Influence of the Biotechnological Process of Mezcal Fermentation on Yeast Diversity in Four palenques of Oaxaca, Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling, Isolation, and Propagation of Yeast
2.2. DNA Extraction and PCR Amplification
2.3. Phylogenetic Analysis
2.4. Multivariable Analysis of Biotechnological Variable Process
3. Results
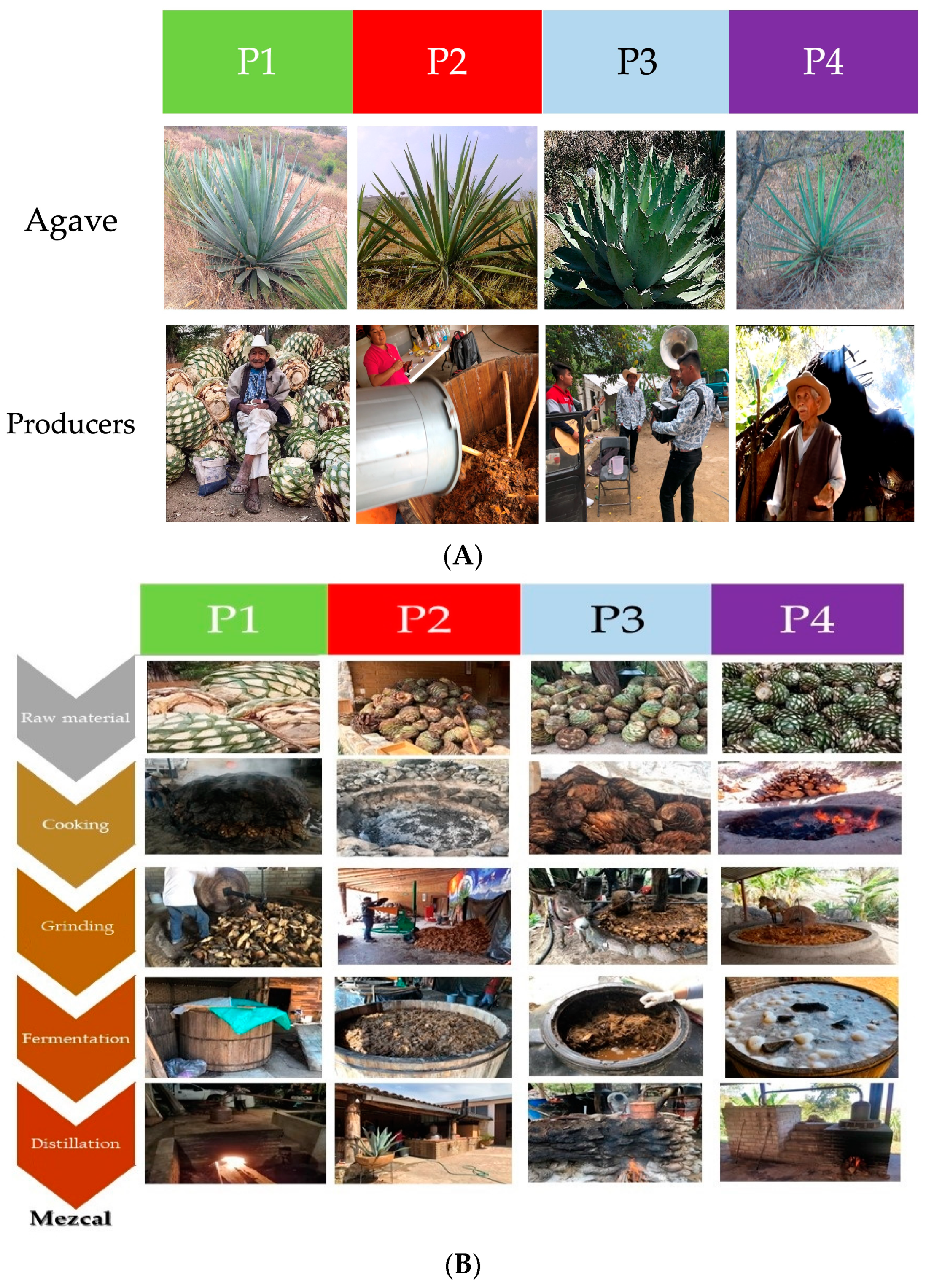
3.1. Study Site
3.2. Mezcal Production Process
3.2.1. The Agave spp.
3.2.2. The Mezcaleros
3.2.3. Treatment to Raw Material
3.2.4. High-Temperature Hydrolysis of Agave spp. Polymers (Cooking)
3.2.5. Shredding and Fermentation Steps
3.2.6. Distillation
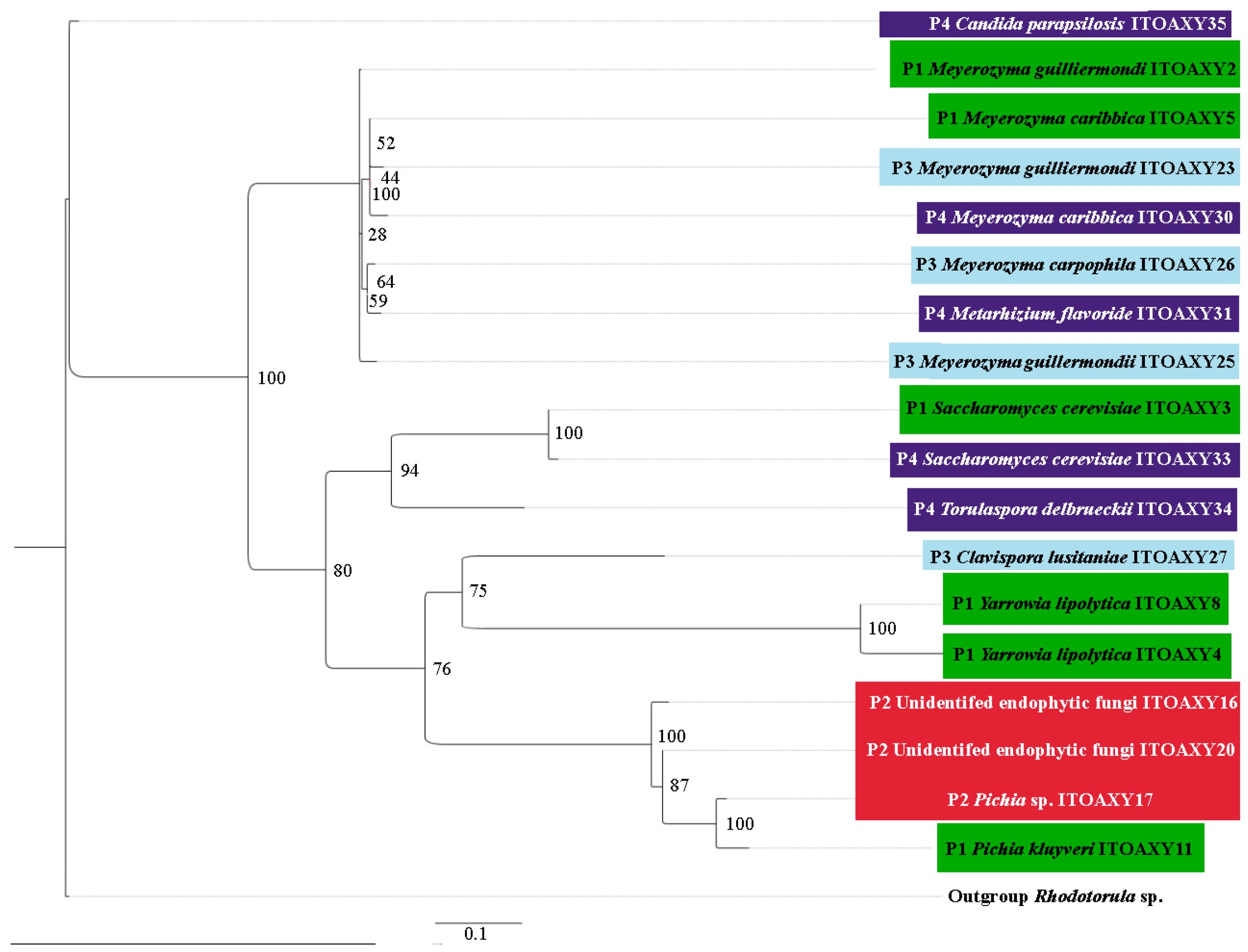
3.3. Yeast Diversity in Four Palenques for Mezcal Production in Oaxaca, Mexico
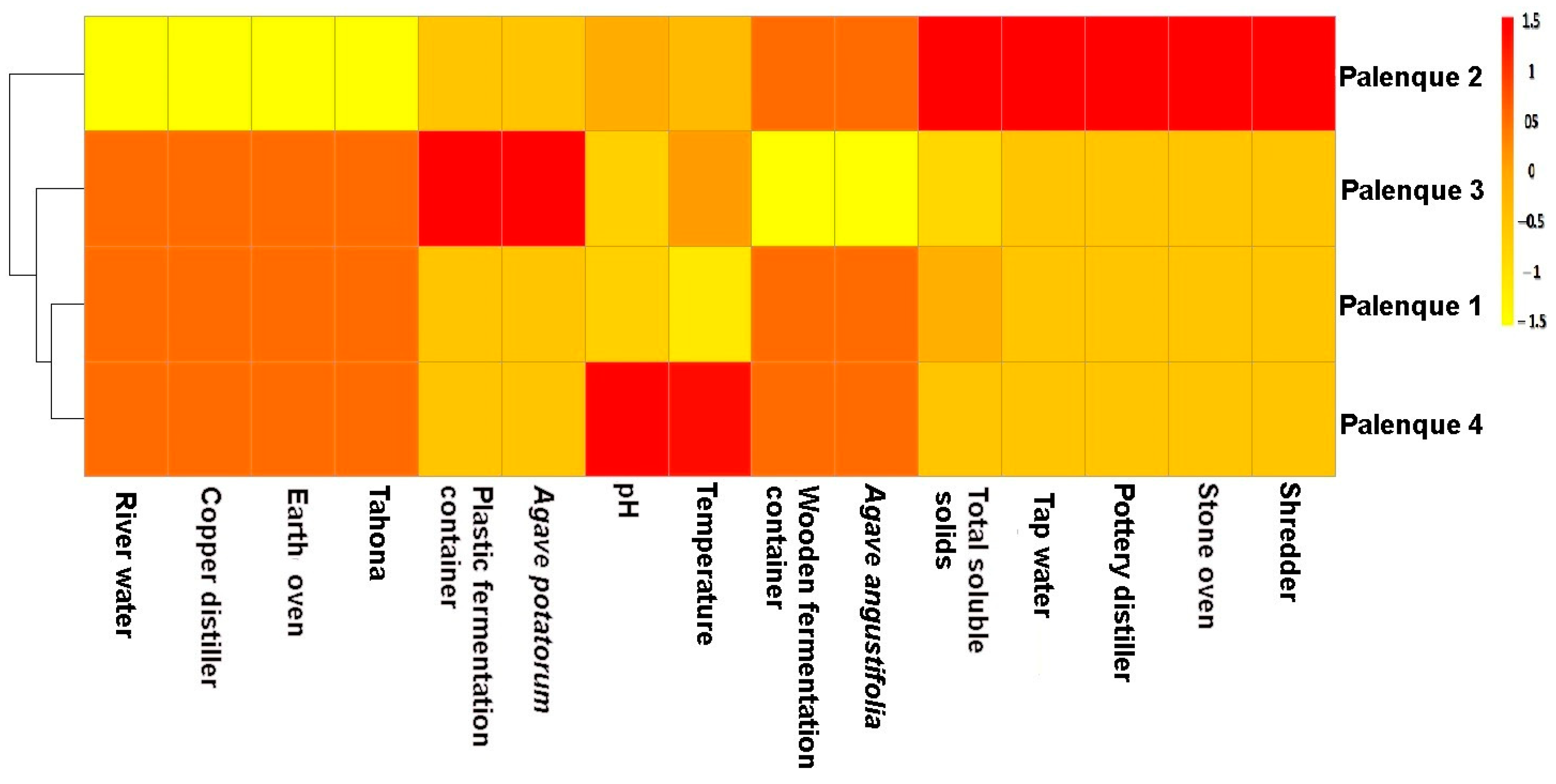
3.4. Relation between Yeast Diversity and the Biotechnological Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Secretaria de Gobernacion. Denominación de origen Mezcal. D. Of. Fed. 1994, 27–30. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=4768551&fecha=28/11/1994#gsc.tab=0 (accessed on 12 June 2023).
- Gentry, H.S. Agaves of Continental North America; University of Arizona Press: Tucson, AZ, USA, 1982. [Google Scholar]
- Secretaría de Economia. NOM-070-SCFI-2016 Bebidas alcohólicas-Mezcal-Especificaciones. 2016. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5472787&fecha=23/02/2017#gsc.tab=0 (accessed on 12 June 2023).
- Molina-Guerrero, J.A.; Botello-Álvarez; Estrada-Baltazar, J.E. Volatile components in mezcal. Rev. Mex. Ing. Quim. 2007, 6, 41–50. Available online: http://www.redalyc.org/articulo.oa?id=62060106 (accessed on 2 February 2023).
- Vera Guzmán, A.M.; Santiago García, P.A.; López, M.G. Compuestos volátiles aromáticos generados durante la elaboración de mezcal de Agave angustifolia y Agave potatorum. Rev. Fitotec. Mex. 2009, 32, 273–279. [Google Scholar] [CrossRef]
- Escalante-Minakata, P.; Blaschek, H.P.; De La Rosa, A.P.B.; Santos, L.; De León-Rodríguez, A. Identification of yeast and bacteria involved in the mezcal fermentation of Agave salmiana. Lett. Appl. Microbiol. 2008, 46, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Lappe-Oliveras, P.; Moreno-Terrazas, R.; Arrizón-Gaviño, J.; Herrera-Suárez, T.; García-Mendoza, A.; Gschaedler-Mathis, A. Yeasts associated with the production of Mexican alcoholic non-distilled and distilled Agave beverages. In FEMS Yeast Research; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; pp. 1037–1052. [Google Scholar] [CrossRef]
- Gallegos-Casillas, P.; García-Ortega, L.F.; Espinosa-Cantú, A.; Torres-Lagunes, C.G.; Avelar-Rivas, J.A.; Cano-Ricárdez, A.; García-Acero, Á.M.; Ruiz-Castro, S.; Flores-Barraza, M.; Castillo, A. Yeast diversity in open agave fermentations across Mexico. bioRxiv 2023. [Google Scholar] [CrossRef]
- Nolasco-Cancino, H.; Santiago-Urbina, J.A.; Wacher, C.; Ruíz-Teran, F. Predominant yeasts during artisanal mezcal fermentation and their capacity to ferment maguey juice. Front. Microbiol. 2018, 9, 2900. [Google Scholar] [CrossRef]
- Casas Acevedo, A.; Aguilar González, C.N.; De la Garza-Toledo, H.; Morlett-Chavez, J.A.; Montet, D.; Rodríguez-Herrera, R. Importancia de las Levaduras no Saccharomyces Durante la Fermentación de Bebidas Alcoholicas. Investig. Cienc. 2015, 23, 73–79. Available online: http://www.redalyc.org/articulo.oa?id=67443217010 (accessed on 8 March 2023).
- Suarez-Machín, C.; Garrido-Carralero, N.A.; Guevara-Rodriguez, C.A. Levadura Saccharomyces cerevisiae y la Producción de Alcohol. ICIDCA. Sobre Los Deriv. Caña 2016, 50, 20–28. Available online: http://www.redalyc.org/articulo.oa?id=223148420004 (accessed on 8 March 2023).
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef]
- Altschup, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1.3.1. Institute of Evolutionary Biology; University of Edinburgh: Edinburgh, UK, 2010; Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 20 June 2023).
- Group. GIMP 2.8.10. Available online: www.gimp.org (accessed on 8 July 2023).
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 8 July 2023).
- Kassambara, A. Practical Guide to Principal Component Methods in R. Available online: http://www.sthda.com (accessed on 8 July 2023).
- R Core Team. Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Garcia-Mendoza, A. Los Agaves de México. Ciencias 2007, 87, 14–23. [Google Scholar]
- Colunga-GarcíaMarín, P.; Larqué Saavedra, A.; Eguiarte, L.E.; Zizumbo-Villarreal, D. (Eds.) En lo Ancestral Hay Futuro: Del Tequila, los Mezcales y Otros Agaves; Centro de Investigación Científica de Yucatán: Mérida, Mexico, 2007. [Google Scholar]
- Márquez-López, R.E.; Santiago-García, P.A.; López, M.G. Agave Fructans in Oaxaca’s Emblematic Specimens: Agave angustifolia Haw. and Agave potatorum Zucc. Plants 2022, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Zizumbo-Villarreal, D.; Colunga-García Marín, P. Early coconut distillation and the origins of mezcal and tequila spirits in west-central Mexico. Genet. Resour. Crop Evol. 2008, 55, 493–510. [Google Scholar] [CrossRef]
- López, J.d.J.H. El mezcal como patrimonio social: De indicaciones geográficas genéricas a denominaciones de origen regionales. Em Questão 2018, 24, 404–433. [Google Scholar] [CrossRef]
- De León-Rodríguez, A.; Escalante-Minakata, P.; de la Rosa, A.P.B.; Blaschek, H.P. Optimization of fermentation conditions for the production of the mezcal from Agave salmiana using response surface methodology. Chem. Eng. Process. Process Intensif. 2008, 47, 76–82. [Google Scholar] [CrossRef]
- Peña-Alvarez, A.; Díaz, L.; Medina, A.; Labastida, C.; Capella, S.; Vera, L.E. Characterization of three Agave species by gas chromatography and solid-phase microextraction-gas chromatography-mass spectrometry. J. Chromatogr. A 2004, 1027, 131–136. [Google Scholar] [CrossRef]
- Wang, J.; Yan, C.; Ma, C.; Huang, S.; Chang, X.; Li, Z.; Chen, X.; Li, X. Effects of two kinds of Bacillus on flavor formation of Baijiu solid-state fermentation with pure mixed bacteria. Int. J. Food Sci. Technol. 2023, 58, 1250–1262. [Google Scholar] [CrossRef]
- Black, S.L.; Thoms, A.V. Hunter-gatherer earth ovens in the archaeological record: Fundamental concepts. Am. Antiq. 2014, 79, 203–226. Available online: http://saa.metapress.com (accessed on 8 July 2023). [CrossRef]
- Ma, M.; Liu, Z.L. Mechanisms of ethanol tolerance in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2010, 87, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-E.; Lee, K.-S.; Yu, B.J.; Sung, Y.-J.; Park, S.M.; Koo, H.M.; Kweon, D.-H.; Park, J.C.; Jin, Y.-S. Identification of gene targets eliciting improved alcohol tolerance in Saccharomyces cerevisiae through inverse metabolic engineering. J. Biotechnol. 2010, 149, 52–59. [Google Scholar] [CrossRef]
- Hoang, D.; Kopp, A.; Chandler, J.A. Interactions between Drosophila and its natural yeast symbionts-Is Saccharomyces cerevisiae a good model for studying the fly-yeast relationship? PeerJ 2015, 3, e1116. [Google Scholar] [CrossRef] [PubMed]
- Lachance, M.A. Yeast communities in a natural tequila fermentation. Antonie Van Leeuwenhoek 1995, 68, 151–160. [Google Scholar] [CrossRef]
- López, M.G. Tequila Aroma. In Flavor Chemistry of Ethnic Foods; Springer: Boston, MA, USA, 1999; pp. 211–217. [Google Scholar] [CrossRef]
- Arrizón, J.; Arizaga, J.J.; Hernandez, R.E.; Estarrón, M.; Gschaedler, A. Production of volatile compounds in Tequila and Raicilla musts by different yeasts isolated from Mexican agave beverages. In ACS Symposium Series; Oxford University Press: Oxford, UK, 2007; pp. 167–177. [Google Scholar] [CrossRef]
- Chadha, A.; Venkataraman, S.; Preetha, R.; Padhi, S.K. Candida parapsilosis: A versatile biocatalyst for organic oxidation-reduction reactions. Bioorg. Chem. 2016, 68, 187–213. [Google Scholar] [CrossRef]
- Perna, M.S.C.; Bastos, R.G.; Ceccato-Antonini, S.R. Single and combined effects of acetic acid, furfural, and sugars on the growth of the pentose-fermenting yeast Meyerozyma guilliermondii. 3 Biotech 2018, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Brito, D.; Magaña-Alvarez, A.; Lappe-Oliveras, P.; Cortes-Velazquez, A.; Torres-Calzada, C.; Herrera-Suarez, T.; Larqué-Saavedra, A.; Tapia-Tussell, R. Genetic diversity of Clavispora lusitaniae isolated from Agave fourcroydes Lem, as revealed by DNA fingerprinting. J. Microbiol. 2015, 53, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Bolaños, J.L.; Serrato-Joya, O.; Chávez-Mireles, H.; Vicente-Magueyal, F.J.; Jiménez-Islas, H. A validated strategy to design efficient fermentation-industrial processes: Agave spirit production. Bioprocess Biosyst. Eng. 2021, 44, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Wyder, M.T.; Arias-Roth, E.; Jakob, E. Cheese yeasts. Yeast 2019, 36, 129–141. [Google Scholar] [CrossRef]
- Valdez, A.V.; Garcia, L.S.; Kirchmayr, M.; Rodríguez, P.R.; Esquinca, A.G.; Coria, R.; Mathis, A.G. Yeast communities associated with artisanal mezcal fermentations from Agave salmiana. Antonie Van Leeuwenhoek 2011, 100, 497–506. [Google Scholar] [CrossRef]
- Páez-Lerma, J.B.; Arias-García, A.; Rutiaga-Quiñones, O.M.; Barrio, E.; Soto-Cruz, N.O. Yeasts Isolated from the Alcoholic Fermentation of Agave duranguensis During Mezcal Production. Food Biotechnol. 2013, 27, 342–356. [Google Scholar] [CrossRef]
- Kirchmayr, M.R.; Segura-García, L.E.; Lappe-Oliveras, P.; Moreno-Terrazas, R.; de la Rosa, M.; Mathis, A.G. Impact of environmental conditions and process modifications on microbial diversity, fermentation efficiency and chemical profile during the fermentation of Mezcal in Oaxaca. LWT 2017, 79, 160–169. [Google Scholar] [CrossRef]
- Benn, S.M.; Peppard, T.L. Characterization of tequila flavor by instrumental and sensory analysis. J. Agric. Food Chem. 1996, 44, 557–566. [Google Scholar] [CrossRef]
- De León-Rodríguez, A.; González-Hernández, L.; Barba de la Rosa, A.; Escalante-Minakata, P.; López, M. Characterization of volatile compounds of mezcal, an ethnic alcoholic beverage obtained from Agave salmiana. J. Agric. Food Chem. 2006, 54, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Andrade Meneses, O.E.; Ruiz Terán, F. Study of yeast populations in a mezcal fermentation. Poster PF 17. In Proceedings of the 11th International Congress on Yeasts. Yeasts in Science and Technology, The Quest for Sustainable De-velopment, Rio de Janeiro, Brazil, 15–20 August 2004; p. 157. [Google Scholar]
- Lappe, P.; Ulloa, M.; Arce Rocha, G.; Cáceres Farfán, M.; Tapia Tussel, R.; Pérez Brito, D.; Larque, A. Isolation and identification of the mycobiota present in Agave fourcroydes. Poster PE 11. In Proceedings of the 11th International Congress on Yeasts. Yeasts in Science and Technology, The Quest for Sustainable De-velopment, Rio de Janeiro, Brazil, 15–20 August 2004; p. 175. [Google Scholar]
- Arrizon, J.; Arizaga, J.J.; Hernández, R.E.; Estarrón, M.; Gschaedler, A. Production of volatile compounds in tequila and raicilla musts by different yeasts isolated from Mexican agave beverages. In Hispanic Foods Chemistry and Flavor; Tunick, M.H., González de Mejía, E., Eds.; American Chemical Society Symposium Series 946; American Chemical Society: Washington, DC, USA, 2007; pp. 167–177. [Google Scholar]
- Martell Nevárez, M.A.; Córdova Gurrola, E.E.; López Miranda, J.; Soto Cruz, N.O.; López Pérez, M.G.; Rutiaga Quiñones, O.M. Effect of fermentation temperature on chemical composition of mescals made from Agave duranguensis juice with different native yeast genera. Afr. J. Microbiol. Res. 2011, 4, 3669–3676. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza-Martinez, V.A.; Alvarez-Gutierrez, P.E.; Palma-Cruz, F.d.J.; Enriquez-Valencia, R.; Ramirez-Lopez, M.P.; Lopez-Sanchez, C.; Vazquez-Lopez, H.G. Influence of the Biotechnological Process of Mezcal Fermentation on Yeast Diversity in Four palenques of Oaxaca, Mexico. Beverages 2023, 9, 99. https://doi.org/10.3390/beverages9040099
Espinoza-Martinez VA, Alvarez-Gutierrez PE, Palma-Cruz FdJ, Enriquez-Valencia R, Ramirez-Lopez MP, Lopez-Sanchez C, Vazquez-Lopez HG. Influence of the Biotechnological Process of Mezcal Fermentation on Yeast Diversity in Four palenques of Oaxaca, Mexico. Beverages. 2023; 9(4):99. https://doi.org/10.3390/beverages9040099
Chicago/Turabian StyleEspinoza-Martinez, Victor Adrian, Peggy Elizabeth Alvarez-Gutierrez, Felipe de Jesus Palma-Cruz, Raul Enriquez-Valencia, Marcos Pedro Ramirez-Lopez, Claudia Lopez-Sanchez, and Hector Gilberto Vazquez-Lopez. 2023. "Influence of the Biotechnological Process of Mezcal Fermentation on Yeast Diversity in Four palenques of Oaxaca, Mexico" Beverages 9, no. 4: 99. https://doi.org/10.3390/beverages9040099
APA StyleEspinoza-Martinez, V. A., Alvarez-Gutierrez, P. E., Palma-Cruz, F. d. J., Enriquez-Valencia, R., Ramirez-Lopez, M. P., Lopez-Sanchez, C., & Vazquez-Lopez, H. G. (2023). Influence of the Biotechnological Process of Mezcal Fermentation on Yeast Diversity in Four palenques of Oaxaca, Mexico. Beverages, 9(4), 99. https://doi.org/10.3390/beverages9040099







