Two Multiplex PCR Methods for Detecting Several Pathogens Associated with Feline Respiratory and Intestinal Tracts
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Bacterial Strains
2.2. Primer Design
2.3. Nucleic Acid Extraction and Reverse Transcription and Construction of Standard Plasmids
2.4. Establishment of the mPCR Methods
2.5. Specificity of the mPCR Methods
2.6. Sensitivity of mPCR Methods
2.7. Examination of Clinical Samples
3. Results
3.1. Optimum Annealing Temperature for the mPCR Methods
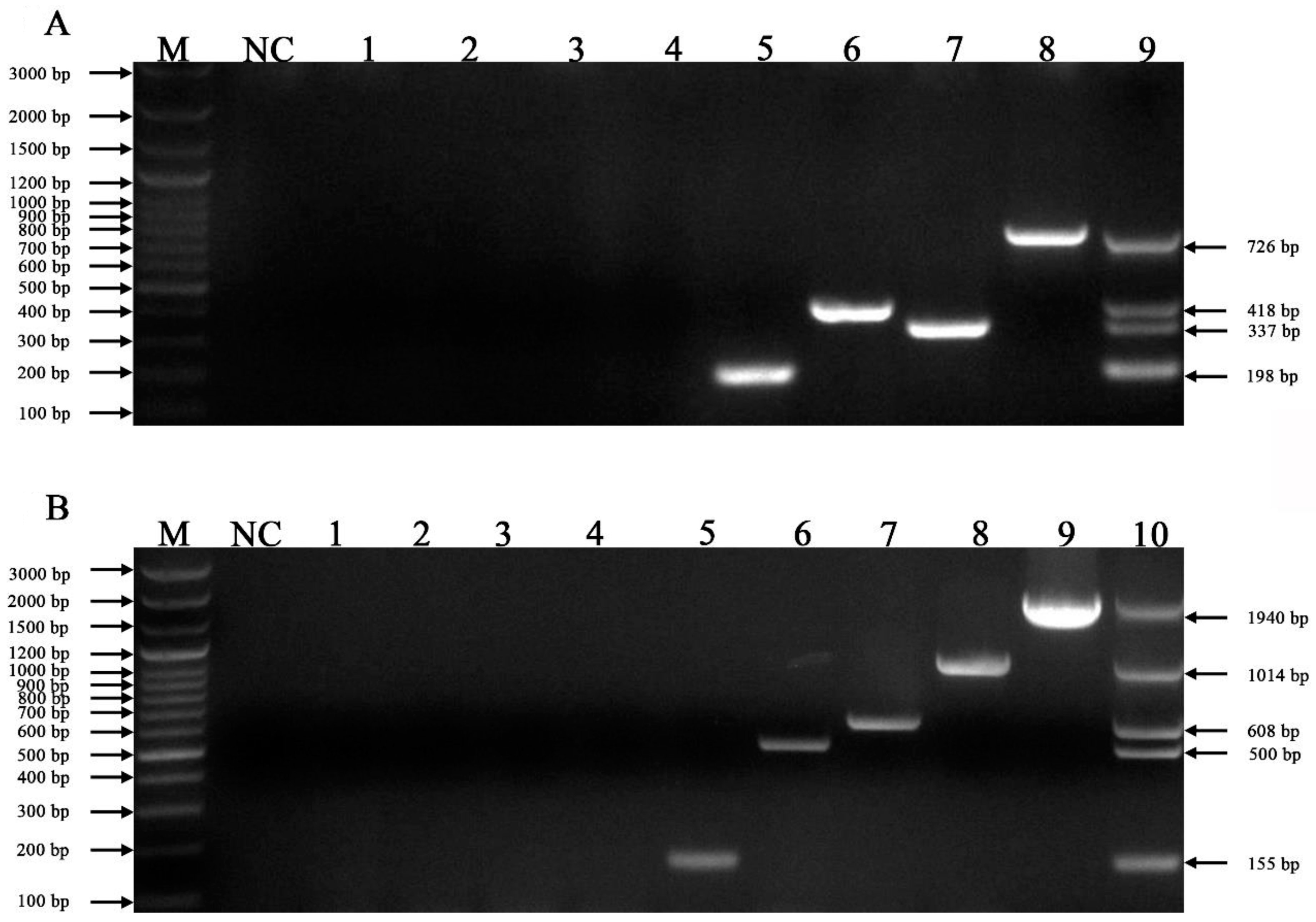
3.2. Specificity of mPCR Methods
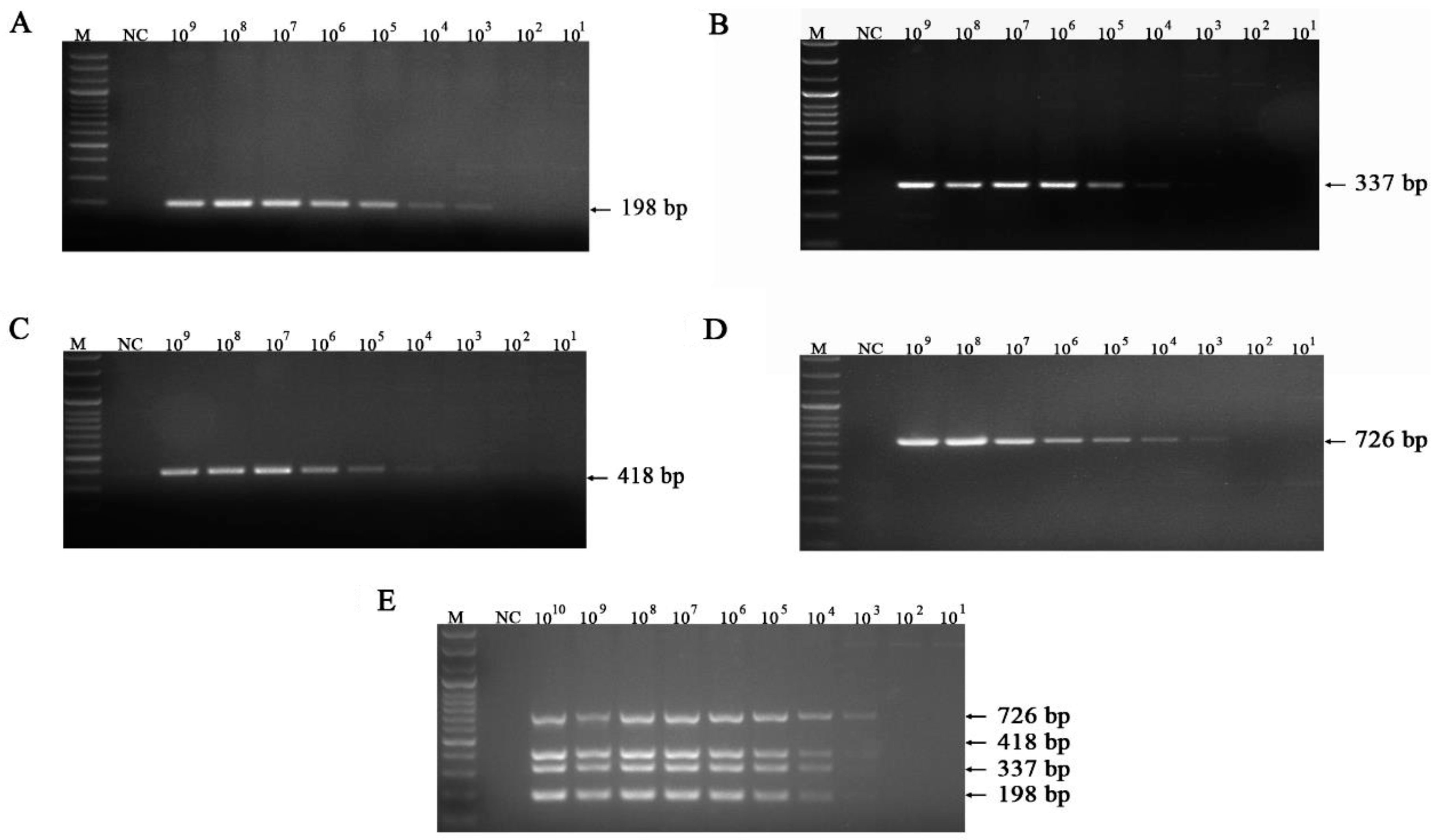
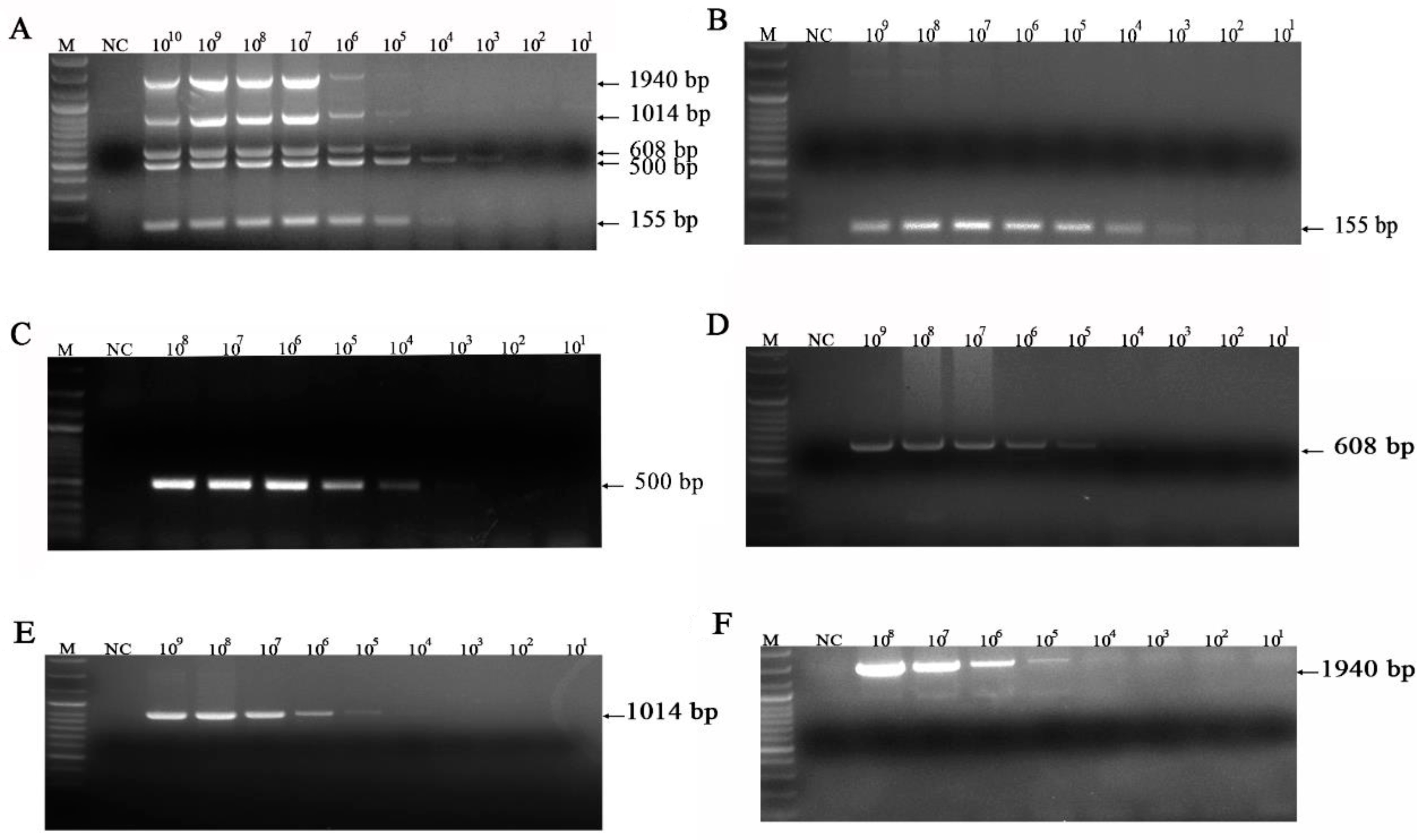
3.3. Sensitivity of the mPCR Methods
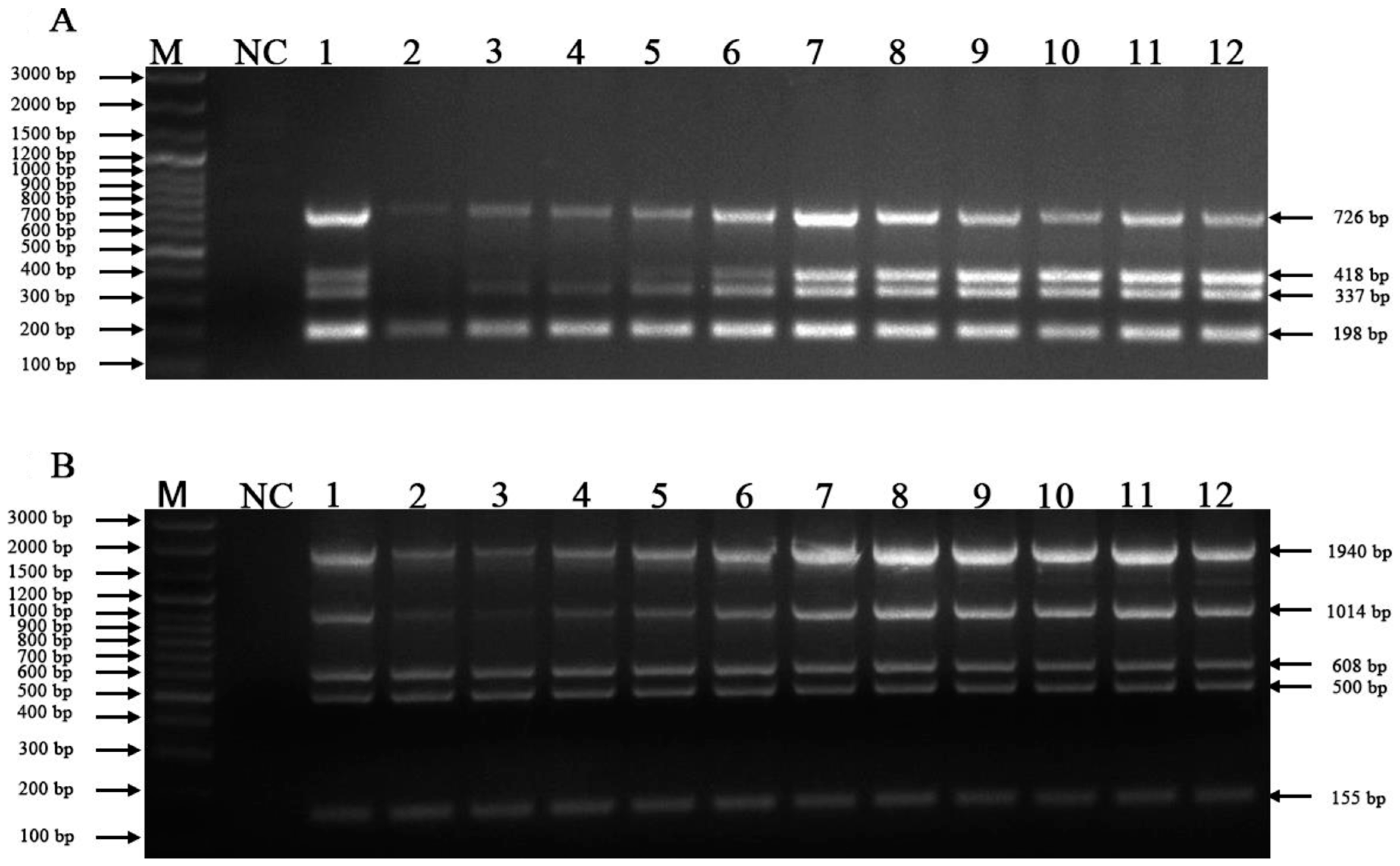
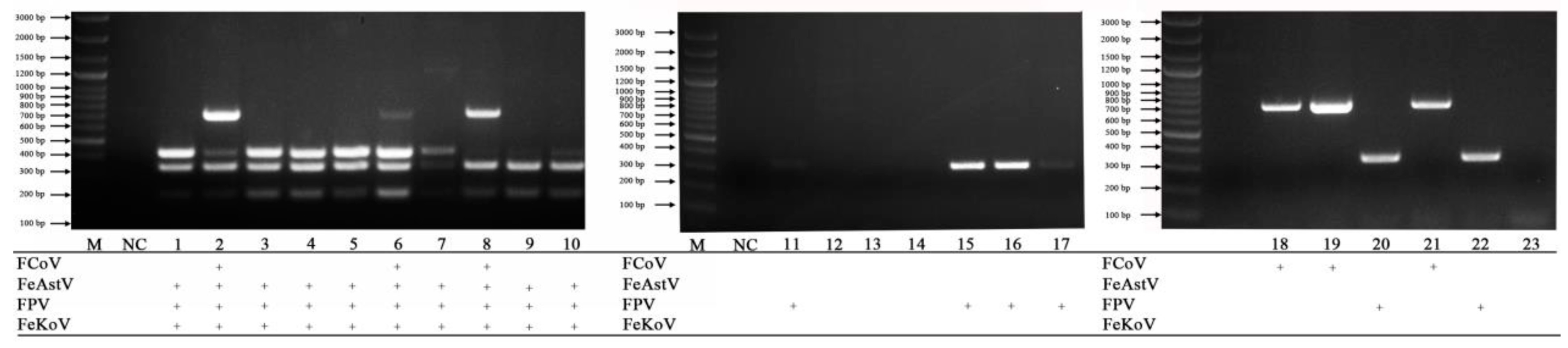
3.4. Examination of Clinical Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, D.; Barrs, V.R.; Kelman, M.; Ward, M.P. Feline upper respiratory tract infection and disease in Australia. J. Feline Med. Surg. 2019, 21, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E. Pediatric Feline Upper Respiratory Disease. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 331–342. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Hosie, M.J.; Hartmann, K.; Egberink, H.; Truyen, U.; Tasker, S.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Calicivirus Infection in Cats. Viruses 2022, 14, 937. [Google Scholar] [CrossRef] [PubMed]
- Bodle, J.E. Feline herpes virus infection. Surv. Ophthalmol. 1976, 21, 209. [Google Scholar] [CrossRef]
- Sykes, J.E. Feline Chlamydiosis. Clin. Tech. Small Anim. Pract. 2005, 20, 129–134. [Google Scholar] [CrossRef]
- Belser, J.A.; Pulit-Penaloza, J.A.; Sun, X.; Brock, N.; Pappas, C.; Creager, H.M.; Zeng, H.; Tumpey, T.M.; Maines, T.R. A Novel A(H7N2) Influenza Virus Isolated from a Veterinarian Caring for Cats in a New York City Animal Shelter Causes Mild Disease and Transmits Poorly in the Ferret Model. J. Virol. 2017, 91, e00672-17. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.; Rimmelzwaan, G.; van Riel, D.; van Amerongen, G.; Baars, M.; Fouchier, R.; Osterhaus, A. Avian H5N1 Influenza in Cats. Science 2004, 306, 241. [Google Scholar] [CrossRef] [PubMed]
- Song, D.S.; An, D.J.; Moon, H.J.; Yeom, M.J.; Jeong, H.Y.; Jeong, W.S.; Park, S.J.; Kim, H.K.; Han, S.Y.; Oh, J.S.; et al. Interspecies transmission of the canine influenza H3N2 virus to domestic cats in South Korea, 2010. J. Gen. Virol. 2011, 92, 2350–2355. [Google Scholar] [CrossRef]
- Songserm, T.; Amonsin, A.; Jam-on, R.; Sae-Heng, N.; Meemak, N.; Pariyothorn, N.; Payungporn, S.; Theamboonlers, A.; Poovorawan, Y. Avian influenza H5N1 in naturally infected domestic cat. Emerg. Infect. Dis. 2006, 12, 681–683. [Google Scholar] [CrossRef]
- Sponseller, B.A.; Strait, E.; Jergens, A.; Trujillo, J.; Harmon, K.; Koster, L.; Jenkins-Moore, M.; Killian, M.; Swenson, S.; Bender, H.; et al. Influenza A Pandemic (H1N1) 2009 Virus Infection in Domestic Cat. Emerg. Infect. Dis. 2010, 16, 534–537. [Google Scholar] [CrossRef]
- Su, S.; Wang, L.; Fu, X.; He, S.; Hong, M.; Zhou, P.; Lai, A.; Gray, G.; Li, S. Equine Influenza A(H3N8) Virus Infection in Cats. Emerg. Infect. Dis. 2014, 20, 2096. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Manzanilla, E.G.; Lloret, A.; León, M.; Thibault, J. Prevalence of feline herpesvirus-1, feline calicivirus, Chlamydophila felis and Mycoplasma felis DNA and associated risk factors in cats in Spain with upper respiratory tract disease, conjunctivitis and/or gingivostomatitis. J. Feline Med. Surg. 2017, 19, 461–469. [Google Scholar] [CrossRef] [PubMed]
- von Bomhard, W.; Polkinghorne, A.; Lu, Z.H.; Vaughan, L.; Vogtlin, A.; Zimmermann, D.R.; Spiess, B.; Pospischil, A. Detection of novel chlamydiae in cats with ocular disease. Am. J. Vet. Res. 2003, 64, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K. Clinical aspects of feline retroviruses: A review. Viruses 2012, 4, 2684–2710. [Google Scholar] [CrossRef]
- Stuetzer, B.; Hartmann, K. Feline parvovirus infection and associated diseases. Vet. J. 2014, 201, 150–155. [Google Scholar] [CrossRef]
- Hoshino, Y.; Zimmer, J.F.; Moise, N.S.; Scott, F.W. Detection of astroviruses in feces of a cat with diarrhea. Brief report. Arch. Virol. 1981, 70, 373–376. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, X.; Luo, J.; Sun, Y.; Xu, H.; Huang, J.; Ou, J.; Li, S. First report and genetic characterization of feline kobuvirus in diarrhoeic cats in China. Transbound. Emerg. Dis. 2018, 65, 1357–1363. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Boyle, J.F.; Floyd, K.; Fudge, A.; Barker, J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis. Am. J. Vet. Res. 1981, 42, 368–377. [Google Scholar]
- Hayashi, T.; Watabe, Y.; Nakayama, H.; Fujiwara, K. Enteritis due to feline infectious peritonitis virus. Nihon Juigaku Zasshi 1982, 44, 97–106. [Google Scholar] [CrossRef]
- Pedersen, N.C. An update on feline infectious peritonitis: Virology and immunopathogenesis. Vet. J. 2014, 201, 123–132. [Google Scholar] [CrossRef]
- Marshall, J.A.; Kennett, M.L.; Rodger, S.M.; Studdert, M.J.; Thompson, W.L.; Gust, I.D. Virus and virus-like particles in the faeces of cats with and without diarrhoea. Aust. Vet. J. 1987, 64, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Harbour, D.A.; Ashley, C.R.; Williams, P.D.; Gruffydd-Jones, T.J. Natural and experimental astrovirus infection of cats. Vet. Rec. 1987, 120, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Tang, Z.; Zhang, X.; Li, W.; Li, B.; Tian, Y.; Xu, D. Development and Application of a Triplex TaqMan Quantitative Real-Time PCR Assay for Simultaneous Detection of Feline Calicivirus, Feline Parvovirus, and Feline Herpesvirus 1. Front. Vet. Sci. 2022, 8, 1639. [Google Scholar] [CrossRef] [PubMed]
- Helps, C.; Reeves, N.; Egan, K.; Howard, P.; Harbour, D. Detection of Chlamydophila felis and feline herpesvirus by multiplex real-time PCR analysis. J. Clin. Microbiol. 2003, 41, 2734–2736. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, Z.; Sun, F.Y.; Guo, L.; Feng, E.; Bai, X.; Cheng, Y. Development of a triple NanoPCR method for feline calicivirus, feline panleukopenia syndrome virus, and feline herpesvirus type I virus. BMC Vet. Res. 2022, 18, 379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Niu, J.; Yi, S.; Dong, G.; Yu, D.; Guo, Y.; Huang, H.; Hu, G. Development and application of a multiplex PCR method for the simultaneous detection and differentiation of feline panleukopenia virus, feline bocavirus, and feline astrovirus. Arch. Virol. 2019, 164, 2761–2768. [Google Scholar] [CrossRef]
- Brownie, J.; Shawcross, S.; Theaker, J.; Whitcombe, D.; Ferrie, R.; Newton, C.; Little, S. The elimination of primer-dimer accumulation in PCR. Nucleic Acids Res. 1997, 25, 3235–3241. [Google Scholar] [CrossRef]
- Hao, X.; Liu, R.; He, Y.; Xiao, X.; Xiao, W.; Zheng, Q.; Lin, X.; Tao, P.; Zhou, P.; Li, S. Multiplex PCR methods for detection of several viruses associated with canine respiratory and enteric diseases. PLoS ONE 2019, 14, e213295. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Qian, P.; Cao, Y.; Wang, J.; Sun, C.; Huang, B.; Cui, N.; Huo, N.; Wu, H.; et al. Molecular and serological investigation of cat viral infectious diseases in China from 2016 to 2019. Transbound. Emerg. Dis. 2020, 67, 2329–2335. [Google Scholar] [CrossRef]
- Francis, D.P.; Essex, M.; Hardy, W.J. Excretion of feline leukaemia virus by naturally infected pet cats. Nature 1977, 269, 252–254. [Google Scholar] [CrossRef]
- Carneiro, R.L.; Farias, J.P.; Pinheiro, J.R.; Farias, J.; Vielmo, A.C.; Birbrair, A.; Belmok, A.; Melo, F.L.; Ribeiro, B.M.; Chaves, G.; et al. First description of a multisystemic and lethal SARS-CoV-2 variant of concern P.1 (Gamma) infection in a FeLV-positive cat. Virol. J. 2022, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yang, F.; Wu, H.; Xu, L. Genetic characterization of novel reassortant H5N6-subtype influenza viruses isolated from cats in eastern China. Arch. Virol. 2017, 162, 3501–3505. [Google Scholar] [CrossRef] [PubMed]

| Pathogen | Primer Name | Primer Sequence (5′-3′) | Target Gene | PCR Products |
|---|---|---|---|---|
| FCV | FCV-F | AACCTGCGCTAACGTGCT | VP1 | 1940 bp |
| FCV-R | TGWATTCCCATGTAGGAGGC | |||
| FHV-1 | FHV-1-F | TACTTCAAGCCTTACGACCACG | gD | 1014 bp |
| FHV-1-R | GATCGAGACCTCTTTACCCTCA | |||
| FeLV | FeLV-F | TGGCACAGGTAAAGCAAGTT | gPr80 | 608 bp |
| FeLV-R | CGTGTCCACCAARAARGTCA | |||
| C.felis | C.felis-F | TTGTCGGATTGATTGGTCTT | ompA | 500 bp |
| C.felis-R | AGTTGGGTTCCAGGTTGTTA | |||
| IAV | IAV-F | CAGAGACTKGAARRTGTCTTTGC | M | 155 bp |
| IAV-R | CTACGCTGCAGTCCTCGCTC | |||
| FCoV | FCoV-F | GATGGAGTMTTCTGGGTTGC | N | 726 bp |
| FCoV-R | TTCCTCAGGCTTCTTATCAG | |||
| FeAstV | FeAstV-F | GAAATGGACTGGACACGTTATGA | ORF1ab | 418 bp |
| FeAstV-R | GGCTTGACCCACATGCCGAA | |||
| FPV | FPV-F | AAGACGTGCAAGCGAGTCC | VP2 | 337 bp |
| FPV-R | GAGCGAAGATAAGCAGCGTAA | |||
| FeKoV | FeKoV-F | CCTCTTTYCTTCGGGACTTTTA | polyprotein | 198 bp |
| FeKoV-R | ACCACATCACTGAYTGTTCGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Hao, X.; Chen, B.; Zhou, P.; Li, S. Two Multiplex PCR Methods for Detecting Several Pathogens Associated with Feline Respiratory and Intestinal Tracts. Vet. Sci. 2023, 10, 14. https://doi.org/10.3390/vetsci10010014
Xiao X, Hao X, Chen B, Zhou P, Li S. Two Multiplex PCR Methods for Detecting Several Pathogens Associated with Feline Respiratory and Intestinal Tracts. Veterinary Sciences. 2023; 10(1):14. https://doi.org/10.3390/vetsci10010014
Chicago/Turabian StyleXiao, Xiangyu, Xiangqi Hao, Bo Chen, Pei Zhou, and Shoujun Li. 2023. "Two Multiplex PCR Methods for Detecting Several Pathogens Associated with Feline Respiratory and Intestinal Tracts" Veterinary Sciences 10, no. 1: 14. https://doi.org/10.3390/vetsci10010014
APA StyleXiao, X., Hao, X., Chen, B., Zhou, P., & Li, S. (2023). Two Multiplex PCR Methods for Detecting Several Pathogens Associated with Feline Respiratory and Intestinal Tracts. Veterinary Sciences, 10(1), 14. https://doi.org/10.3390/vetsci10010014





