculated using the following formula: BC [mg/L] = (A × DF × MW × 1000/Ꜫ × l), where A represents the absorption value at the absorption maximum, corrected for the absorption at 600 nm; DF is the dilution factor; and l is the path length of the cuvette (which was 1 cm). The molecular weights (MW) and molar extinction coefficients (Ꜫ) of betanin (MW = 550 g/mol; Ꜫ = 60,000 L/(mol.cm) in H2O; λ = 538 nm) and indicaxanthin (MW = 308 g/mol; Ꜫ = 48,000 L/(mol.cm) in H2O; λ = 480 nm) were used for the quantification of betacyanins and betaxanthins. All measurements were performed in duplicate using a UV-VIS automated plate reader (Biotek Instrument Inc, Winooski, VT, USA).
2.6. Antioxidant Activity
2.6.1. ABTS•+ Discoloration Assay
The total antioxidant activity of the infusions was measured using an adaptation of the ABTS•+ discoloration assay, as previously described [27,35]. Briefly, the solution of ABTS•+ produced with 7 mM ABTS-NH4 and 2.45 mM potassium persulfate was diluted with distilled water until an absorbance of 0.70 ± 0.05 at 734 nm was obtained. Afterward, 50 μL of each sample was combined with 250 μL of the diluted ABTS•+ solution in a 96-well microplate (Biotek Instrument Inc., Winooski, VT, USA). The mixture was allowed to react for 20 min in the dark, at room temperature, and the absorbance was then measured at 734 nm, and the results were expressed in milligrams of GAE per mL of the infusion.
2.6.2. Superoxide (SO•) Scavenging Assay
The SO• scavenging capacity of prickly pear infusions was determined, as described by Catarino et al. [36]. To a mix of 75 µL of nitroblue tetrazolium (NBT), 100 µL of β-NADH and 75 µL of phenazine methosulfate (PMS), 75 µL of each sample/standard was added, left to incubate at room temperature for 5 min, and the absorbance was measured at 560 nm in a plate reader (Biotek Instrument Inc., Winooski, VT, USA) [36]. The results were expressed in the milligrams of GAE per mL of the infusion.
2.6.3. Nitric oxide (NO•) Scavenging Assay
The NO• scavenging method was adapted from Catarino et al. [36] with the absorbance of the prickly pear infusions measured at 562 nm in an automated plate reader (Biotek Instrument Inc., Winooski, VT, USA) after mixing with 100 µL of sodium nitroprusside and the Griess reagent (0.5% sulphanilamide and 0.05% naphthyletylenediamine dihydrochloride in 2.5% H3PO4). Gallic was used as a reference, and results were expressed in milligrams of GAE per mL of the infusion.
2.7. Sensory Analysis
An untrained panel of 32 tasters, consisting of 18 females and 14 males, aged between 21 and 70, was used to evaluate the infusions, which were prepared as follows: 4 g of small fragments of peel was added to 400 mL of boiling water, and the mixture was left to infuse for 5 min, after which it was transferred to a washed container to remove any sediments. The tasters were asked to evaluate the infusion based on the following parameters: color, aroma, flavor, visual appearance, and overall acceptance. A qualitative scale was used to describe their degree of like or dislike for each of the tested infusions. Additionally, the tasters were asked a few more questions to determine their overall acceptance of the drink. The survey used in the evaluation is provided in the supplementary material (Table S2).
2.8. Statistical Analysis
Principal component analysis (PCA) and cluster analysis were conducted using the online software MetaboAnalyst 5.0 [37]. One-factor statistical analysis was applied, and standard normalization was utilized within the software. The results of the physicochemical and antioxidant analyses are presented as the mean ± standard deviation based on a minimum of three independent assays performed in duplicate. All experimental data underwent statistical analysis using GraphPad prism 6.1 software to assess the significant differences between samples. The analysis employed a two-way ANOVA test with a significance level set at 0.05.
3. Results and Discussion
3.1. Proximal Composition of Opuntia ficus-indica Peels
The proximate composition of prickly pear peels from three cultivars (cv ‘Rosa’, ‘Gialla’, and ‘Bianca’) is summarized in Table 1. The moisture and ash content of the samples was similar to those described by other authors [38,39]. The protein content was about 5%, which is a value consistent with those reported for dried prickly pear peel from Egypt [9]. The peel contained approximately 75% of carbohydrates and 1% of lipids, which is a carbohydrate content similar to that reported by Parafati et al. (73%) [39], while that of lipids was considerably higher (2%). The mineral content of the samples was found to be generally consistent with data from the literature for plants of the same cultivars, with the exception of iron (Fe), copper (Cu), and zinc (Zn), which were ten times lower than those found by other authors [9,40]. Moreover, in agreement with the existing literature, we also observed clear variations in the mineral content of the three cultivars. Notably, the ‘Rossa’ cultivar had a higher concentration of magnesium (Mg), manganese (Mn), iron (Fe), and zinc (Zn); ‘Gialla’ was richer in selenium (Se); and the ‘Bianca’ cultivar contained higher levels of nickel (Ni). Note that the plants used in this study were not fertilized, and the mineral content of the soil could have affected the mineral uptake by the plants, as suggested by previous studies on the traceability of Portuguese wine grapes [41].

Table 1.
Proximal composition (moisture, carbohydrates, lipids, protein, ash and minerals) of the prickly pear peels—cv ‘Rossa’ (red peel), cv ‘Gialla’ (orange peel), and cv ‘Bianca’ (white peels)—after drying at 35 °C. Results are expressed as averages ± SD. (n = 3). Different lowercase letters above each bar indicate statistically significant differences (p < 0.05).
3.2. Effects of Drying Process on Prickly Pear Peels
3.2.1. Drying Curves and Visual Aspect
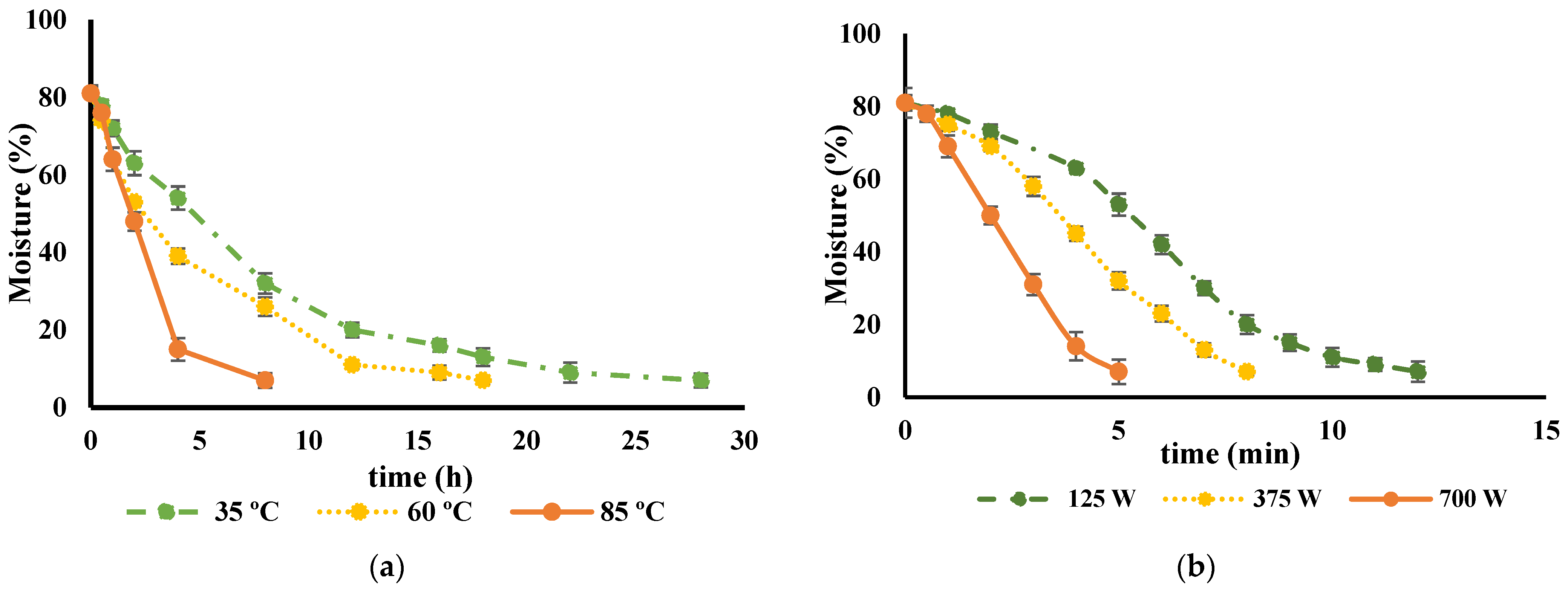
Preserving food products by drying is a widely used technique that helps retain the nutritional value and bioactive compounds of the food. The primary objective of this method is to remove water from the food, which, in turn, prevents the degradation of biological compounds due to chemical oxidation and enzymatic activity. The drying curves of various types of cultivar peel were found to be quite similar. As an example, the impact of different drying techniques on the drying kinetics of the ‘Rossa’ cultivar is illustrated in Figure 1. It took approximately 28 h to decrease the moisture content from 81.1% to 8% at 35 °C, whereas increasing the drying temperature to 60 °C or 85 °C resulted in reduced drying times of 18 and 8 h, respectively. Previous studies have reported analogous findings concerning the rates of water loss and drying durations when employing diverse temperature conditions for the dehydration of alternative fruit peels, including citrus peels and roselle fruits [42,43,44].
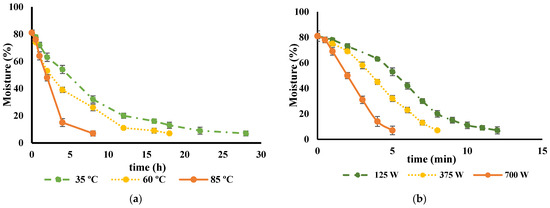
Figure 1.
Drying curve of Opuntia ficus-indica peels from cv ‘Rossa’ (a) at different temperatures, 35 °C (green), 60 °C (yellow) and 85 °C (orange) and (b) at different microwave potencies, 120 W (green), 375 W (yellow) and 700 W (orange).
The kinetics of O. ficus-indica peel drying was initially fast, possibly due to their high water content, and the drying rate decreased toward the end of the process with less water available to evaporate. Other herbs, such as Alpinia zerumbet, Etlingera elatior, Curcuma longa, Kaempferia galanga, and Vitex leaves, with lower-water activity, were reported to have faster drying rates than O. ficus-indica peel [45].
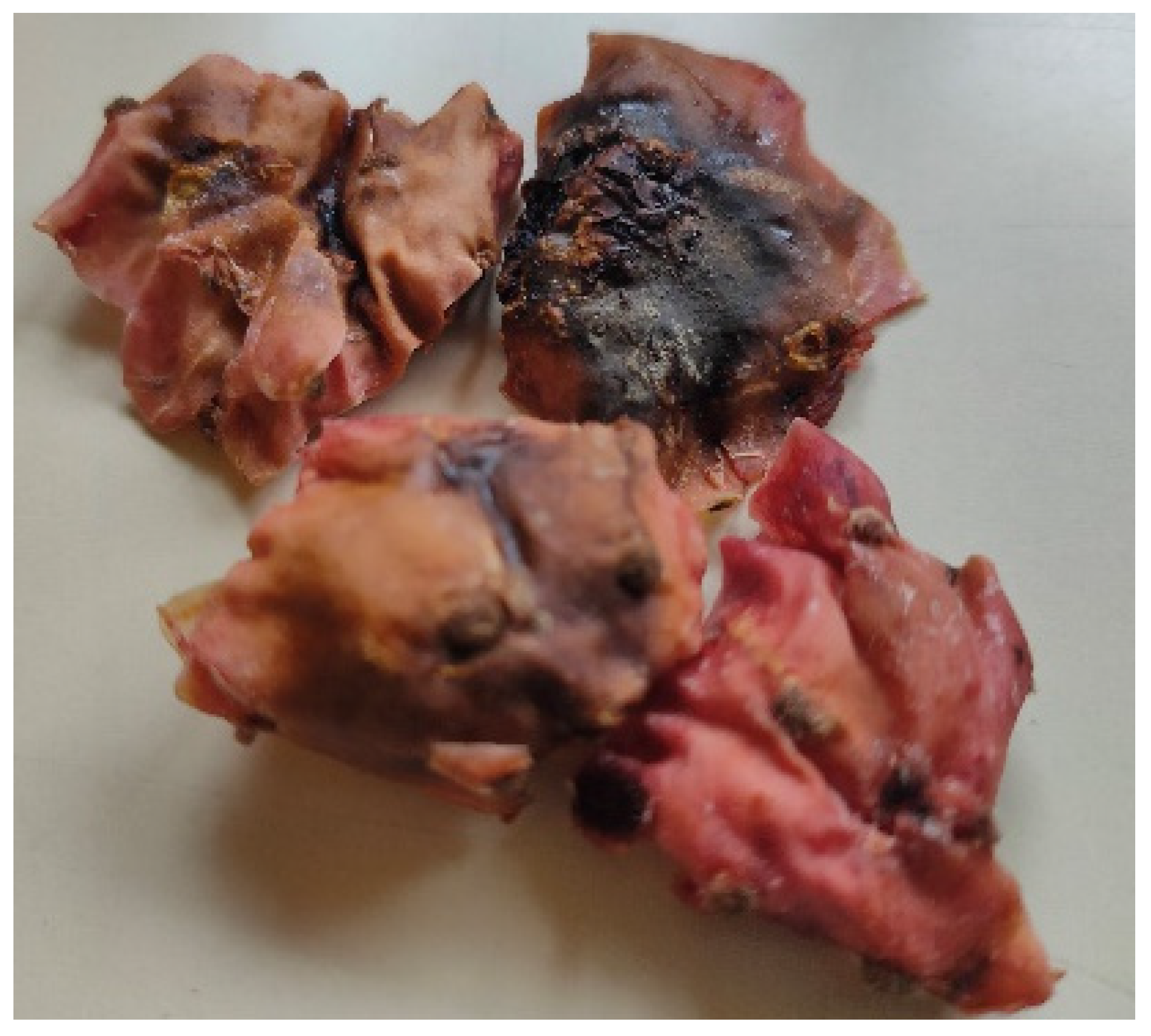
Regarding the use of MW, there are no data from the literature focusing on the effects of MW potencies on O. ficus-indica peel. This drying technology significantly reduced the drying time compared to the food dehydration methods, which is in agreement with previous findings for other fruits and vegetables [42,46,47]. In this study, drying times of around 12, 8, and 5 min were achieved for all the cultivars tested when using MW potencies of 125 W, 375 W, and 700 W, respectively. However, despite the ability of MW heating to efficiently generate heat within moist food portions, its non-uniform electromagnetic field within the MW cavity can result in excessive temperatures at product edges and corners, leading to irreversible drying and scorching and may cause the development of off-flavors [48]. This was observed in MW-dried peel, as evidenced by the dark, burned spots shown in Figure 2.
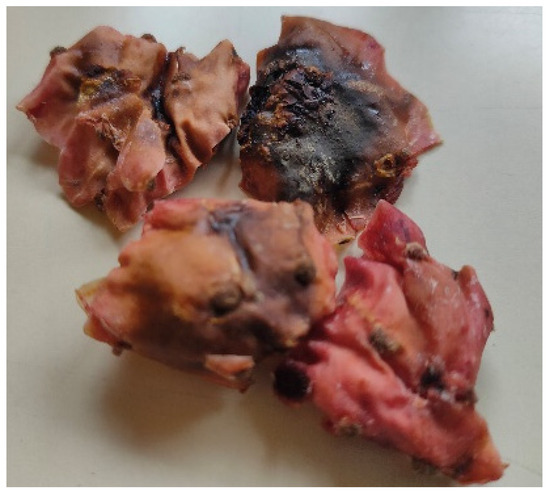
Figure 2.
Impact of the electromagnetic field present in a microwave oven on Opuntia ficus-indica cv ‘Rossa’ peel.
3.2.2. Influence of Drying on the Phytochemical Composition of Infusions
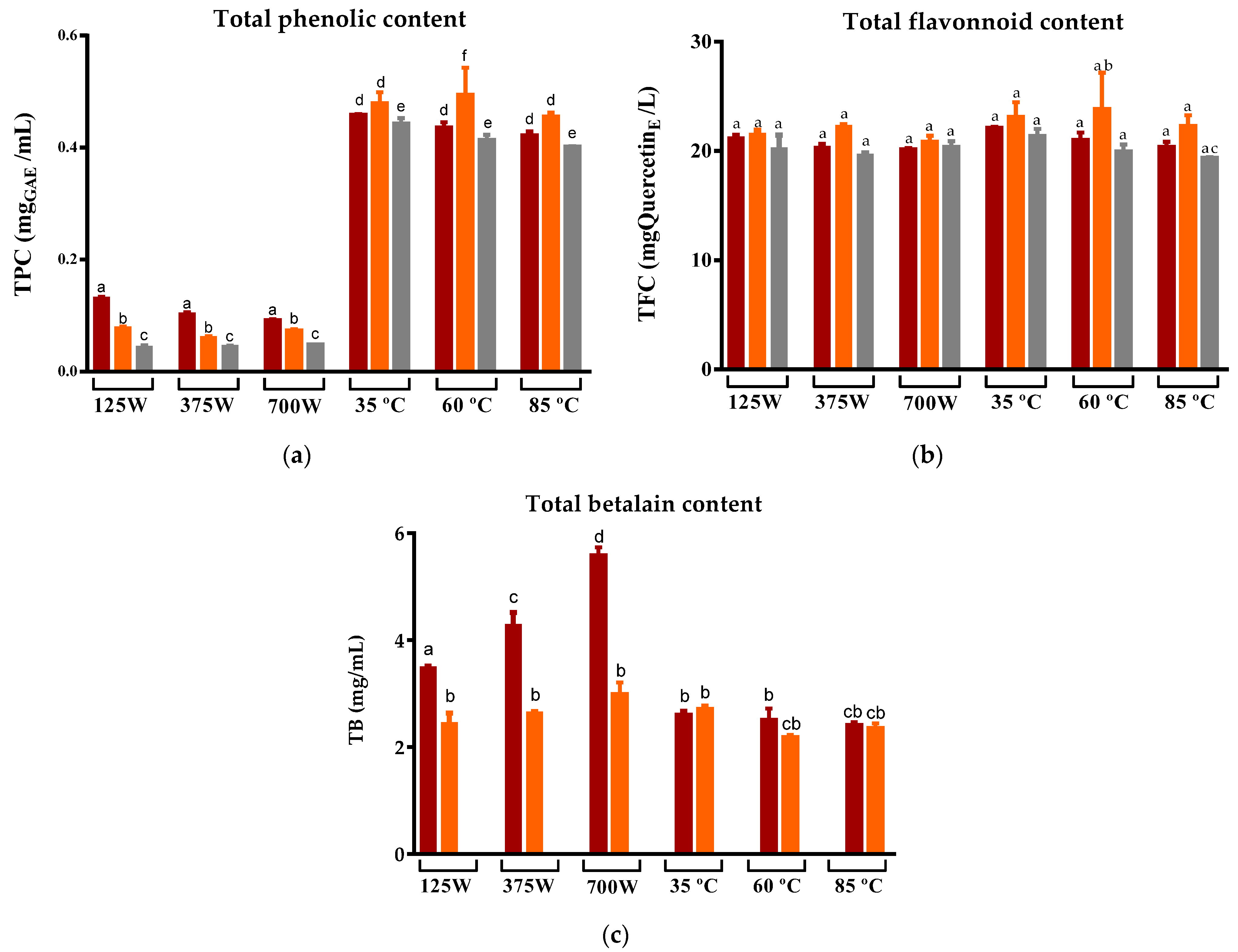
The impact of two different drying methods (DEHY and MW) on the phytochemical composition and antioxidant capacity of three cultivars of prickly pear peels (cv ‘Rossa’, ‘Gialla’, and ‘Bianca’) was investigated by producing infusions obtained with water at 100 °C. The TPC of the infusions was significantly different between the two drying methods, with MW-dried peel resulting in infusions with a lower TPC compared to those obtained from DEHY-dried peel (Figure 3a). On the other hand, the variation in the potency of MW in the range of 125 W to 700 W caused insignificant variations in the phenolic levels of the respective infusions. This behavior is similar to that reported by Wojdyło et al. for Ziziphus jujube Mill fruits, where an increase in the MW potency from 120 to 480 W tended to reduce TPC in jujube fruits from all three cultivars tested but not significantly [49]. Furthermore, Ghanem et al. also demonstrated that the MW-drying of citrus peel sourced from distinct cultivars, with a gradual increase in the microwave power within the range of 100 to 450, yielded a non-significant reduction in TPC. However, when compared to the fresh samples, a more pronounced reduction in TPC was evident. This decline in TPC could be attributed to the uninterrupted exposure of the peel to electromagnetic radiation, which induced thermal degradation and the subsequent combustion of the peel, thereby resulting in a concomitant reduction in TPC [43].
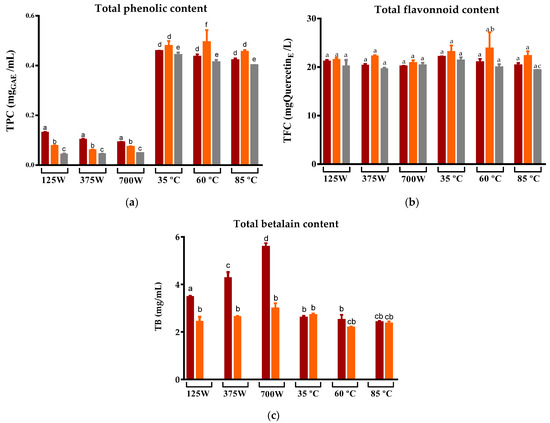
Figure 3.
Variation in the (a) Total phenolic compounds, (b) Total flavonoids, and (c) Total betalains compounds of prickly pear peels infusions cv ‘Rossa’ (red peel) in red (▬), cv ‘Gialla’ (orange peel) in orange (▬), and cv ‘Bianca’ (white peel) in grey (▬) obtained after MW and DEHY-drying at different potencies/temperatures, respectively. Results are expressed as the average ± SD (n = 3). Different lowercase letters above each bar indicate statistically significant differences (p < 0.05).
Regarding the DEHY-dried peel, in general, there was no significant variation in the TPC for the three temperatures tested (35 °C, 60 °C and 85 °C) and for the three prickly pear cultivars, suggesting that the increase in the drying temperature up to 85 °C did not degrade the phenolic compounds present in the peel [50]. Curiously, the levels of total flavonoids in all the infusions were not affected by the drying method, the MW potencies, or the dehydrator temperature. Nevertheless, when comparing the results of this study with those reported by other researchers for fruit juices, infusions, and flavored teas also prepared in the same ratio (1:100), all the samples evaluated in this work exhibited higher flavonoid concentrations [51,52].
Betalains, i.e., water-soluble nitrogen-containing pigments, are important metabolites in prickly pear figs, determining their reddish-purple and yellow-orange colors (betacyanin and betaxanthin, respectively). These compounds are claimed to exhibit strong antioxidant properties but are susceptible to pH, oxygen, metal ions, temperature, water activity, light exposure, and enzymatic activities, which limit their industrial applications [53]. As expected, the infusions of cv ‘Rossa’ origin presented the highest levels of betalains due to the fact that the fruit of this cultivar is quite rich in betacyanin [54], while that of cv “Gialla” contained moderate amounts of betaxanthin and cv “Bianca” does not contain betalains, as it does not present the metabolic pathway for their production [55]. Curiously, the levels of these metabolites were improved in infusions that originated from MW-dried peels compared to those produced from prickly pear peels submitted to DEHY-drying. Moreover, as opposed to TPC, the increase in MW potencies in the drying process allowed a greater recuperation of betalains in the infusions, suggesting that the shortening of time provided at high MW potencies is a key factor for their preservation, possibly due to their consequent reduction in exposure to factors such as oxygen, light, enzymes, and water activity. These findings are consistent with a previous study reporting that the use of MW-drying resulted in the extraction of higher quantities of betalains compared to the conventional temperature drying of Beta vulgaris [56]. In the case of DEHY-drying, it is expected that an increase in the drying temperature from 10 to 50 °C could lead to the greater retention of betalains due to a reduction in the drying time and the consequent decrease in exposure time [57]. At higher temperatures, despite the increase in extraction, there is also a degradation of betalains. This negative effect of temperature on the preservation of betalains has been previously reported [58,59,60]. Thus, our results consolidate the preference for low temperatures for the preservation of betalains.
3.2.3. Antioxidant Activity of the Infusion
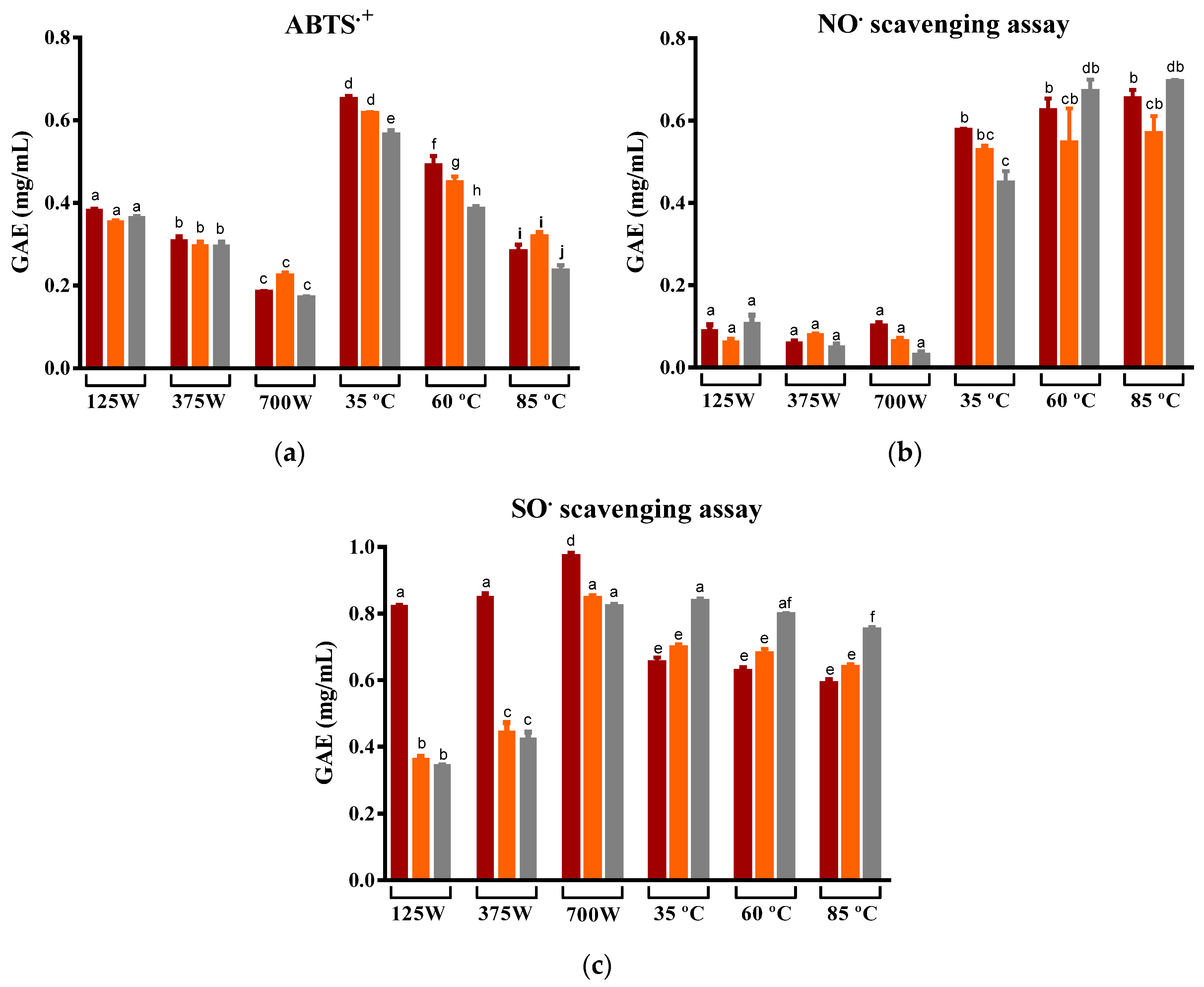
As mentioned, the nutritional and beneficial properties of the prickly pear are largely associated with the antioxidant compounds it contains, including phenolic compounds and betalains. In this work, three different antiradical tests (ABTS●+, NO●, and SO●) were performed to evaluate the antioxidant capacity of the prickly pear infusions. In the vast majority, infusions produced from MW-drying peels had an inferior antioxidant capacity compared to those obtained from DEHY-dried peels (Figure 4). Yet, there were distinct tendencies among the three executed assays.
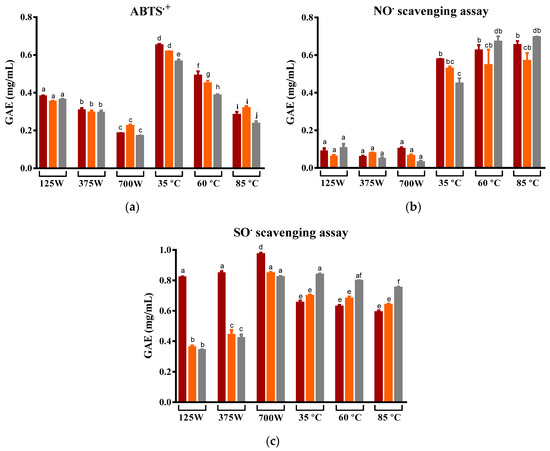
Figure 4.
Variation in the capacity to scavenge the (a) ABTS•+, (b) NO•, and (c) SO• of prickly pear peel infusions cv ´Rossa´ (red peel) in red (▬), cv ‘Gialla’ (orange peel) in orange (▬), and cv ‘Bianca’ (white peel) in grey (▬) obtained after MW and DEHY-drying at different potencies/temperatures, respectively. The results are expressed as the average ± SD (n = 3). Different lowercase letters above each bar indicate statistically significant differences (p < 0.05).
The ABTS•+ assay relies on electron transfer principles, i.e., upon the addition of the samples to the reaction mixture, they donate electrons to neutralize ABTS radicals, reducing them back to colorless ABTS. The degree of discoloration directly corresponds to the substance’s antioxidant capacity [61]. In this study, MW-drying led to a significantly reduced antioxidant capacity. As shown in Figure 2a, samples dried using MW exhibited an approximately 1.5 times lower ability to scavenge the ABTS●+ radical compared to those dried using a DEHY at the lowest temperature. In more detail, both the increment of MW potencies and temperature clearly decreased the ability of the infusions to scavenge the ABTS●+, which is a fact that may be attributed to the degradation of some antioxidant compounds present in the peel and caused by its long exposure to oxygen, light and moderate temperatures, and the application of electromagnetic radiation [48,62]. Still, this effect was not evident in the other two antioxidant tests.
The NO radical is pivotal in comprehending the body’s anti-inflammatory responses, and, in particular, in the NO• scavenging assay, a sample is introduced into the reaction mixture, serving as an electron donor that neutralizes the radicals. This electron transfer process converts NO radicals back to nitric oxide or related species. The degree of this reduction directly indicates the substance’s anti-inflammatory potential. Hence, within anti-inflammatory processes, compounds with robust NO radical-scavenging abilities are essential [63]. In this particular case, for NO• scavenging, the increase in MW potencies showed no significant correlation with the capacity of samples to scavenge the radical, whereas the rising DEHY temperatures resulted in infusions with a better ability to scavenge this radical (approximately 0.1 mgGAE/mL and 0.6 mgGAE/mL for MW and DEHY, respectively). Similar results have been reported in the literature for the drying of other fruits belonging to the genus Opuntia [64,65] and for various teas and herbal infusions [66,67,68]. Superoxide radicals, highly reactive molecules involved in oxidative stress and various diseases, are targeted in the superoxide radical assay. In this assay, a substance is introduced into the reaction mixture, where it scavenges superoxide radicals through electron donation. This electron transfer process transforms superoxide radicals into stable molecules, effectively neutralizing their harmful effects [69]. When exploring the SO• scavenging ability, it was evidenced that MW-drying peels from the ‘Rossa’ cultivar were the most promising sample, with the highest recorded antioxidant capacity. At lower MW potencies, the ‘Rossa’ infusion exhibited twice the ability to reduce the superoxide radical when compared to the infusion produced with the peel from the ‘Gialla’ and ‘Bianca’ cultivars. This finding is likely due to the microwave’s known capacity to extract betalains from beetroot [56], which is associated with higher superoxide scavenging capacities [70,71]. In turn, the increase in temperature caused little effect on the capacity of the infusions to scavenge SO•. The cultivars ‘Rossa’ and ‘Gialla’ exhibited a lower capacity to scavenge SO• when compared to cv ‘Bianca’, quantifying approximately 0.6 mgGAE/mL for ‘Rossa’ and ‘Gialla,’ and 0.8 mgGAE/mL for ‘Bianca’. The application of higher temperatures during dehydration was observed to decrease the recovery of phytochemicals, as seen in the results presented earlier and previously reported by Bassama et al. for Opuntia dillenii Haw [72]. Notably, the ‘Bianca’ cultivar is linked with a higher ascorbic acid content [73] that is associated with higher resilience to high temperatures, potentially contributing to its greater capacity to scavenge superoxide radicals.
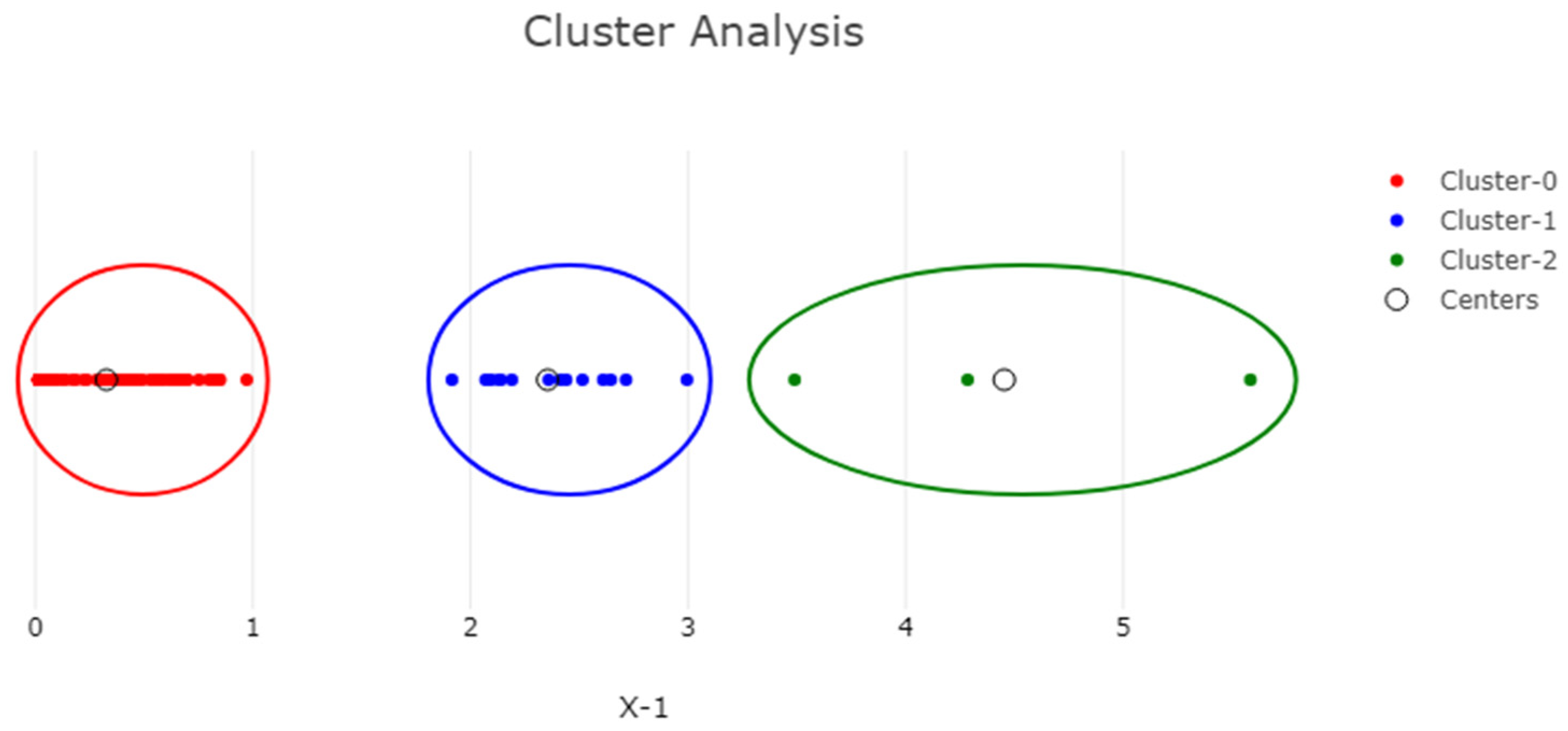
3.2.4. K-Means Cluster Analysis and Principal Components Analysis (PCA)
The PCA and cluster analysis of the phytochemical composition and antioxidant capacity of prickly pear infusions is illustrated in Figure 5 and Figure 6, as well as in Figures S1 and S2 in the Supplementary Materials.
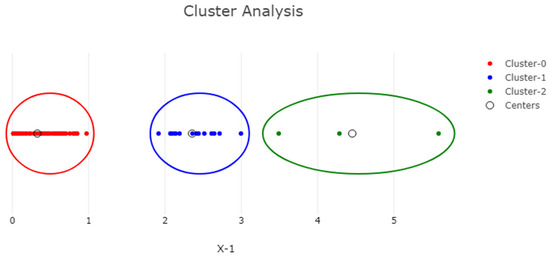
Figure 5.
K-means cluster analysis of Opuntia ficus-indica peel infusion from ‘Rossa’, ‘Gialla’ and ‘Bianca’ cultivars after DEHY-drying and MW-drying showing the clusters formed between the different drying methods and the different phytochemical, and antioxidant components of Opuntia ficus-indica peel infusion (K = 3).
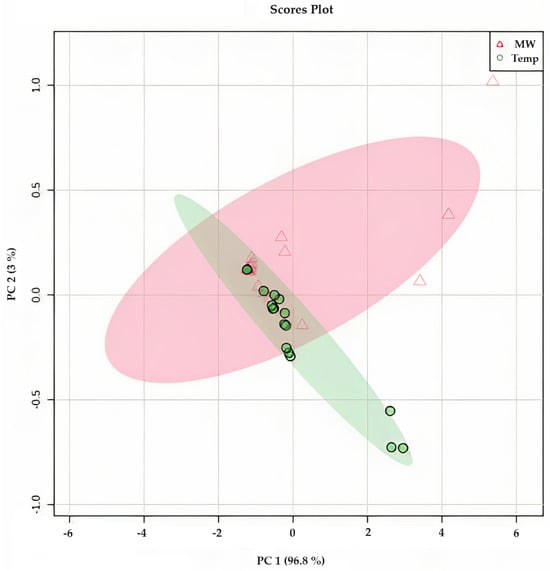
Figure 6.
Principal component analysis (PCA) of Opuntia ficus-indica cv ‘Rossa’, ‘Gialla’ and ‘Bianca’ peel infusions after DEHY-drying (●—in green) and MW-drying (∆—in red), showing the correlation between the different drying methods and the different phytochemical, and antioxidant components of Opuntia ficus-indica peel infusion.
The statistical analysis conducted yielded three distinct clusters, i.e., K = 3, with the first two being more densely populated than the third; the latter is highlighted in green on the right-hand side of the figure. This green cluster (Cluster 2) is composed of the results obtained for betalains, which were significantly different, presenting high variability between the cultivars and drying temperatures compared to the other tests performed. The other two populated clusters corresponded to the values obtained for the TPC, TFC and antioxidant activity. The choice of the number of K-means clusters was based on ensuring a variance of at least 90%, while the sum of squared distances from all the points to the centers (SSE) was 10.38, indicating a considerable level of variability within the different clusters. These findings highlight the inherent variability associated with the methods of drying and the specific tests performed, as previously noted.
Principal component analysis (PCA) is a statistical technique used to reduce the dimensionality of large datasets while maintaining as much variance as possible. In this study, PCA was employed to analyze the infusions of O. ficus-indica obtained from different drying techniques and cultivars. Specifically, three different drying temperatures (35, 60, and 85 °C) and three microwave potencies (125, 375, and 700 W) were investigated, and the resulting clusters identified in the PCA (Figure 6) demonstrated a correlation superior to 95%, that both the drying techniques and cultivar composition had an impact on the observed variation in the results. Figure S1 (supplementary materials) also depicts the effects of different cultivars on the PCA. Further, the Biplot PCA, illustrated in Figure S2, highlights that the concentration of betalains in the infusions was the main difference between the techniques, and this was represented by the points farthest from the central cluster. These findings are consistent with the results obtained from the cluster analysis (Figure 5). Additionally, the scavenging of superoxide and nitric oxide radicals also contributed to some variation in the drying methods, as discussed in Section 3.2.3.
Overall, the comparison of the effects of various temperatures and microwave potency settings allowed us to conclude that the selection of the drying method had a greater impact on the phytochemical composition and antioxidant activity of the prickly pear infusion from the three cultivars evaluated, whereas changes in temperature, particularly at 35 °C and 60 °C, had a slightly higher influence on the majority of the tests conducted. Notably, using higher temperatures (more particularly, 80 °C) caused a more noticeable negative impact on the extracted materials. Also of note, preliminary sensory tests indicated that they differed in odor and taste, with a consumer preference for the infusion obtained from cv ‘Rossa’ due to its more appealing color and taste. Moreover, all infusions produced from MW-dried peel had a slightly burnt taste, which was pointed as unpleasant.
3.3. Effects of the Steeping Temperature Conditions on the Contents of Phytochemicals and Antioxidant Activity of the Infusions
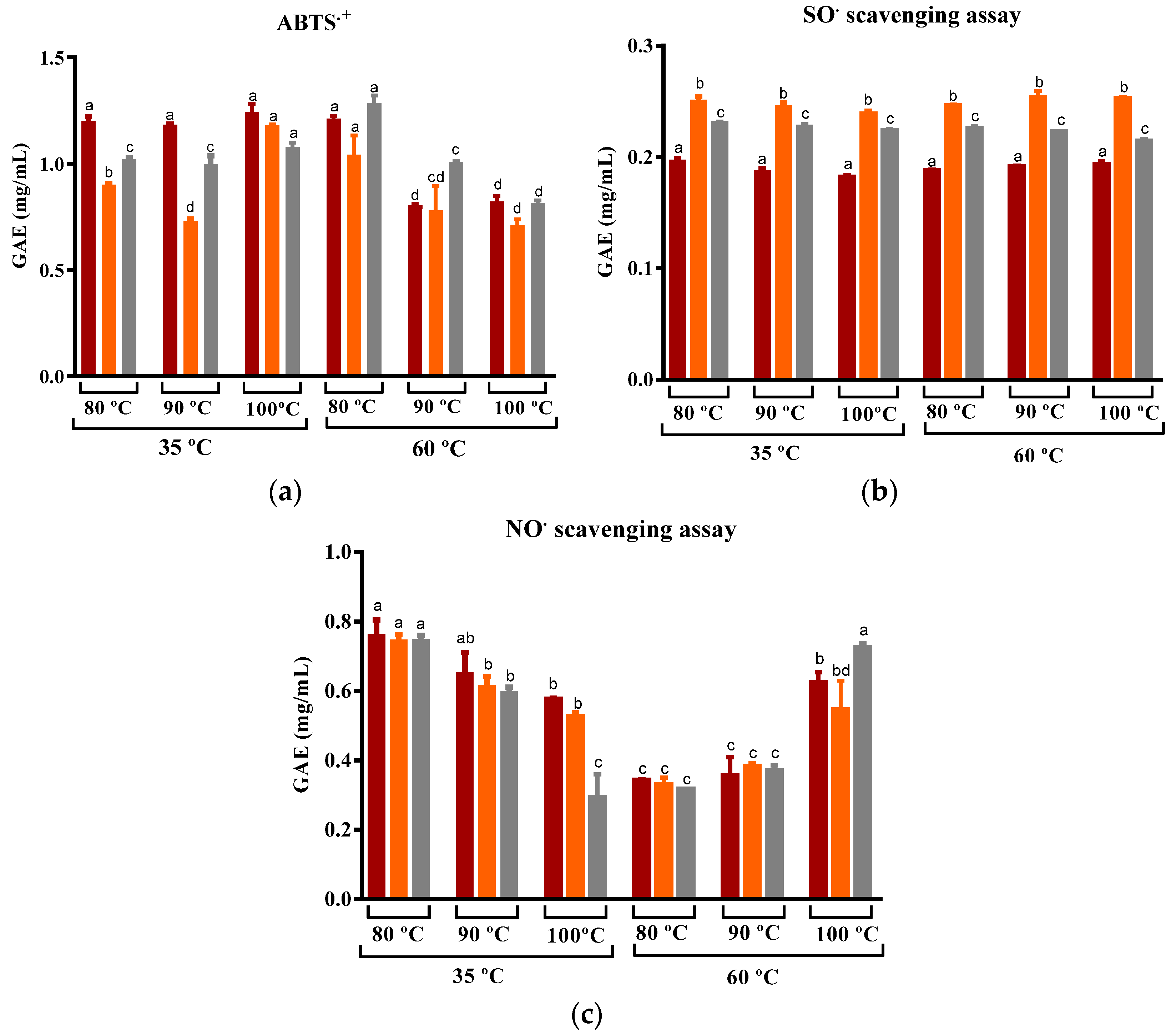
Based on the previous results, which showed a higher phytochemical content and antioxidant activity for DEHY-dried peel at low temperatures (35 °C and 60 °C), these conditions were then chosen to study the effects of three different steeping conditions by infusing the peel in water (1:100) at 80, 90, and 100 °C for 5 min on the phytochemical content and antioxidant capacity of prickly pear peel infusions.
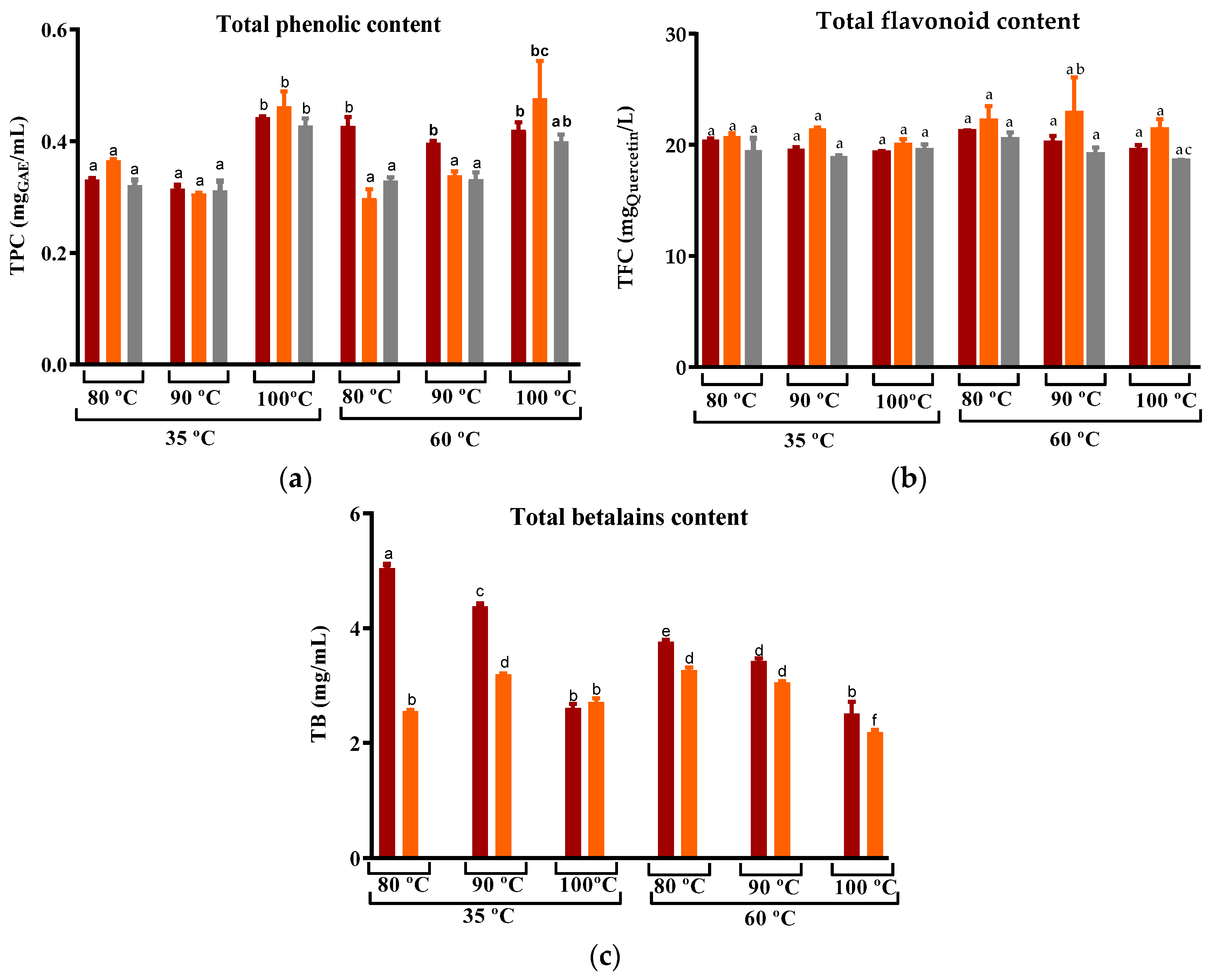
For the peel dried at 35 °C, the use of water temperatures at 80 °C and 90 °C yielded a comparable extraction of phenolic compounds (Figure 7). As for peel dried at 60 °C, with the exception of ‘Rossa’ cv, an increase in the water temperature resulted in an improvement in the recovery of TPC from the infusion. This trend is consistent with findings reported for white and oolong tea as well as rice leaf infusion, where an increase in steeping temperature led to an increased recovery of TPC after 3–5 min of steeping [74,75,76]. Globally, for both the drying temperatures, the maximum of phenolic compounds recovered was obtained with the use of water at 100 °C, which was around 0.5 mgGAE/mL and, thus, higher than the values obtained by Nikniaz et al. for commercial black teas (Camellia sinensis L.) (0.1–0.3 mgGAE/mL) [21]. Nevertheless, levels of flavonoids were similar in all infusions and significantly lower than those found by Chong and Nyam for kenaf leaves tea (57–68 mgCHE/L).
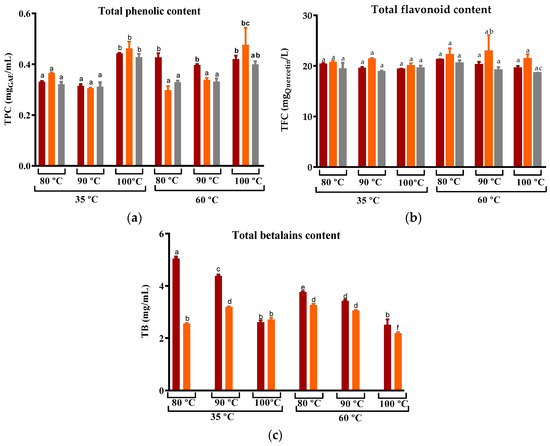
Figure 7.
Variation in the (a) Total phenolic compounds, (b) Total flavonoids, and (c) Total betalains compounds of prickly pear peel infusions cv ´Rossa´ (red peel) in red (▬), cv ‘Gialla’ (orange peel) in orange (▬), and cv ‘Bianca’ (white peel) in grey (▬) obtained from peel dried at 35 and 60 °C and steeped at 80, 90, and 100 °C. Results are expressed as the average ± SD (n = 3). Different lowercase letters above each bar indicate statistically significant differences (p < 0.05).
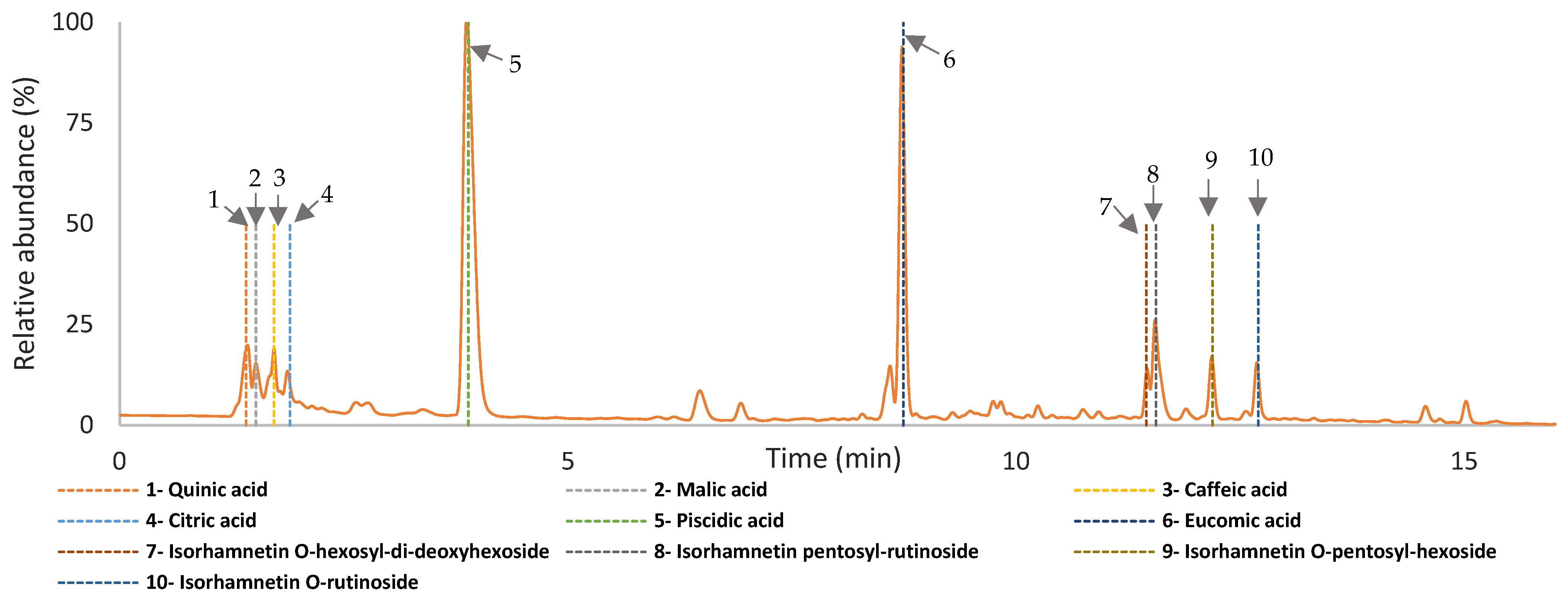
The chromatographic analysis of all infusions revealed similar peaks at 280 nm, suggesting that the same phenolic compounds were extracted regardless of the steeping conditions employed. The identification of the main phenolic compounds was based on UV data plus MS/MS data and further comparison with data from the literature. As observed in Figure 8 (a representative chromatogram of all infusions produced) and Table S1, the main compounds identified in the three Opuntia cultivars included piscidic acid, eucomic acid, and isorhamnetin derivatives. Not surprisingly, all these compounds matched the main phenolic components previously reported in juices with a fermented beverage and betalain-rich microparticles from the peel flour of O. ficus-indica [8,27,31,32].
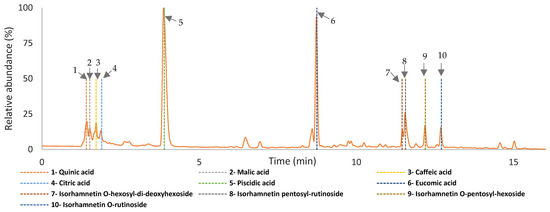
Figure 8.
The chromatogram with the identification of the components detected in the on Opuntia ficus-indica infusion from cv ‘Rossa´ (red peel), cv ‘Gialla’ (orange peel), and cv ‘Bianca’ (white peel) produced from peel dried at 35 °C and steeped at 100 °C, at 280 nm using UHPLC-DAD-ESI-MSn.
In all tested conditions, the levels of total betalains in cv ‘Rossa’ infusions were higher than those of cv ‘Gialla’ (Figure 7c). Moreover, maximum levels were obtained from cv ‘Rossa’ samples dried at 35 °C. This is in accordance with Bassama et al.’s description of the degradation of betacyanins from Opuntia dillenii Haw during pasteurization at 60–90 °C and storage [72]. In the case of the ‘Gialla’ cultivar, the total betalain content was found to be higher for peel that was dried at 35 °C and steeped at 90 °C. However, when the peel was DEHY-dried at 60 °C, an increase in the steeping temperature resulted in a decrease in the betalain extraction for both the red and orange cultivars.
The antioxidant activity of the infusions obtained at 80, 90, and 100 °C against ABTS•+, SO•, and NO• is shown in Figure 9. The results suggest that there was no statistically significant difference in the capacity to scavenge SO• among infusions produced at various steeping temperatures. Similarly, Hajiaghaalipour et al. found that the ability to scavenge SO• using cold and hot water extracts was similar. These findings imply that the steeping temperature may not affect the capacity to scavenge SO• [77].
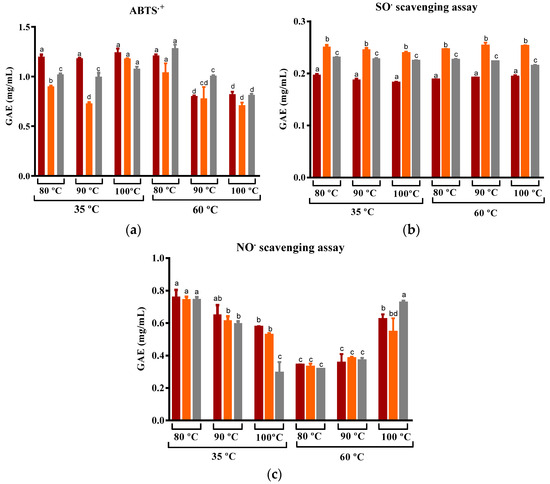
Figure 9.
Variation in the (a) ABTS•+, (b) SO•, and (c) NO• scavenging capacity of prickly pear peel infusions cv ´Rossa´ (red peel) in red (▬), cv ‘Gialla’ (orange peel) in orange (▬), and cv ‘Bianca’ (white peel) in grey (▬) obtained from peel dried at 35 and 60 °C and steeped at 80, 90, and 100 °C. Results are expressed as the average ± SD (n = 3). Different lowercase letters above each bar indicate statistically significant differences (p < 0.05).
In turn, our study reveals that the infusions obtained from ‘Gialla’ and ‘Bianca’ cultivars, which were subjected to DEHY-drying at 35 °C, exhibited a variation in their ability to scavenge ABTS•+. A slight increase in the scavenging capacity was observed at higher steeping temperatures, while the opposite trend was observed for infusions obtained from the peel that was DEHY-dried at 60 °C. Similar values to those observed for peel dried at 35 °C were reported by Pérez-Burillo et al. for white tea. The authors found that the variation in water temperature from 60 to 100 °C resulted in an increase in the ability to scavenge ABTS•+ [74]. It is possible that the superior retention of these compounds observed for samples dried at 35 °C, compared to those dried at 60 °C, may lead to a higher capacity to scavenge ABTS cation radicals.
As for the capacity of the prickly pear infusion to act as a scavenging agent of nitric oxide radical, the steeping temperature also had opposite effects depending on the drying temperature. Drying at lower temperatures led to a decrease in the NO• scavenging capacity with an increase in the steeping temperature, while peel dried at higher temperatures showed an increase in the NO• scavenging capacity with a higher water temperature. Nevertheless, except for the infusions obtained at 100 °C, those obtained from DEHY-dried at 60 °C were low and promising compared to infusions from DEHY-dried at 35 °C. Hajiaghaalipour et al. reported comparable outcomes when using infusions of distinct white, green, and black teas. Their study demonstrated that steeping the dried teas at varying times and at different temperatures resulted in different effects for each tea variety. Steeping at higher temperatures of silver needle white tea resulted in a reduction in the antioxidant activity of the infusions, whereas the opposite trend was registered for black tea [77]. These observations suggest that the ability of different tea samples to scavenge NO radicals is dependent on the properties of the raw material as well as the drying and steeping temperatures.
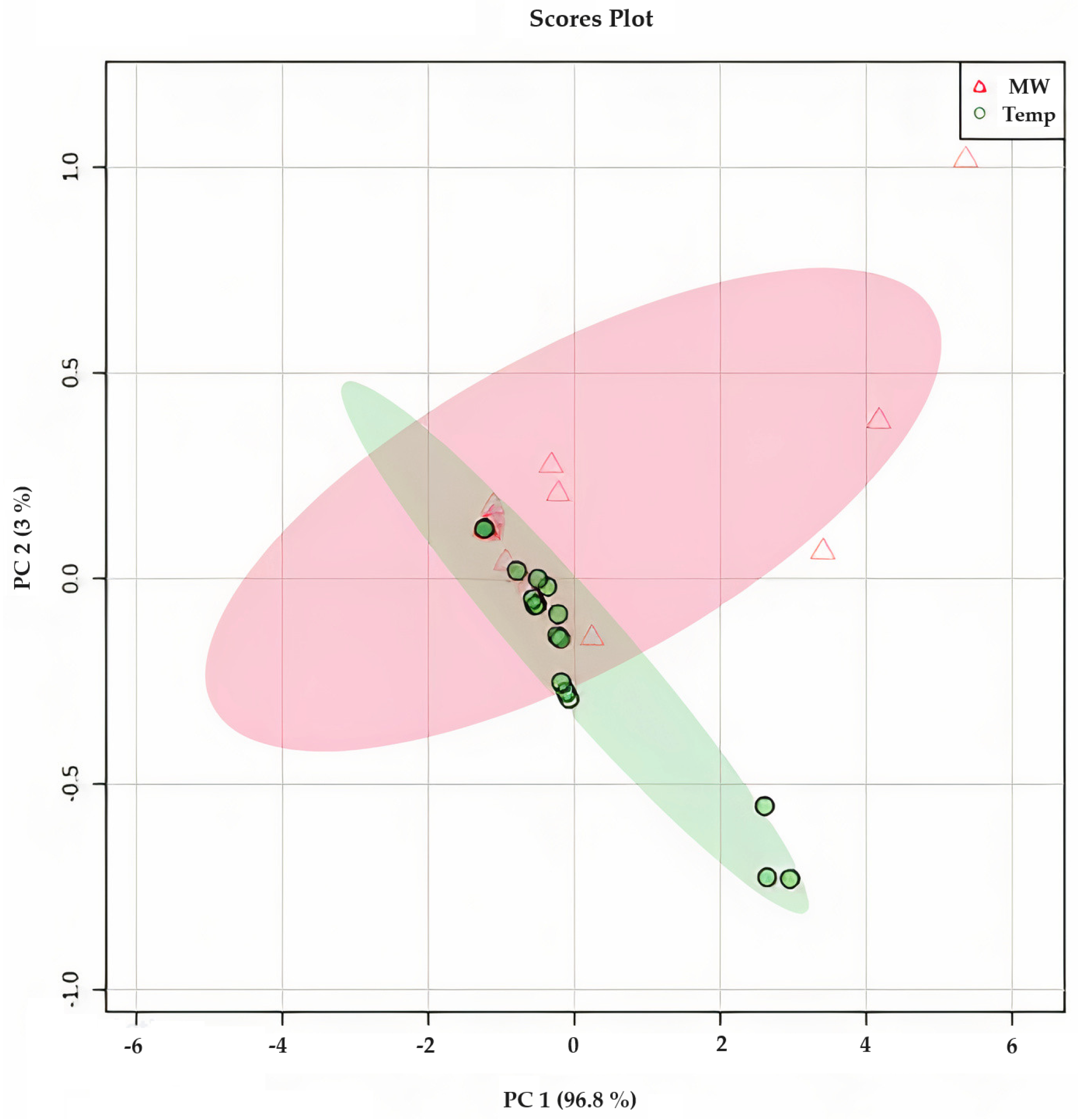
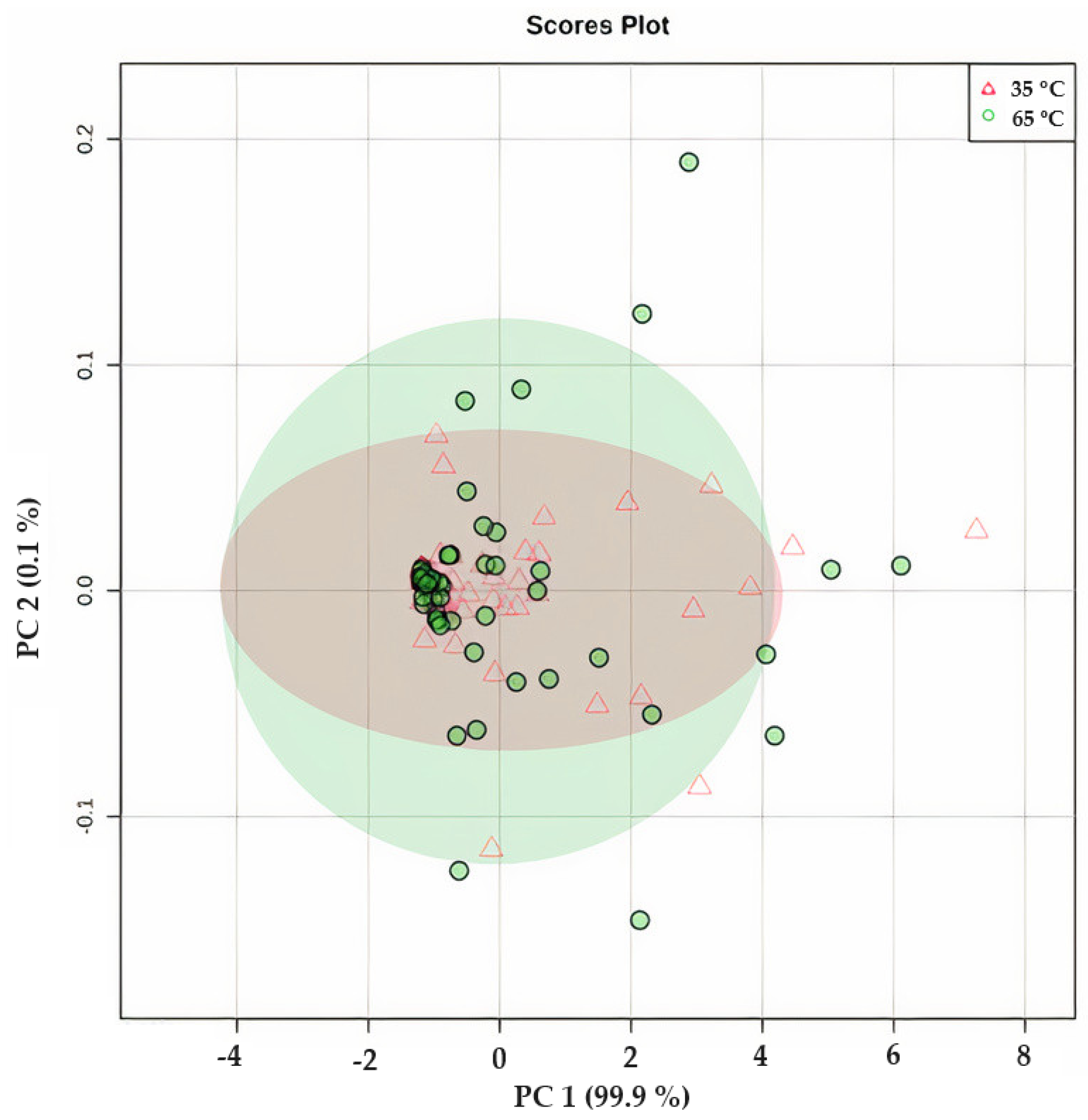
Principal Components Analysis of Steeping Conditions
Figure 10 presents the results of the principal component analysis (PCA) of O. ficus-indica infusions, which were prepared by steeping at 80, 90, and 100 °C. The distribution of the points in the figure reveals that water temperature had little impact on the variations observed in the phytochemical composition and antioxidant capacity. With a correlation of 99.9%, it can be concluded that the biggest changes were observed for the TPC at 100 °C, the betalain content, and SO● scavenging. This finding was also corroborated by the Biplot shown in Figure S3 (Supplementary Materials). The clustering observed was primarily attributed to the differences in the composition of various cultivars, such as the variation in the total betalain content, which had a more significant influence on the results obtained. Therefore, it can be concluded that the use of different steeping conditions does not affect the phytochemical and antioxidant capacity of O. ficus-indica peel infusion. Moreover, a preliminary sensory analysis performed in our group indicated that the overall characteristics, including color, odor, and taste, of the infusions of each cultivar produced under different steeping conditions were indistinguishable from each other.
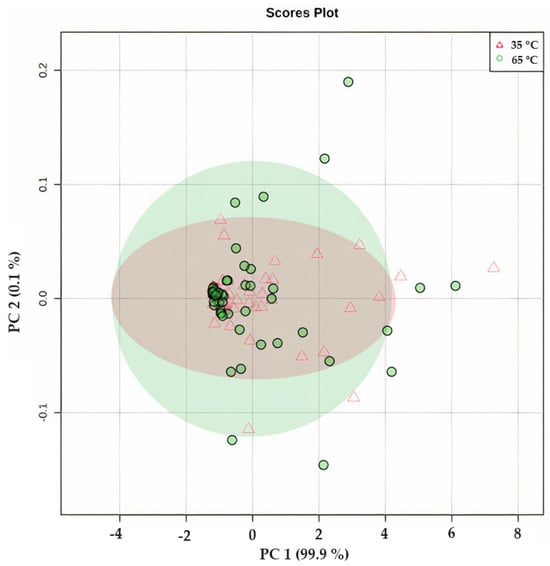
Figure 10.
Principal component analysis (PCA) of Opuntia ficus-indica peel infusions from cv ‘Rossa’, ‘Gialla’, and ‘Bianca’ dried at 35 °C (∆—in red) and 60 °C (●—in green) in a food dehydrator and after steeping at three different temperatures (80, 90, and 100 °C) showing the correlation between the different steeping conditions and the different phytochemical and antioxidant components of Opuntia ficus-indica peel infusion.
3.4. Sensorial Analysis of the infusions
In order to understand the consumers’ acceptability of the infusion produced, a sensory evaluation was conducted on the prickly pear peel infusion. This study involved the utilization of three different cultivars and the use of peel dried at 35 °C and steeped at 100 °C, as these conditions yielded superior overall results, namely regarding the content of phenolic compounds, betalains, and the antioxidant capacity. The global appearance of the prickly pear infusions from the three cultivars was clearly distinguishable in terms of their color (Figure 11).

Figure 11.
Infusions of Opuntia ficus-indica cultivars ‘Gialla’ (left), ‘Rossa’ (middle) and ‘Bianca’ (right) produced with approximately 1 g of dried peel and 100 mL of water at 100 °C.
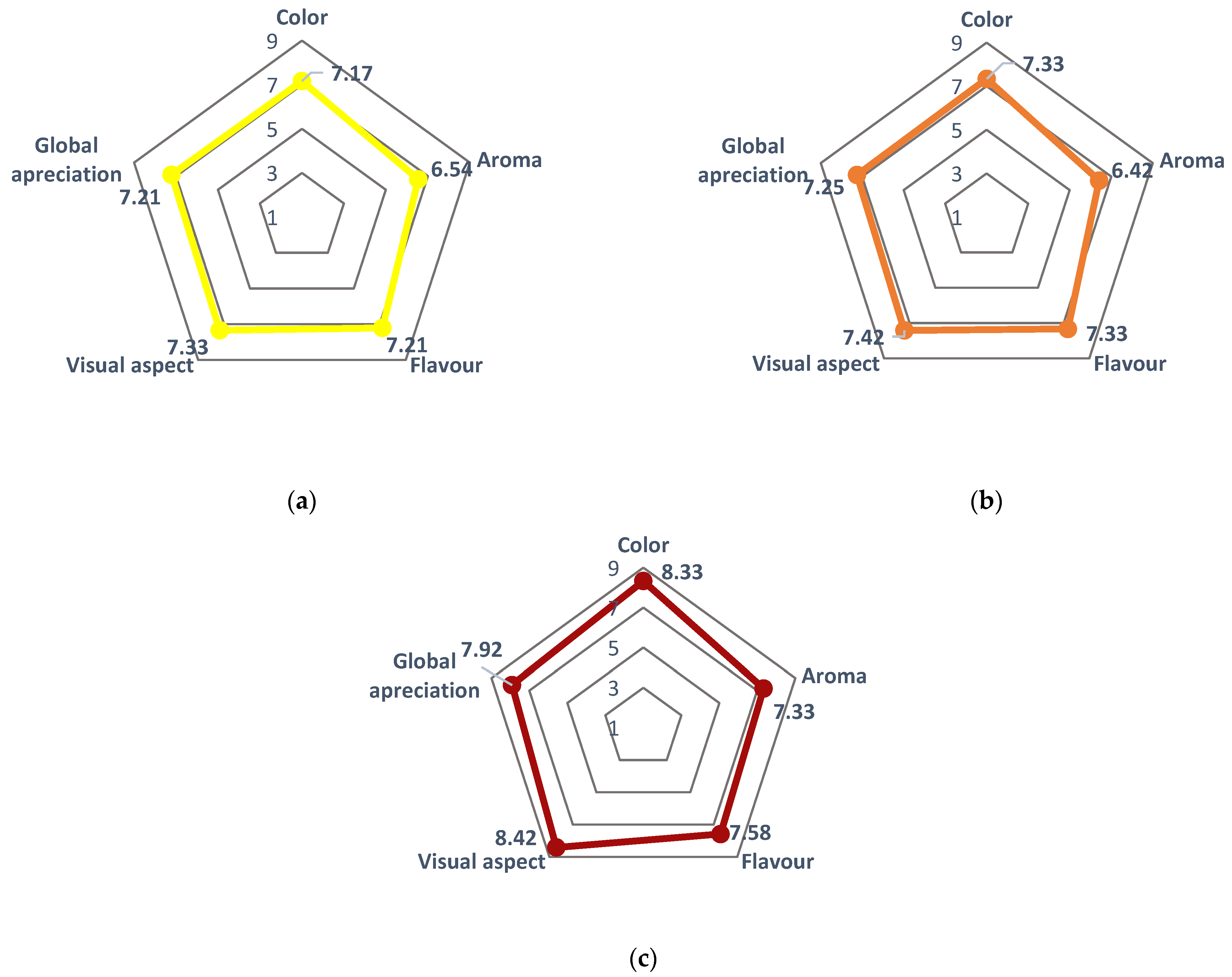
The sensory evaluation was performed by an untrained panel consisting of 32 tasters ranging in age from 24 to 70 years. For this, a qualitative scale ranging from 1 to 9, with each value corresponding to a specific criterion, was used as follows: (1) Dislike extremely; (2) Dislike very much; (3) Dislike moderately; (4) Dislike slightly; (5) Neither like nor dislike; (6) Like slightly; (7) Like moderately; (8) Like very much; and (9) Like extremely (Supplementary material, Table S2). This scale enabled the assessment of the infusion based on color, aroma, flavor, visual expectation, and global appreciation. The results, depicted in Figure 12, demonstrated slight variations among the different cultivars, with the ‘Rossa’ cultivar exhibiting higher ratings across all evaluated characteristics. The color displayed the highest variation, with ‘Rossa’ achieving an average score of 8.33, surpassing the scores of 7.33 and 7.13 obtained for ‘Gialla’ and ‘Bianca’ cultivars, respectively. Conversely, aroma received the least favorable ratings from the tasters for all cultivars, ranging from 6.43 to 7.33. Notably, the levels of approval for sweetness, flavor, and overall appreciation were consistently close for each cultivar.
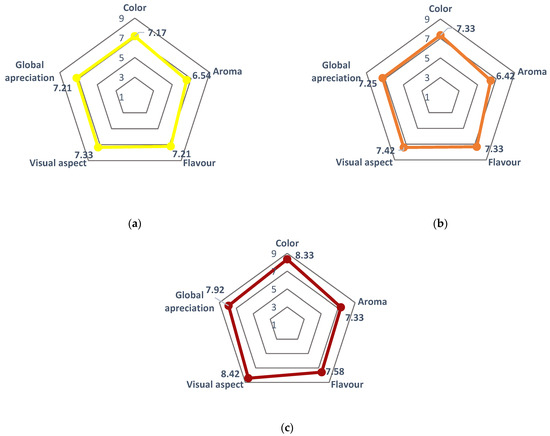
Figure 12.
Sensory analysis of Opuntia ficus-indica peel infusion from cultivars (a) ‘Bianca’ (white peel) in yellow (▬), (b) ‘Gialla’ (orange peel) in orange (▬), and (c) ‘Rossa’ (red peel) in red (▬) obtained from peels dried at 35 and 60 °C and steeped at 80, 90, and 100 °C. Results are expressed as the average ± SD (n = 3).
4. Conclusions
In summary, this study highlights the critical influence of drying methods on the quality and phytochemical composition of O. ficus-indica peel beverages, particularly infusions. MW-drying was found to have a detrimental impact on the peeling quality, resulting in undesired burnt spots and infusions with reduced TPC, TFC, and antioxidant capacity when compared to food dehydrator drying. For the latter capacity, drying at elevated temperatures (85 °C) also caused a decline in both the antioxidant activity and bioactive compound content of the resulting infusions. On the other hand, using lower drying temperatures between 35 and 60 °C efficiently maintained the biological and sensory properties of the resultant beverage. Therefore, careful temperature control is essential for preserving the integrity of beneficial bioactive components and antioxidant activity in the resulting infusions while processing O. ficus-indica peel. Infusions produced at temperatures ranging from 80 to 100 °C exhibited similar concentrations of bioactive compounds across the O. ficus-indica cultivars, with 100 °C yielding the highest concentration and proving to be the most practical option. Among the infusions, those produced from the ‘Rossa’ cultivar were preferred by consumers, followed by ‘Gialla,’ while ‘Bianca’ received comparatively lower scores. These findings collectively contribute to the understanding of how processing methods and cultivar choice influence the production of Opuntia ficus-indica peel beverages, particularly infusions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/beverages9040097/s1, Figure S1: Principal components analysis (PCA) of Opuntia ficus-indica peel infusions from cv ‘Rossa’ (◊—in red), ‘Gialla’ (∆—in orange) and ‘Bianca’ (◊—in green) after oven-drying (35 °C, 60 °C and 85 °C) and Mw-drying (125 W, 375 W and 700 W) showing the correlation between the different cultivars and the drying methods with the different phytochemical and antioxidant components of Opuntia ficus-indica peel infusion. Figure S2: Biplot of the Principal components analysis (PCA) of Opuntia ficus-indica cv ‘Rossa’, ‘Gialla’ and ‘Bianca’ peel infusions after food dehydrator drying and microwave drying showing the correlation between the different drying methods and the different phytochemical and antioxidant components of Opuntia ficus-indica peel infusion. Table S1: Identification of the components detected in the chromatogram of Opuntia ficus-indica infusion from cv ‘Rossa´ (red peel), cv ‘Gialla’ (orange peel), and cv ‘Bianca’ (white peel) produced from peels dried at 35 °C and steeped at 100 °C, at 280 nm, by UHPLC-DAD-ESI-MSn. Figure S3: Biplot of the principal components analysis (PCA) of Opuntia ficus-indica peel infusions from cv ‘Rossa’, ‘Gialla’, and ‘Bianca’ dried at 35 °C (∆—in red) and 60 °C (●—in green) in a food dehydrator and after steeping at three different temperatures (80, 90, and 100 °C) showing the correlation between the different steeping conditions and the different phytochemical and antioxidant components of Opuntia ficus-indica peel infusion. Table S2: Sensorial analysis of Opuntia ficus-indica peel infusions from cultivars ‘Bianca’, ‘Gialla’ and ‘Rossa’.
Author Contributions
Conceptualization, R.M.F., D.F.W. and S.M.C.; methodology, R.M.F.; software, R.M.F.; validation, R.M.F.; formal analysis, R.M.F.; investigation, R.M.F.; data curation, R.M.F.; writing—original draft preparation, R.M.F.; writing—review and editing, D.F.W., A.M.S.S., J.A.S. and S.M.C.; supervision, J.A.S. and S.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported through the projects UIDB/50006/2020 and UIDP/50006/2020, funded by FCT/MCTES through national funds. Project “INOVFARMER.MED—Improving Mediterranean supply chain through innovative agro-food business models to strengthen small-scale farmers competitiveness”, which is part of the PRIMA Program, is supported by the European Union, Grant Agreement No.1733, FCT/PRIMA/0005/2021. R.F. and S.M.C. thank FCT/MCTES for the PhD grant ref. SFRH/BD/137057/2018 and the labor contract under the Scientific Employment Stimulus—Institutional Call, respectively.
Data Availability Statement
The data that support the findings of this study are available within the article.
Acknowledgments
We acknowledge the company Figo d’Idanha for supplying us with the raw material used in the development of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Díaz-Rivas, J.O.; Gallegos-Infante, J.A.; Valdez-Fragoso, A.; Rocha-Guzmán, N.E.; González-Laredo, R.F.; Rodríguez-Ramírez, A.; Gamboa-Gómez, C.I.; Moreno-Jiménez, M.R. Comparative study of phenolic profile and content in infusions and concentrated infusions of buddleja scordioides treated by high-intensity pulsed electric fields (Hipef). Beverages 2018, 4, 81. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, J.W.; Chen, Y.W.; Li, S.P. Advanced phytochemical analysis of herbal tea in China. J. Chromatogr. A 2013, 1313, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Pham, H.N.T.; Negus, C. From Herbal Teabag to Infusion—Impact of Brewing on Polyphenols and Antioxidant Capacity. Beverages 2022, 8, 81. [Google Scholar] [CrossRef]
- Cota-Sánchez, J.H. Nutritional Composition of the Prickly Pear (Opuntia ficus-indica) Fruit; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124081178. [Google Scholar]
- Feugang, J.M.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and medicinal use of Cactus pear (Opuntia spp.) cladodes and fruits. Front. Biosci. 2006, 11, 2574–2589. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.G. Chemical Composition and Functional Properties of Prickly Pear (Opuntia ficus-indica) Seeds Flour and Protein Concentrate. World J. Dairy Food Sci. 2008, 3, 11–16. [Google Scholar]
- Jiménez-Aguilar, D.M.; López-Martínez, J.M.; Hernández-Brenes, C.; Gutiérrez-Uribe, J.A.; Welti-Chanes, J. Dietary fiber, phytochemical composition and antioxidant activity of Mexican commercial varieties of cactus pear. J. Food Compos. Anal. 2015, 41, 66–73. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.P.; Semedo, C.; Serra, T.; Duarte, C.M.M.; Bronze, M.R. Contribution to the characterization of Opuntia spp. juices by LC-DAD-ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef]
- El-Said, N.M.; Ashraf, I.N.; Sahar, A.R.; Deraz, S.F. Prickly pear [Opuntia ficus-indica (L.) Mill] peels: Chemical composition, nutritional value and protective effects on liver and kidney functions and cholesterol in rats. Funct. Plant Sci. Biotecnol. 2011, 5, 30–35. [Google Scholar]
- Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M.; Koubaa, M. Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: From preservation of beverages to valorization of by-products. Trends Food Sci. Technol. 2017, 67, 260–270. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Escobedo-Avellaneda, Z.; Martín-Belloso, O.; Gutiérrez-Uribe, J.; Valdez-Fragoso, A.; García-García, R.; Torres, J.A.; Welti-Chanes, J. Effect of High Hydrostatic Pressure on the Content of Phytochemical Compounds and Antioxidant Activity of Prickly Pears (Opuntia ficus-indica) Beverages. Food Eng. Rev. 2015, 7, 198–208. [Google Scholar] [CrossRef]
- García-García, R.; Escobedo-Avellaneda, Z.; Tejada-Ortigoza, V.; Martín-Belloso, O.; Valdez-Fragoso, A.; Welti-Chanes, J. Hurdle technology applied to prickly pear beverages for inhibiting Saccharomyces cerevisiae and Escherichia coli. Lett. Appl. Microbiol. 2015, 60, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.M.; Sallam, E.M. Utilization of Prickly Pear Peels to Improve Quality of Pan Bread. Arab J. Nucl. Sci. Appl. 2016, 42, 151–163. [Google Scholar]
- Durance, T. Handbook of Food Preservation. Food Res. Int. 2002, 35, 409. [Google Scholar] [CrossRef]
- Talebzadeh, S.L.; Fatemi, H.; Azizi, M.; Kaveh, M.; Salavati Nik, A.; Szymanek, M.; Kulig, R. Interaction of Different Drying Methods and Storage on Appearance, Surface Structure, Energy, and Quality of Berberis vulgaris var. asperma. Foods 2022, 11, 3003. [Google Scholar] [CrossRef] [PubMed]
- MicroDried: Fruits & Vegetables. Available online: https://microdried.com/ (accessed on 11 September 2023).
- ENWAVE. Available online: https://www.enwave.net/ (accessed on 11 September 2023).
- Omari, A.; Behroozi-Khazaei, N.; Sharifian, F. Drying kinetic and artificial neural network modeling of mushroom drying process in microwave-hot air dryer. J. Food Process Eng. 2018, 41, e12849. [Google Scholar] [CrossRef]
- Mbondo, N.N.; Owino, W.O.; Ambuko, J.; Sila, D.N. Effect of drying methods on the retention of bioactive compounds in African eggplant. Food Sci. Nutr. 2018, 6, 814–823. [Google Scholar] [CrossRef]
- Gąsecka, M.; Siwulski, M.; Magdziak, Z.; Budzyńska, S.; Stuper-Szablewska, K.; Niedzielski, P.; Mleczek, M. The effect of drying temperature on bioactive compounds and antioxidant activity of Leccinum scabrum (Bull.) Gray and Hericium erinaceus (Bull.) Pers. J. Food Sci. Technol. 2020, 57, 513–525. [Google Scholar] [CrossRef]
- Nikniaz, Z.; Mahdavi, R.; Ghaemmaghami, S.J.; Lotfi Yagin, N.; Nikniaz, L. Effect of different brewing times on antioxidant activity and polyphenol content of loosely packed and bagged black teas (Camellia sinensis L.). Avicenna J. Phytomed. 2016, 6, 313–321. [Google Scholar]
- Sharpe, E.; Hua, F.; Schuckers, S.; Andreescu, S.; Bradley, R. Effects of brewing conditions on the antioxidant capacity of twenty-four commercial green tea varieties. Food Chem. 2016, 192, 380–387. [Google Scholar] [CrossRef]
- Gouws, C.A.; D’Cunha, N.M.; Georgousopoulou, E.N.; Mellor, D.D.; Naumovski, N. The effect of different drying techniques on phytochemical content and in vitro antioxidant properties of Australian-grown prickly pears (Opuntia ficus-indica). J. Food Process. Preserv. 2019, 43, e13900. [Google Scholar] [CrossRef]
- Patlán, J.; Gomez, J.; Ceron, A.; Sosa, M. Moisture diffusivity during hot-air drying of red prickly pear peel (Opuntia streptacantha). In Proceedings of the CSBE/SCGAB 2017 Annual Conference, Winnipeg, MB, Canada, 6–10 August 2017. [Google Scholar]
- Ammar, I.; Ennouri, M.; Bouaziz, M.; Ben Amira, A.; Attia, H. Phenolic Profiles, Phytchemicals and Mineral Content of Decoction and Infusion of Opuntia ficus-indica Flowers. Plant Foods Hum. Nutr. 2015, 70, 388–394. [Google Scholar] [CrossRef]
- Amrane-Abider, M.; Nerín, C.; Tamendjari, A.; Serralheiro, M.L.M. Phenolic composition, antioxidant and antiacetylcholinesterase activities of Opuntia ficus-indica peel and flower teas after in vitro gastrointestinal digestion. J. Sci. Food Agric. 2022, 102, 4401–4409. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Amaral, R.A.; Silva, A.M.S.; Cardoso, S.M.; Saraiva, J.A. Effect of high pressure and thermal pasteurization on microbial and physico-chemical properties of Opuntia ficus-indica juices. Beverages 2022, 8, 84. [Google Scholar] [CrossRef]
- Marçal, C.; Pinto, C.A.; Silva, A.M.S.; Monteiro, C.; Saraiva, J.A.; Cardoso, S.M. Macroalgae-fortified sausages: Nutritional and quality aspects influenced by non-thermal high-pressure processing. Foods 2021, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Yeddes, N.; Chérif, J.K.; Guyot, S.; Sotin, H.; Ayadi, M.T. Comparative study of antioxidant power, polyphenols, flavonoids and betacyanins of the peel and pulp of three Tunisian Opuntia forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Queffelec, J.; Flórez-Fernández, N.; Saraiva, J.A.; Torres, M.D.; Cardoso, S.M.; Domínguez, H. Production of betalain-rich Opuntia ficus-indica peel flour microparticles using spray-dryer: A holist approach. Lebensm.-Wiss. Technol. 2023, 186, 115241. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Costa, A.M.; Pinto, C.A.; Cardoso, S.M.; Silva, A.M.S.; Saraiva, J.A. Impact of Fermentation and Pasteurization on the Physico-Chemical and Phytochemical Composition of Opuntia ficus-indica Juices. Foods 2023, 12, 2096. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Monteiro, F.; Passos, C.P.; Silva, A.M.S.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Blanching impact on pigments, glucosinolates, and phenolics of dehydrated broccoli by-products. Food Res. Int. 2020, 132, 109055. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva, A.; Cruz, M.T.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins from Fucus vesiculosus: Modulation of inflammatory response by blocking nf-κb signaling pathway. Int. J. Mol. Sci. 2020, 21, 6897. [Google Scholar] [CrossRef] [PubMed]
- MetaboAnalyst. Available online: https://www.metaboanalyst.ca/ (accessed on 24 October 2023).
- Bouazizi, S.; Montevecchi, G.; Antonelli, A.; Hamdi, M. Effects of prickly pear (Opuntia ficus-indica L.) peel flour as an innovative ingredient in biscuits formulation. Lebensm.-Wiss. Technol. 2020, 124, 109155. [Google Scholar] [CrossRef]
- Parafati, L.; Restuccia, C.; Palmeri, R.; Fallico, B.; Arena, E. Characterization of prickly pear peel flour as a bioactive and functional ingredient in bread preparation. Foods 2020, 9, 1189. [Google Scholar] [CrossRef]
- Albergamo, A.; Potortí, A.G.; Di Bella, G.; Amor, N.B.; Lo Vecchio, G.; Nava, V.; Rando, R.; Ben Mansour, H.; Turco, V. Lo Chemical Characterization of Different Products from the Tunisian Opuntia ficus-indica (L.) Mill. Foods 2022, 11, 155. [Google Scholar] [CrossRef]
- Catarino, S.; Madeira, M.; Monteiro, F.; Caldeira, I.; de Sousa, R.B.; Curvelo-Garcia, A. Mineral composition through soil-wine system of portuguese vineyards and its potential for wine traceability. Beverages 2018, 4, 85. [Google Scholar] [CrossRef]
- Erdem, T.; Karaaslan, S.; Öztekin, S.; Zeynep, Ş.; Çiftçi, H. Microwave Drying of Orange Peels and Its Mathematical Models. Tarım Makinaları Bilim. Derg. 2014, 10, 329–333. [Google Scholar]
- Ghanem, N.; Mihoubi, D.; Kechaou, N.; Mihoubi, N.B. Microwave dehydration of three citrus peel cultivars: Effect on water and oil retention capacities, color, shrinkage and total phenols content. Ind. Crops Prod. 2012, 40, 167–177. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Chuyen, H. Van Processing of herbal tea from roselle (Hibiscus sabdariffa L.): Effects of drying temperature and brewing conditions on total soluble solid, phenolic content, antioxidant capacity and sensory quality. Beverages 2020, 6, 2. [Google Scholar] [CrossRef]
- Dryden, I.G.C. (Ed.) Drying, conditioning and industrial space heating. In The Efficient Use of Energy; Butterworth-Heinemann: Oxford, UK, 1982; pp. 166–198. ISBN 9780408012508. [Google Scholar]
- Chong, K.L.; Lim, Y.Y. Effects of drying on the antioxidant properties of herbal tea from selected Vitex species. J. Food Qual. 2012, 35, 51–59. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Mujumdar, A.S.; Wang, S. Trends in microwave-related drying of fruits and vegetables. Trends Food Sci. Technol. 2006, 17, 524–534. [Google Scholar] [CrossRef]
- Wojdyło, A.; Lech, K.; Nowicka, P.; Hernandez, F.; Figiel, A.; Carbonell-barrachina, A.A. Influence of Different Drying Techniques on Phenolic Compounds, Antioxidant Capacity and Colour of Ziziphus jujube Mill. Fruits. Molecules 2019, 24, 2361. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Dróżdż, P.; Biesaga, M.; Pyrzynska, K. Evaluation of the antioxidant properties of fruit and flavoured black teas. Eur. J. Nutr. 2011, 50, 681–688. [Google Scholar] [CrossRef]
- Pavun, L.; Uskoković-Marković, S.; Jelikić-Stankov, M.; Dikanović, D.; Durdević, P. Determination of flavonoids and total polyphenol contents in commercial apple juices. Czech J. Food Sci. 2018, 36, 233–238. [Google Scholar] [CrossRef]
- Sawicki, T.; Wiczkowski, W. The effects of boiling and fermentation on betalain profiles and antioxidant capacities of red beetroot products. Food Chem. 2018, 259, 292–303. [Google Scholar] [CrossRef]
- Chavez-Santoscoy, R.A.; Gutierrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic composition, antioxidant capacity and in vitro cancer cell cytotoxicity of nine prickly pear (Opuntia spp.) juices. Plant Foods Hum. Nutr. 2009, 64, 146–152. [Google Scholar] [CrossRef]
- Felker, P.; Stintzing, F.C.; Müssig, E.; Leitenberger, M.; Carle, R.; Vogt, T.; Bunch, R. Colour inheritance in cactus pear (Opuntia ficus-indica) fruits. Ann. Appl. Biol. 2008, 152, 307–318. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E.; Ballard, T.; Liceaga, A.; San Martín-González, M.F. Microwave-assisted extraction of betalains from red beet (Beta vulgaris). Lebensm.-Wiss. Technol. 2014, 59, 276–282. [Google Scholar] [CrossRef]
- Gokhale, S.V.; Lele, S.S. Betalain Content and Antioxidant Activity of Beta vulgaris: Effect of Hot Air Convective Drying and Storage. J. Food Process. Preserv. 2014, 38, 585–590. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation—Structural and chromatic aspects. J. Food Sci. 2006, 71, 41–50. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Protoggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an imporved ABTS radical cation decolorization assay. Free 1999, 26, 1231–1237. [Google Scholar]
- Yi, W.; Wetzstein, H.Y. Effects of drying and extraction conditions on the biochemical activity of selected herbs. HortScience 2011, 46, 70–73. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Izuegbuna, O.; Otunola, G.; Bradley, G. Chemical composition, antioxidant, antiinflammatory, and cytotoxic activities of Opuntia stricta cladodes. PLoS ONE 2019, 14, e0209682. [Google Scholar] [CrossRef]
- Cho, J.Y.; Park, S.-C.; Kim, T.-W.; Kim, K.-S.; Song, J.-C.; Lee, H.-M.; Sung, H.-J.; Rhee, M.-H.; Kim, S.-K.; Park, H.-J.; et al. Radical scavenging and anti-inflammatory activity of extracts from Opuntia humifusa Raf. J. Pharm. Pharmacol. 2010, 58, 113–119. [Google Scholar] [CrossRef]
- Sirichaiwetchakoon, K.; Lowe, G.M.; Eumkeb, G. The Free Radical Scavenging and Anti-Isolated Human LDL Oxidation Activities of Pluchea indica (L.) Less. Tea Compared to Green Tea (Camellia sinensis). Biomed Res. Int. 2020, 2020, 4183643. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, H.; Zhang, M.; Li, C.J.; Lin, X.Z.; Sheng, J.; Shi, W. Variations of antioxidant properties and NO scavenging abilities during fermentation of tea. Int. J. Mol. Sci. 2011, 12, 4574–4590. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Yokozawa, T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem. Toxicol. 2002, 40, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Ielciu, I.; Sevastre, B.; Olah, N.K.; Turdean, A.; Chișe, E.; Marica, R.; Oniga, I.; Uifălean, A.; Sevastre-Berghian, A.C.; Niculae, M.; et al. Evaluation of hepatoprotective activity and oxidative stress reduction of Rosmarinus officinalis L. Shoots tincture in rats with experimentally induced hepatotoxicity. Molecules 2021, 26, 1737. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Longhi-Balbinot, D.T.; Zarpelon, A.C.; Staurengo-Ferrari, L.; Baracat, M.M.; Georgetti, S.R.; Sassonia, R.C.; Verri, W.A.; Casagrande, R. Anti-inflammatory activity of betalain-rich dye of Beta vulgaris: Effect on edema, leukocyte recruitment, superoxide anion and cytokine production. Arch. Pharm. Res. 2015, 38, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.V.T.; Pereira, A.D.; Boaventura, G.T.; Ribeiro, R.S.D.A.; Verícimo, M.A.; De Carvalho-Pinto, C.E.; Baião, D.D.S.; Del Aguila, E.M.; Paschoalin, V.M.F. Short-term betanin intake reduces oxidative stress in wistar rats. Nutrients 2019, 11, 1978. [Google Scholar] [CrossRef]
- Bassama, J.; Tamba, A.; Ndong, M.; Sarr, K.D.D.; Cissé, M. Degradation kinetics of betacyanins during the pasteurization and storage of cactus pear (Opuntia dillenii haw.) juice using the arrhenius, eyring, and ball models. Beverages 2021, 7, 2. [Google Scholar] [CrossRef]
- Giraldo-Silva, L.; Ferreira, B.; Rosa, E.; Dias, A.C.P. Opuntia ficus-indica Fruit: A Systematic Review of Its Phytochemicals and Pharmacological Activities. Plants 2023, 12, 543. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Giménez, R.; Rufián-Henares, J.A.; Pastoriza, S. Effect of brewing time and temperature on antioxidant capacity and phenols of white tea: Relationship with sensory properties. Food Chem. 2018, 248, 111–118. [Google Scholar] [CrossRef]
- Su, X.; Duan, J.; Jiang, Y.; Duan, X.; Chen, F. Polyphenolic profile and antioxidant activities of oolong tea infusion under various steeping conditions. Int. J. Mol. Sci. 2007, 8, 1196–1205. [Google Scholar] [CrossRef]
- Uthai, N. Effects of temperature, steeping time and particle size used in infusion brewing on total phenolic content and antioxidant activity of tea produced from young upland rice leaves. Afr. J. Food Agric. Nutr. Dev. 2021, 21, 17477–17491. [Google Scholar] [CrossRef]
- Hajiaghaalipour, F.; Sanusi, J.; Kanthimathi, M.S. Temperature and Time of Steeping Affect the Antioxidant Properties of White, Green, and Black Tea Infusions. J. Food Sci. 2016, 81, H246–H254. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).